Medication Usage Record-Based Predictive Modeling of Neurodevelopmental Abnormality in Infants under One Year: A Prospective Birth Cohort Study
Abstract
:1. Introduction
2. Methods
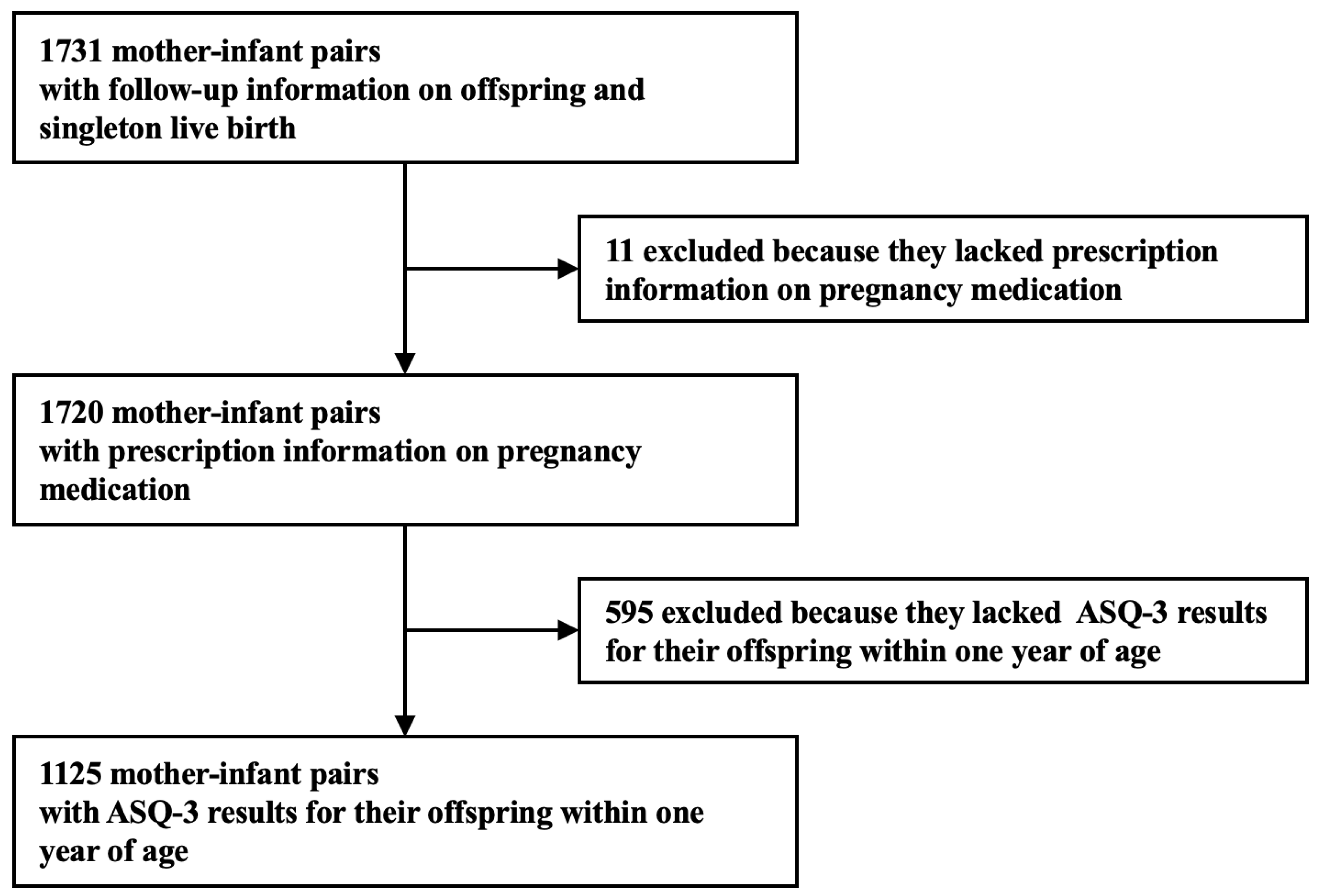
2.1. Study Design and Setting
2.2. Definition of Diagnosis Groups
2.3. Maternal Medications Exposure during Pregnancy
2.4. Definition of Maternal Characteristics
2.5. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Poling, A.; Methot, L.L.; Lesage, M.G. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Fein, D.; Barton, M.; Eigsti, I.M.; Kelley, E.; Naigles, L.; Schultz, R.T.; Stevens, M.; Helt, M.; Orinstein, A.; Rosenthal, M.; et al. Optimal outcome in individuals with a history of autism. J. Child. Psychol. Psychiatry 2013, 54, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Risi, S.; DiLavore, P.S.; Shulman, C.; Thurm, A.; Pickles, A. Autism from 2 to 9 years of age. Arch. Gen. Psychiatry 2006, 63, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.; Goodson, S. How well does early diagnosis of autism stand the test of time? Follow-up study of children assessed for autism at age 2 and development of an early diagnostic service. Autism 2003, 7, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill. Summ. 2012, 61, 1–19. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems: Alphabetical Index; World Health Organization: Geneva, Switzerland, 2004; Volume 3.
