Low Dietary Betaine Intake Is Associated with Increased Blood Cholesterol in Mexican Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometrics and Blood Pressure Measurements
2.3. Dietary Intake and Physical Exercise
2.4. Biochemical Tests
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta 2016, 1860, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules 2023, 28, 4824. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med. 2005, 43, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, S.; Zhao, Y.; Wang, H.; Feng, J. Dietary Betaine Addition Promotes Hepatic Cholesterol Synthesis, Bile Acid Conversion, and Export in Rats. Nutrients 2020, 12, 1399. [Google Scholar] [CrossRef]
- Xu, L.; Huang, D.; Hu, Q.; Wu, J.; Wang, Y.; Feng, J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br. J. Nutr. 2015, 113, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.M.; Neves, J.A.; Freitas, A.; Tirapicos, J.L. Effect of long-term betaine supplementation on chemical and physical characteristics of three muscles from the Alentejano pig. J. Sci. Food Agric. 2012, 92, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Millard, H.R.; Musani, S.K.; Dibaba, D.T.; Talegawkar, S.A.; Taylor, H.A.; Tucker, K.L.; Bidulescu, A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: The Jackson Heart Study. Eur. J. Nutr. 2018, 57, 51–60. [Google Scholar] [CrossRef]
- Detopoulou, P.; Panagiotakos, D.B.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2008, 87, 424–430. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; Olthof, M.R.; Verhoef, P.; Bots, M.L.; van der Schouw, Y.T. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur. J. Clin. Nutr. 2008, 62, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Kawachi, T.; Tsuji, M.; Nakamura, K. Choline and Betaine Intakes Are Not Associated with Cardiovascular Disease Mortality Risk in Japanese Men and Women. J. Nutr. 2015, 145, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Rajaie, S.; Esmaillzadeh, A. Dietary choline and betaine intakes and risk of cardiovascular diseases: Review of epidemiological evidence. ARYA Atheroscler. 2011, 7, 78–86. [Google Scholar]