- Woo, E.; Lam, L. CAS Epidemiological Data on Autistic Spectrum Disorder from 2003–2005; Child Assessment Service Epidemiology and Research Gnlletin; Child Assessment Service, Hong Kong Special Adminstrative Region of the People’s Republic of China, Department of Health: Hong Kong, China, 2007.
- Khambadkone, S.G.; Cordner, Z.A.; Tamashiro, K.L.K. Maternal stressors and the developmental origins of neuropsychiatric risk. Front. Neuroendocrinol. 2020, 57, 100834. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fallin, M.D.; Riley, A.; Landa, R.; Walker, S.O.; Silverstein, M.; Caruso, D.; Pearson, C.; Kiang, S.; Dahm, J.L.; et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics 2016, 137, e20152206. [Google Scholar] [CrossRef]
- Jenabi, E.; Bashirian, S.; Khazaei, S.; Basiri, Z. The maternal prepregnancy body mass index and the risk of attention deficit hyperactivity disorder among children and adolescents: A systematic review and meta-analysis. Korean J. Pediatr. 2019, 62, 374–379. [Google Scholar] [CrossRef]
- Buchmayer, S.; Johansson, S.; Johansson, A.; Hultman, C.M.; Sparen, P.; Cnattingius, S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 2009, 124, e817–e825. [Google Scholar] [CrossRef]
- Yamamoto, J.M.; Benham, J.L.; Dewey, D.; Sanchez, J.J.; Murphy, H.R.; Feig, D.S.; Donovan, L.E. Neurocognitive and behavioural outcomes in offspring exposed to maternal pre-existing diabetes: A systematic review and meta-analysis. Diabetologia 2019, 62, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Maher, G.M.; O’Keeffe, G.W.; Kearney, P.M.; Kenny, L.C.; Dinan, T.G.; Mattsson, M.; Khashan, A.S. Association of Hypertensive Disorders of Pregnancy with Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 809–819. [Google Scholar] [CrossRef]
- Mann, J.R.; McDermott, S.; Bao, H.; Hardin, J.; Gregg, A. Pre-eclampsia, birth weight, and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 548–554. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Manning, S.E.; Hillier, A.; Anderson, S.L.; Nordgren, R.E.; Walters, S.E.; Nagaraja, H.N.; Cooley, W.C.; Gaelic, S.E.; Bauman, M.L. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 2005, 35, 471–478. [Google Scholar] [CrossRef]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; O’Keeffe, G.W.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1299–1309. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Peng, C.T.; Zhang, X.; Ruan, B. Antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in the children: A meta-analysis of cohort studies. BJOG 2018, 125, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Lee, B.K.; Dalman, C.; Golding, J.; Lewis, G.; Magnusson, C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: Population based case-control study. BMJ 2013, 346, f2059. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Wang, Y.; Tang, Y.; Qu, Y.; Tang, J.; Shi, J.; Xiao, D.; Mu, D. Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: A meta-analysis. Aust. N. Z. J. Psychiatry 2019, 53, 195–206. [Google Scholar] [CrossRef]
- Franz, A.P.; Bolat, G.U.; Bolat, H.; Matijasevich, A.; Santos, I.S.; Silveira, R.C.; Procianoy, R.S.; Rohde, L.A.; Moreira-Maia, C.R. Attention-Deficit/Hyperactivity Disorder and Very Preterm/Very Low Birth Weight: A Meta-analysis. Pediatrics 2018, 141, e20171645. [Google Scholar] [CrossRef]
- Johnson, S.; Hollis, C.; Kochhar, P.; Hennessy, E.; Wolke, D.; Marlow, N. Autism spectrum disorders in extremely preterm children. J. Pediatr. 2010, 156, 525–531.e2. [Google Scholar] [CrossRef]
- Guinchat, V.; Thorsen, P.; Laurent, C.; Cans, C.; Bodeau, N.; Cohen, D. Pre-, peri- and neonatal risk factors for autism. Acta Obstet. Gynecol. Scand. 2012, 91, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Jeste, S.S. Neurodevelopmental behavioral and cognitive disorders. Continuum 2015, 21, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, N.; Thind, R.; Mohammad, H.; Thabtah, F. Assessing Autistic Traits in Toddlers Using a Data-Driven Approach with DSM-5 Mapping. Bioengineering 2023, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.L.; Wang, S.H.; Liu, W.B.; Zhu, H.L.; Li, M.; Zou, X.B. A multimodal machine learning system in early screening for toddlers with autism spectrum disorders based on the response to name. Front. Psychiatry 2023, 14, 1039293. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Duan, H.; Wang, G. Application of Machine Learning Techniques to Detect the Children with Autism Spectrum Disorder. J. Healthc. Eng. 2022, 2022, 9340027. [Google Scholar] [CrossRef]
- Moradi, E.; Khundrakpam, B.; Lewis, J.D.; Evans, A.C.; Tohka, J. Predicting symptom severity in autism spectrum disorder based on cortical thickness measures in agglomerative data. Neuroimage 2017, 144, 128–141. [Google Scholar] [CrossRef]
- Oztekin, I.; Finlayson, M.A.; Graziano, P.A.; Dick, A.S. Is there any incremental benefit to conducting neuroimaging and neurocognitive assessments in the diagnosis of ADHD in young children? A machine learning investigation. Dev. Cogn. Neurosci. 2021, 49, 100966. [Google Scholar] [CrossRef]
- Siugzdaite, R.; Bathelt, J.; Holmes, J.; Astle, D.E. Transdiagnostic Brain Mapping in Developmental Disorders. Curr. Biol. 2020, 30, 1245–1257.e4. [Google Scholar] [CrossRef]
- Santos, A.; Caramelo, F.; Melo, J.B.; Castelo-Branco, M. Dopaminergic Gene Dosage Reveals Distinct Biological Partitions between Autism and Developmental Delay as Revealed by Complex Network Analysis and Machine Learning Approaches. J. Pers. Med. 2022, 12, 1579. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Alipanahi, B.; Lee, L.J.; Bretschneider, H.; Merico, D.; Yuen, R.K.; Hua, Y.; Gueroussov, S.; Najafabadi, H.S.; Hughes, T.R. The human splicing code reveals new insights into the genetic determinants of disease. Science 2015, 347, 1254806. [Google Scholar] [CrossRef]
- Abbas, H.; Garberson, F.; Glover, E.; Wall, D.P. Machine learning approach for early detection of autism by combining questionnaire and home video screening. J. Am. Med. Inform. Assoc. 2018, 25, 1000–1007. [Google Scholar] [CrossRef]
- Song, I.; Marsh, N.V. Anonymous indexing of health conditions for a similarity measure. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Das, W.; Khanna, S. A Robust Machine Learning Based Framework for the Automated Detection of ADHD Using Pupillometric Biomarkers and Time Series Analysis. Sci. Rep. 2021, 11, 16370. [Google Scholar] [CrossRef]