- Abbasi, M.S.P.; Tousi, A.Z.; Yazdani, Y.; Vahdat, S.; Gharebakhshi, F.; Nikrad, N.; Manzouri, A.; Ardekani, A.M.; Jafarzadeh, F. Dietary choline and betaine intake, cardio-metabolic risk factors and prevalence of metabolic syndrome among overweight and obese adults. BMC Endocr. Disord. 2023, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Paredez, B.; Aparicio-Bautista, D.I.; Argoty-Pantoja, A.D.; Patiño, N.; Flores Morales, J.; Salmerón, J.; León-Reyes, G.; Velázquez-Cruz, R. Association of MARC1, ADCY5, and BCO1 Variants with the Lipid Profile, Suggests an Additive Effect for Hypertriglyceridemia in Mexican Adult Men. Int. J. Mol. Sci. 2022, 23, 11815. [Google Scholar] [CrossRef] [PubMed]
- Fierro, N.A.; Gonzalez-Aldaco, K.; Torres-Valadez, R.; Martinez-Lopez, E.; Roman, S.; Panduro, A. Immunologic, metabolic and genetic factors in hepatitis C virus infection. World J. Gastroenterol. 2014, 20, 3443–3456. [Google Scholar] [CrossRef]
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Gomez, B.; Almeda-Valdés, P.; Tussié-Luna, M.T.; Aguilar-Salinas, C.A. Dyslipidemia in mexico, a call for action. Rev. Investig. Clin. 2018, 70, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ramos-López, O.; Ojeda-Granados, C.; Román, S.; Panduro, A. Influencia genética en las preferencias alimentarias. Rev. Endocrinol. Nutr. 2013, 21, 74–83. [Google Scholar]
- Roman, S.; Ojeda-Granados, C.; Ramos-Lopez, O.; Panduro, A. Genome-based nutrition: An intervention strategy for the prevention and treatment of obesity and nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3449–3461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Milner, M.; Klonizakis, M. Physiological effects of a short-term lifestyle intervention based on the Mediterranean diet: Comparison between older and younger healthy, sedentary adults. Nutrition 2018, 55–56, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Bugg, A.; Hunt, B.; Theodoridis, X.; Bogdanos, D.P.; Grammatikopoulou, M.G. Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions. Nutrients 2021, 13, 3034. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aceviz, Y.; Sobrevilla-Navarro, A.A.; Ramos-Lopez, O. Dietary Intake of Capsaicin and Its Association with Markers of Body Adiposity and Fatty Liver in a Mexican Adult Population of Tijuana. Healthcare 2023, 11, 3001. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Mejia-Godoy, R.; Frías-Delgadillo, K.J.; Torres-Valadez, R.; Flores-García, A.; Sánchez-Enríquez, S.; Aguiar-García, P.; Martínez-López, E.; Zepeda-Carrillo, E.A. Interactions between DRD2/ANKK1 TaqIA Polymorphism and Dietary Factors Influence Plasma Triglyceride Concentrations in Diabetic Patients from Western Mexico: A Cross-sectional Study. Nutrients 2019, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Panduro, A.; Rivera-Iñiguez, I.; Roman, S. Dopamine D2 receptor polymorphism (C957T) is associated with sugar consumption and triglyceride levels in West Mexicans. Physiol. Behav. 2018, 194, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Panduro, A.; Martinez-Lopez, E.; Roman, S. Sweet Taste Receptor TAS1R2 Polymorphism (Val191Val) Is Associated with a Higher Carbohydrate Intake and Hypertriglyceridemia among the Population of West Mexico. Nutrients 2016, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Rios, D.; Panduro, A.; Roman, S.; Ramos-Lopez, O. CD36 polymorphism, sugary drinks, and sedentarism are associated with hypertriglyceridemic waist phenotype. Int. J. Vitam. Nutr. Res. 2024, 94, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Ranganathan, P. Common pitfalls in statistical analysis: The use of correlation techniques. Perspect. Clin. Res. 2016, 7, 187–190. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Lever, M.; George, P.M.; Atkinson, W.; Molyneux, S.L.; Elmslie, J.L.; Slow, S.; Richards, A.M.; Chambers, S.T. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PLoS ONE 2011, 6, e21666. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, S.V.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Bleie, Ø.; Ueland, P.M. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J. Nutr. 2008, 138, 914–920. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Sikand, G.; Petersen, K.S.; Anderson, C.A.M.; Aspry, K.E.; Bolick, J.P.; Kris-Etherton, P.M.; Maki, K.C. Nutrition interventions for adults with dyslipidemia: A Clinical Perspective from the National Lipid Association. J. Clin. Lipidol. 2023, 17, 428–451. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Törrönen, A.; Toppinen, L.; Alfthan, G.; Saarinen, M.; Aro, A.; Uusitupa, M. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am. J. Clin. Nutr. 2002, 76, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Grizales, A.M.; Patti, M.E.; Lin, A.P.; Beckman, J.A.; Sahni, V.A.; Cloutier, E.; Fowler, K.M.; Dreyfuss, J.M.; Pan, H.; Kozuka, C.; et al. Metabolic Effects of Betaine: A Randomized Clinical Trial of Betaine Supplementation in Prediabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3038–3049. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; van Vliet, T.; Verhoef, P.; Zock, P.L.; Katan, M.B. Effect of homocysteine-lowering nutrients on blood lipids: Results from four randomised, placebo-controlled studies in healthy humans. PLoS Med. 2005, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Zawieja, E.E.; Zawieja, B.; Chmurzynska, A. Betaine Supplementation Moderately Increases Total Cholesterol Levels: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2021, 18, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Zawieja, E.; Durkalec-Michalski, K.; Muzsik-Kazimierska, A.; Chmurzynska, A. The Effect of 3-Week Betaine Supplementation on Blood Biomarkers of Cardiometabolic Health in Young Physically Active Males. Metabolites 2022, 12, 731. [Google Scholar] [CrossRef]
- Zeisel, S.H. Betaine supplementation and blood lipids: Fact or artifact? Nutr. Rev. 2006, 64, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Ghanavati, M.; Asbaghi, O.; Tinsley, G.M.; Mombaini, D.; Kooti, W.; Kashkooli, S.; Wong, A. Effects of betaine supplementation on cardiovascular markers: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6516–6533. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.W.; Choi, Y.J.; Hong, S.H.; Jun, D.S.; Na, J.D.; Choi, Y.J.; Kim, Y.C. Involvement of multiple pathways in the protection of liver against high-fat diet-induced steatosis by betaine. J. Funct. Foods 2015, 17, 66–72. [Google Scholar] [CrossRef]
- Idriss, A.A.; Hu, Y.; Hou, Z.; Hu, Y.; Sun, Q.; Omer, N.A.; Abobaker, H.; Ni, Y.; Zhao, R. Dietary betaine supplementation in hens modulates hypothalamic expression of cholesterol metabolic genes in F1 cockerels through modification of DNA methylation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 217, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Abobaker, H.; Hu, Y.; Hou, Z.; Sun, Q.; Idriss, A.A.; Omer, N.A.; Zong, Y.; Zhao, R. Dietary betaine supplementation increases adrenal expression of steroidogenic acute regulatory protein and yolk deposition of corticosterone in laying hens. Poult. Sci. 2017, 96, 4389–4398. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Fan, R.; Du, Y.; Hou, M.; Tang, Z.; Ling, W.; Zhu, H. Betaine supplementation attenuates atherosclerotic lesion in apolipoprotein E-deficient mice. Eur. J. Nutr. 2009, 48, 205–212. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Asare, E.; Yang, Y.; Yang, J.J.; Yang, H.M.; Wang, Z.Y. Dietary supplementation of betaine promotes lipolysis by regulating fatty acid metabolism in geese. Poult. Sci. 2021, 100, 101460. [Google Scholar] [CrossRef]