- Sardaar, S.; Qi, B.; Dionne-Laporte, A.; Rouleau, G.A.; Rabbany, R.; Trakadis, Y.J. Machine learning analysis of exome trios to contrast the genomic architecture of autism and schizophrenia. BMC Psychiatry 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Lin, L.; Han, N.; Zhao, Z.; Liu, Z.; Luo, S.; Xu, X.; Liu, J.; Wang, H. Effects of dynamic change in fetuin-A levels from the first to the second trimester on insulin resistance and gestational diabetes mellitus: A nested case-control study. BMJ Open Diabetes Res. Care 2020, 8, e000802. [Google Scholar] [CrossRef]
- Blackwell, C.K.; Wakschlag, L.S.; Gershon, R.C.; Cella, D.; with the ECHO PRO Core. Measurement framework for the Environmental influences on Child Health Outcomes research program. Curr. Opin. Pediatr. 2018, 30, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Department of Disease Control Ministry of Health. Guidelines for the Prevention and Control of Overweight and Obesity in Chinese Adults; People’s Medical Publishing House Co., Ltd.: Beijing, China, 2006. (In Chinese) [Google Scholar]
- American College of Obstetricians and Gynecologists’ Committee on Clinical Consensus-Obstetrics; Gantt, A.; Society for Maternal-Fetal Medicine; Metz, T.D.; Kuller, J.A.; Louis, J.M.; Society for Maternal-Fetal Medicine; Cahill, A.G.; Turrentine, M.A.; American College of Obstetricians and Gynecologists. Obstetric Care Consensus #11, Pregnancy at age 35 years or older. Am. J. Obstet. Gynecol. 2023, 228, B25–B40. [Google Scholar] [CrossRef]
- Ismail, E.; Gad, W.; Hashem, M. A hybrid Stacking-SMOTE model for optimizing the prediction of autistic genes. BMC Bioinform. 2023, 24, 379. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Lundberg, S.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar]
- Yeung, C.; Ho, D.; Zhang, Z.; Raman, A.P.; Pham, B.; Fountaine, K.T.; Levy, K. Enhancing Adjoint Optimization-Based Photonic Inverse Design with Explainable Machine Learning. ACS Photonics 2022, 9, 1577–1585. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Larsson, H.; Sariaslan, A.; Langstrom, N.; D’Onofrio, B.; Lichtenstein, P. Family income in early childhood and subsequent attention deficit/hyperactivity disorder: A quasi-experimental study. J. Child. Psychol. Psychiatry 2014, 55, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.S.; Skipper, B.J.; Rabiner, D.L.; Qeadan, F.; Campbell, R.A.; Naftel, A.J.; Umbach, D.M. Attention-Deficit/Hyperactivity Disorder (ADHD): Interaction between socioeconomic status and parental history of ADHD determines prevalence. J. Child. Psychol. Psychiatry 2018, 59, 213–222. [Google Scholar] [CrossRef]
- Walton, K.M. Risk Factors for Behavioral and Emotional Difficulties in Siblings of Children With Autism Spectrum Disorder. Am. J. Intellect. Dev. Disabil. 2016, 121, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef]
- Getz, K.D.; Anderka, M.T.; Werler, M.M.; Jick, S.S. Maternal Pre-pregnancy Body Mass Index and Autism Spectrum Disorder among Offspring: A Population-Based Case-Control Study. Paediatr. Perinat. Epidemiol. 2016, 30, 479–487. [Google Scholar] [CrossRef]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.; Uher, R.; Reichenberg, A.; Sandin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy With the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176–184. [Google Scholar] [CrossRef]
- Ji, Y.; Raghavan, R.; Wang, X. Early Life Origins of ASD and ADHD. Sex. Reprod. Health Spec. Popul. 2021. [Google Scholar] [CrossRef]
- Kane, R.C. A possible association between fetal/neonatal exposure to radiofrequency electromagnetic radiation and the increased incidence of autism spectrum disorders (ASD). Med. Hypotheses 2004, 62, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Candelas, C.; Hwei, T.; Lee, S.; Lucchini, M.; Smaniotto Aizza, A.; Kahn, L.G.; Buss, C.; O’Connor, T.G.; Ghassabian, A.; Padula, A.M.; et al. Prenatal sleep health and risk of offspring ADHD symptomatology and associated phenotypes: A prospective analysis of timing and sex differences in the ECHO cohort. Lancet Reg. Health Am. 2023, 27, 100609. [Google Scholar] [CrossRef]
- Glover, V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 25–35. [Google Scholar] [CrossRef]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef]
- Shaw, K.N.; Commins, S.; O’Mara, S.M. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broad-spectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. Eur. J. Neurosci. 2003, 17, 2438–2446. [Google Scholar] [CrossRef]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; McCarthy, M.M. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur. J. Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef]
- Ji, Y.; Riley, A.W.; Lee, L.C.; Hong, X.; Wang, G.; Tsai, H.J.; Mueller, N.T.; Pearson, C.; Thermitus, J.; Panjwani, A.; et al. Maternal Biomarkers of Acetaminophen Use and Offspring Attention Deficit Hyperactivity Disorder. Brain Sci. 2018, 8, 127. [Google Scholar] [CrossRef]
- Ji, Y.; Azuine, R.E.; Zhang, Y.; Hou, W.; Hong, X.; Wang, G.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry 2020, 77, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ding, R.; Wang, J. The Association between Vitamin D Status and Autism Spectrum Disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients 2020, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Gusso, D.; Prauchner, G.R.K.; Rieder, A.S.; Wyse, A.T.S. Biological Pathways Associated with Vitamins in Autism Spectrum Disorder. Neurotox. Res. 2023, 41, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.J. Prevalence, consequences and prevention of childhood nutritional iron deficiency: A child public health perspective. Clin. Lab. Haematol. 2006, 28, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Han, X.; Wang, J.; Sun, J. Baicalin may have a therapeutic effect in attention deficit hyperactivity disorder. Med. Hypotheses 2015, 85, 761–764. [Google Scholar] [CrossRef] [PubMed]
- dela Pena, I.C.; Young Yoon, S.; Kim, Y.; Park, H.; Man Kim, K.; Hoon Ryu, J.; Young Shin, C.; Hoon Cheong, J. 5,7-Dihydroxy-6-methoxy-4′-phenoxyflavone, a derivative of oroxylin A improves attention-deficit/hyperactivity disorder (ADHD)-like behaviors in spontaneously hypertensive rats. Eur. J. Pharmacol. 2013, 715, 337–344. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.E.; Soriano, S.G. Does general anesthesia affect neurodevelopment in infants and children? BMJ 2019, 367, l6459. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mathena, R.P.; Singh, S.; Kim, J.; Long, J.J.; Li, Q.; Junn, S.; Blaize, E.; Mintz, C.D. Early Developmental Exposure to Repetitive Long Duration of Midazolam Sedation Causes Behavioral and Synaptic Alterations in a Rodent Model of Neurodevelopment. J. Neurosurg. Anesthesiol. 2019, 31, 151–162. [Google Scholar] [CrossRef]
- Iqbal O’Meara, A.M.; Miller Ferguson, N.; Zven, S.E.; Karam, O.L.; Meyer, L.C.; Bigbee, J.W.; Sato-Bigbee, C. Potential Neurodevelopmental Effects of Pediatric Intensive Care Sedation and Analgesia: Repetitive Benzodiazepine and Opioid Exposure Alters Expression of Glial and Synaptic Proteins in Juvenile Rats. Crit. Care Explor. 2020, 2, e0105. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Meyer, D.; Meyer, L.; Chand, S.; Jagadesan, S.; Miravite, M.; Guda, C.; Yelamanchili, S.V.; Pendyala, G. Identification of YWHAH as a Novel Brain-Derived Extracellular Vesicle Marker Post Long-Term Midazolam Exposure during Early Development. Cells 2023, 12, 966. [Google Scholar] [CrossRef]
- Boscolo, A.; Milanovic, D.; Starr, J.A.; Sanchez, V.; Oklopcic, A.; Moy, L.; Ori, C.C.; Erisir, A.; Jevtovic-Todorovic, V. Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology 2013, 118, 1086–1097. [Google Scholar] [CrossRef]
- Slikker, W., Jr.; Paule, M.G.; Wright, L.K.; Patterson, T.A.; Wang, C. Systems biology approaches for toxicology. J. Appl. Toxicol. 2007, 27, 201–217. [Google Scholar] [CrossRef]
- Edwards, D.A.; Shah, H.P.; Cao, W.; Gravenstein, N.; Seubert, C.N.; Martynyuk, A.E. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology 2010, 112, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.; Chiu, P.H.; Lin, C.Y.; Chen, H.H. Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr. Res. 2012, 136, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, Z.; Zhang, F.; Liu, Y.; Zhang, Y.; Sun, X.; Sang, M.; Luo, H. Ropivacaine mesylate exerts neurotoxicity via up-regulation of Fas/FasL expression in rat pheochromocytoma PC12 cells. Am. J. Transl. Res. 2019, 11, 1626–1634. [Google Scholar] [PubMed]
- Vassal, O.; Del Carmine, P.; Beuriat, P.A.; Desgranges, F.P.; Gadot, N.; Allaouchiche, B.; Timour-Chah, Q.; Stewart, A.; Chassard, D. Neurotoxicity of intrathecal 6% hydroxyethyl starch 130/0.4 injection in a rat model. Anaesthesia 2015, 70, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (%) |
|---|---|
| Total | 1125 |
| Male = yes | 572 (50.8) |
| Advanced maternal age = yes | 128 (11.4) |
| Maternal pre-pregnancy BMI | |
| Low | 91 (8.1) |
| Norm | 677 (60.2) |
| Fat | 357 (31.7) |
| Sum of people (mean (SD)) | 3.35 (1.31) |
| Family income (mean (SD)) | 17.03 (13.89) |
| Smoke | |
| Non-Smoker | 1058 (94.0) |
| Ex-Smoker | 61 (5.4) |
| Smoker | 6 (0.5) |
| Alcohol = ever drink | 34 (3.0) |
| Folate | |
| Never supplemented folate | 237 (21.1) |
| Formerly supplemented folate | 90 (8.0) |
| Currently supplemented folate | 798 (70.9) |
| Iron | |
| Never supplemented iron | 1079 (95.9) |
| Formerly supplemented iron | 8 (0.7) |
| Currently supplemented iron | 38 (3.4) |
| Anxiety | |
| Never anxiety | 1011 (89.9) |
| Subthreshold anxiety | 110 (9.8) |
| Severe anxiety | 4 (0.4) |
| PSQI = yes | 140 (12.4) |
| Pressure exposure = yes | 109 (9.7) |
| Chemical exposure = yes | 481 (42.8) |
| Physical exposure = yes | 303 (26.9) |
| Animal exposure = yes | 271 (24.1) |
| Medication types taken (mean (SD)) | 17.23 (5.96) |
| ASQ-3 result | |
| Communication disorder = yes | 55 (4.9) |
| Gross Motor disorder = yes | 83 (7.4) |
| Fine Motor disorder = yes | 26 (2.3) |
| Problem Solving disorder = yes | 33 (2.9) |
| Personal-Social disorder = yes | 77 (6.8) |
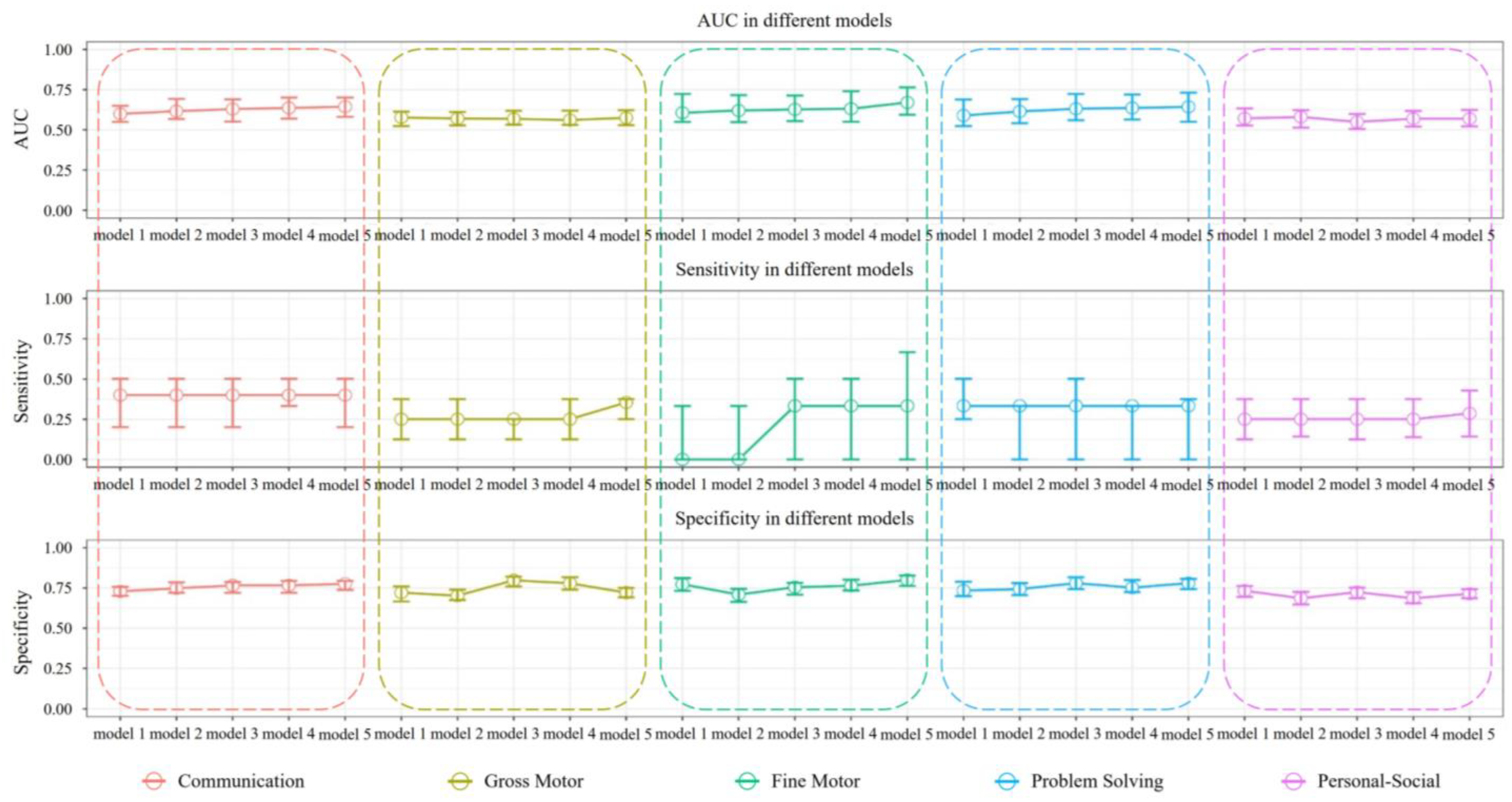
| Area | Model | AUC Median (25%, 75%) | Specificity Median (25%, 75%) | Sensitivity Median (25%, 75%) |
|---|---|---|---|---|
| Communication | model 1 | 0.599 (0.549, 0.649) | 0.729 (0.701, 0.757) | 0.400 (0.200, 0.500) |
| model 2 | 0.616 (0.567, 0.692) | 0.748 (0.720, 0.785) | 0.400 (0.200, 0.500) | |
| model 3 | 0.629 (0.551, 0.690) | 0.766 (0.720, 0.787) | 0.400 (0.200, 0.500) | |
| model 4 | 0.636 (0.569, 0.701) | 0.766 (0.720, 0.794) | 0.400 (0.333, 0.600) | |
| model 5 | 0.644 (0.581, 0.701) | 0.776 (0.738, 0.794) | 0.400 (0.200, 0.500) | |
| Gross Motor | model 1 | 0.577 (0.523, 0.613) | 0.721 (0.666, 0.760) | 0.250 (0.125, 0.375) |
| model 2 | 0.570 (0.528, 0.611) | 0.702 (0.675, 0.740) | 0.250 (0.125, 0.375) | |
| model 3 | 0.568 (0.532, 0.618) | 0.798 (0.760, 0.821) | 0.250 (0.125, 0.250) | |
| model 4 | 0.561 (0.531, 0.619) | 0.779 (0.740, 0.817) | 0.250 (0.125, 0.375) | |
| model 5 | 0.574 (0.529, 0.622) | 0.721 (0.692, 0.750) | 0.354 (0.250, 0.375) | |
| Fine Motor | model 1 | 0.606 (0.549, 0.723) | 0.773 (0.732, 0.811) | 0.000 (0.000, 0.333) |
| model 2 | 0.620 (0.548, 0.716) | 0.709 (0.664, 0.745) | 0.000 (0.000, 0.333) | |
| model 3 | 0.627 (0.554, 0.713) | 0.755 (0.709, 0.782) | 0.333 (0.000, 0.500) | |
| model 4 | 0.631 (0.550, 0.740) | 0.764 (0.734, 0.802) | 0.333 (0.000, 0.500) | |
| model 5 | 0.670 (0.594, 0.764) | 0.800 (0.763, 0.827) | 0.333 (0.000, 0.667) | |
| Problem Solving | model 1 | 0.589 (0.523, 0.689) | 0.734 (0.699, 0.789) | 0.333 (0.250, 0.500) |
| model 2 | 0.614 (0.541, 0.691) | 0.743 (0.706, 0.780) | 0.333 (0.000, 0.333) | |
| model 3 | 0.631 (0.560, 0.723) | 0.780 (0.743, 0.817) | 0.333 (0.000, 0.500) | |
| model 4 | 0.636 (0.564, 0.720) | 0.752 (0.725, 0.799) | 0.333 (0.000, 0.333) | |
| model 5 | 0.643 (0.550, 0.731) | 0.780 (0.743, 0.807) | 0.333 (0.000, 0.375) | |
| Personal-Social | model 1 | 0.571 (0.527, 0.633) | 0.733 (0.695, 0.762) | 0.250 (0.125, 0.375) |
| model 2 | 0.580 (0.514, 0.623) | 0.686 (0.648, 0.726) | 0.250 (0.143, 0.375) | |
| model 3 | 0.549 (0.505, 0.598) | 0.724 (0.686, 0.752) | 0.250 (0.125, 0.375) | |
| model 4 | 0.569 (0.520, 0.617) | 0.686 (0.655, 0.724) | 0.250 (0.138, 0.375) | |
| model 5 | 0.569 (0.521, 0.624) | 0.714 (0.686, 0.743) | 0.286 (0.143, 0.429) |
| Size a | Decay b | Accuracy Median (25%, 75%) | Sensitivity Median (25%, 75%) | Specificity Median (25%, 75%) | AUC Median (25%, 75%) |
|---|---|---|---|---|---|
| 4 | 0.2 | 0.721 (0.696, 0.739) | 0.700 (0.597, 0.797) | 0.742 (0.680, 0.748) | 0.821 (0.716, 0.833) |
| 6 | 0.4 | 0.730 (0.699, 0.746) | 0.700 (0.562, 0.738) | 0.731 (0.723, 0.757) | 0.812 (0.715, 0.832) |
| 2 | 0.01 | 0.721 (0.695, 0.734) | 0.770 (0.588, 0.837) | 0.704 (0.669, 0.753) | 0.811 (0.727, 0.833) |
| 3 | 0.05 | 0.720 (0.653, 0.747) | 0.718 (0.550, 0.825) | 0.715 (0.659, 0.741) | 0.809 (0.709, 0.823) |
| 6 | 0.5 | 0.720 (0.690, 0.763) | 0.750 (0.575, 0.750) | 0.726 (0.712, 0.749) | 0.808 (0.722, 0.829) |
| 2 | 0.4 | 0.735 (0.701, 0.765) | 0.700 (0.550, 0.738) | 0.751 (0.734, 0.761) | 0.808 (0.713, 0.829) |
| 4 | 0.4 | 0.733 (0.701, 0.767) | 0.693 (0.532, 0.750) | 0.753 (0.699, 0.770) | 0.808 (0.708, 0.830) |
| 8 | 0.3 | 0.719 (0.688, 0.749) | 0.641 (0.545, 0.788) | 0.726 (0.710, 0.748) | 0.807 (0.713, 0.821) |
| 7 | 0.3 | 0.717 (0.684, 0.740) | 0.718 (0.550, 0.788) | 0.726 (0.680, 0.747) | 0.806 (0.705, 0.825) |
| 4 | 0.5 | 0.732 (0.684, 0.756) | 0.700 (0.512, 0.738) | 0.735 (0.704, 0.772) | 0.806 (0.713, 0.828) |
| Variable Name | Mean(|SHAP Value|) | Num | ||||
|---|---|---|---|---|---|---|
| Communication | Gross Motor | Fine Motor | Problem Solving | Personal-Social | ||
| Family income | 0.095 | 0.083 | - | 0.125 | 0.088 | 4 |
| Sum of people | 0.060 | 0.099 | - | 0.070 | 0.057 | 4 |
| Chemical exposure | 0.059 | - | - | 0.060 | 0.111 | 3 |
| Maternal pre-pregnancy BMI | 0.057 | 0.062 | - | - | - | 2 |
| Acetaminophen | - | 0.073 | - | - | - | 1 |
| Blue Scutellaria Oral Liquid | - | - | - | 0.057 | - | 1 |
| Calcium Acetate Granules | - | - | - | - | 0.086 | 1 |
| Chuanbai Anti-Itch Wash | - | 0.062 | - | - | - | 1 |
| Currently supplemented folate | - | - | - | 0.075 | - | 1 |
| Ferrous Succinate | - | - | 0.080 | - | - | 1 |
| Hydroxyethyl Starch Sodium Chloride Injection | - | - | 0.062 | - | - | 1 |
| Midazolam injection | - | - | 0.097 | - | - | 1 |
| Physical exposure | - | - | - | - | 0.060 | 1 |
| PSQI | 0.104 | - | - | - | - | 1 |
| Ropivacaine mesylate injection | - | - | 0.097 | - | - | 1 |
| Subthreshold anxiety | - | - | 0.068 | - | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Shen, Y.; Lyu, J.; Yang, L.; Wang, H.-J.; Hong, S.; Ji, Y. Medication Usage Record-Based Predictive Modeling of Neurodevelopmental Abnormality in Infants under One Year: A Prospective Birth Cohort Study. Healthcare 2024, 12, 713. https://doi.org/10.3390/healthcare12070713
Zhou T, Shen Y, Lyu J, Yang L, Wang H-J, Hong S, Ji Y. Medication Usage Record-Based Predictive Modeling of Neurodevelopmental Abnormality in Infants under One Year: A Prospective Birth Cohort Study. Healthcare. 2024; 12(7):713. https://doi.org/10.3390/healthcare12070713
Chicago/Turabian StyleZhou, Tianyi, Yaojia Shen, Jinlang Lyu, Li Yang, Hai-Jun Wang, Shenda Hong, and Yuelong Ji. 2024. "Medication Usage Record-Based Predictive Modeling of Neurodevelopmental Abnormality in Infants under One Year: A Prospective Birth Cohort Study" Healthcare 12, no. 7: 713. https://doi.org/10.3390/healthcare12070713
APA StyleZhou, T., Shen, Y., Lyu, J., Yang, L., Wang, H.-J., Hong, S., & Ji, Y. (2024). Medication Usage Record-Based Predictive Modeling of Neurodevelopmental Abnormality in Infants under One Year: A Prospective Birth Cohort Study. Healthcare, 12(7), 713. https://doi.org/10.3390/healthcare12070713