- Liu, J.; Ding, L.; Zhai, X.; Wang, D.; Xiao, C.; Hui, X.; Sun, T.; Yu, M.; Zhang, Q.; Li, M.; et al. Maternal Dietary Betaine Prevents High-Fat Diet-Induced Metabolic Disorders and Gut Microbiota Alterations in Mouse Dams and Offspring From Young to Adult. Front. Microbiol. 2022, 13, 809642. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tan, X.; Liang, X.; Chen, H.; Ou, Q.; Wu, Q.; Yu, X.; Zhao, H.; Huang, Q.; Yi, Z.; et al. Maternal Betaine Supplementation Mitigates Maternal High Fat Diet-Induced NAFLD in Offspring Mice through Gut Microbiota. Nutrients 2023, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, W.; Feng, Y.; Wu, L.; Jia, Y.; Zhao, R. Betaine Alleviates High-Fat Diet-Induced Disruptionof Hepatic Lipid and Iron Homeostasis in Mice. Int. J. Mol. Sci. 2022, 23, 6263. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Jia, Y.; Lu, J.; Yuan, M.; Sui, S.; Song, H.; Zhao, R. Maternal dietary betaine supplementation modifies hepatic expression of cholesterol metabolic genes via epigenetic mechanisms in newborn piglets. Br. J. Nutr. 2014, 112, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Armstrong, M.E.G.; Papadaki, A. Adherence to a traditional Mexican diet and non-communicable disease-related outcomes: Secondary data analysis of the cross-sectional Mexican National Health and Nutrition Survey. Br. J. Nutr. 2022, 129, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
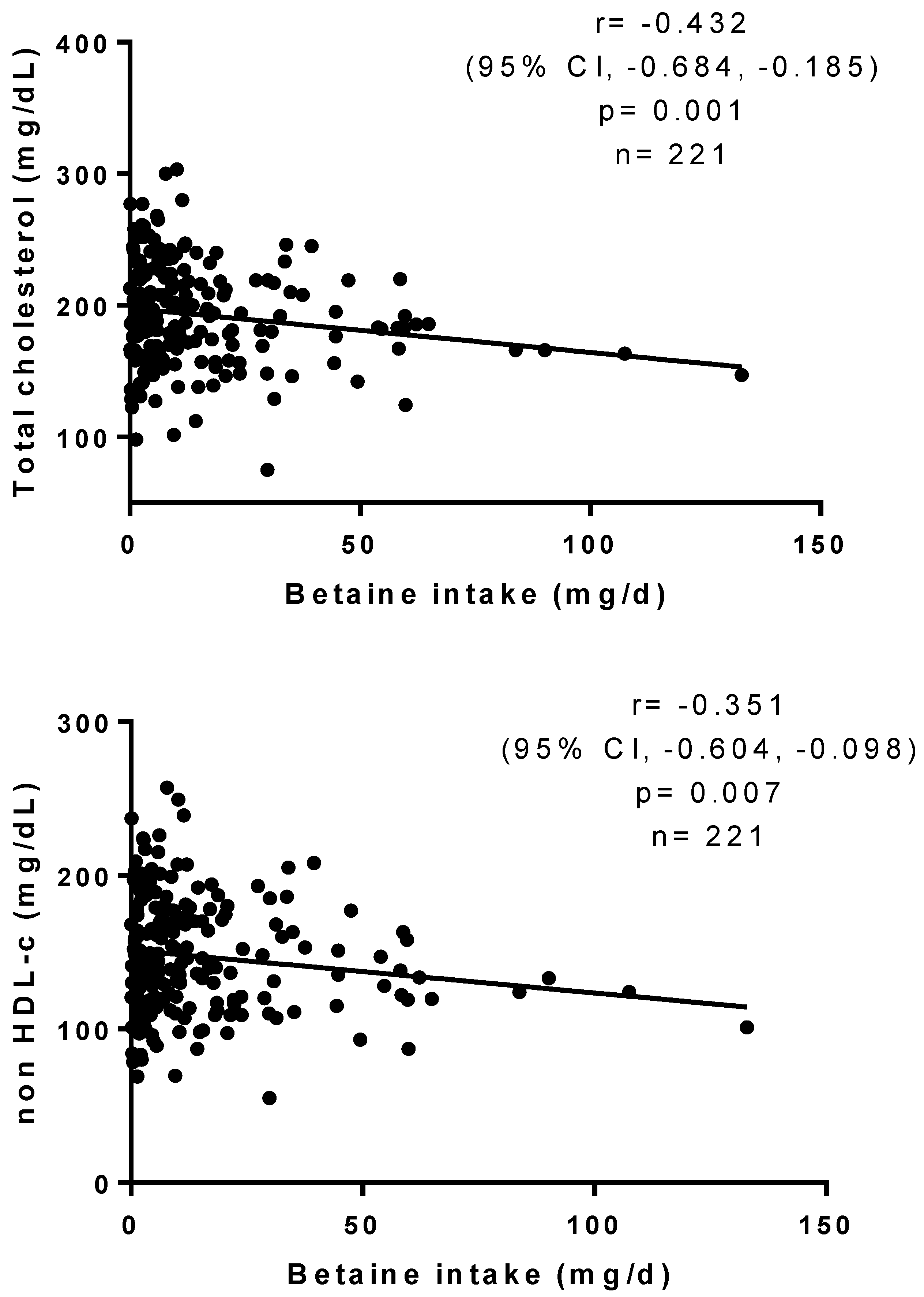
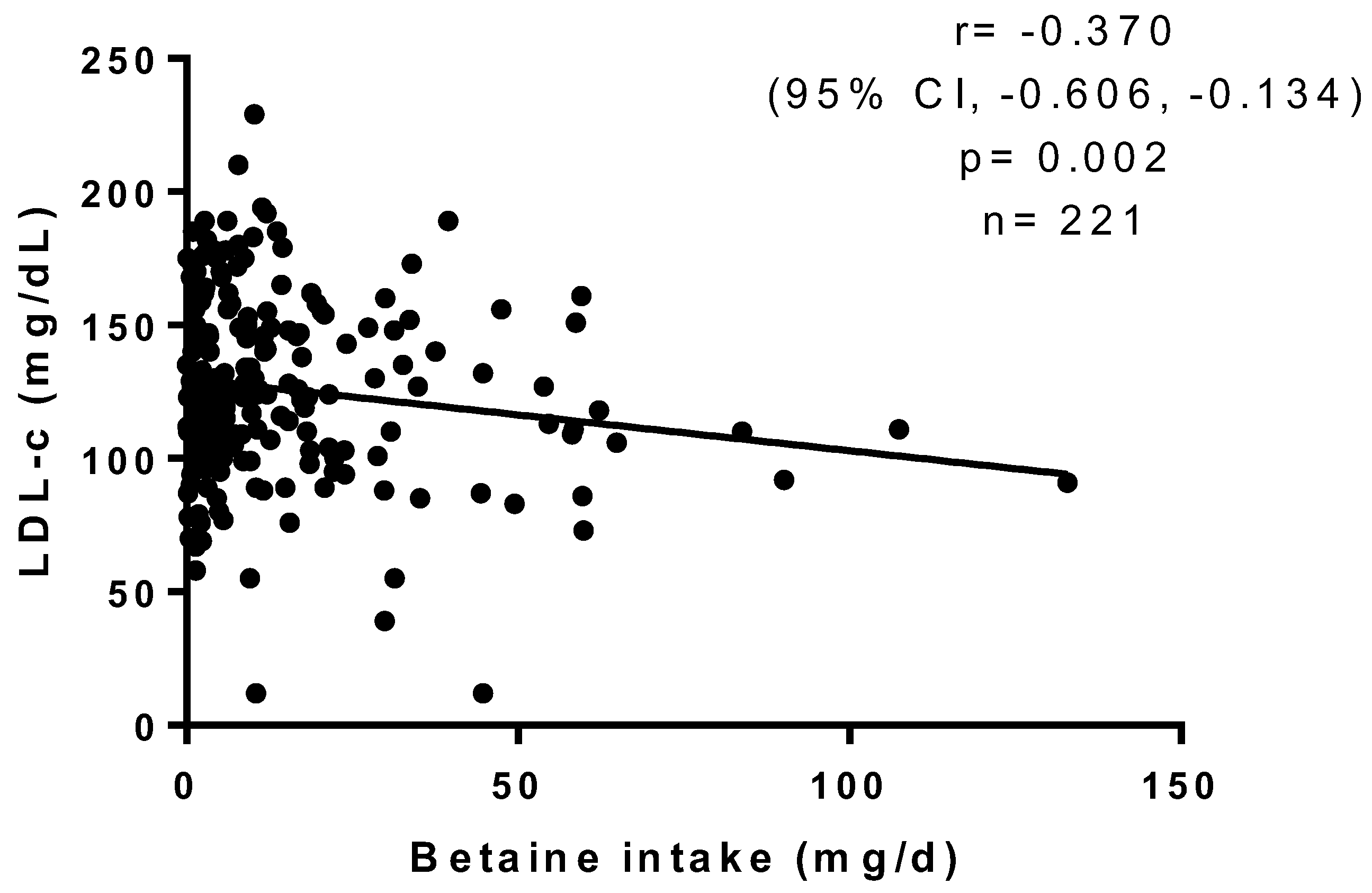
| Variable | Group 1 (n = 70) | Group 2 (n = 71) | Group 3 (n = 71) | p |
|---|---|---|---|---|
| Age (years) | 35.7 ± 12.5 | 39.2 ± 12.8 | 37.9 ± 12.1 | 0.247 |
| Sex (F/M) | 44/26 | 45/26 | 41/30 | 0.749 |
| BMI (kg/m2) | 27.9 ± 5.4 | 29.2 ± 6.1 | 27.9 ± 4.8 | 0.273 |
| Body fat (%) | 35.8 ± 9.0 | 36.1 ± 9.0 | 32.6 ± 9.4 | 0.284 |
| Waist (cm) | 89.1 ± 14.6 | 92.4 ± 15.5 | 89.3 ± 13.4 | 0.319 |
| Hip (cm) | 105 ± 10 | 107 ± 11 | 103 ± 9 | 0.120 |
| Neck (cm) | 37.1 ± 8.7 | 36.4 ± 4.4 | 36.4 ± 4.2 | 0.796 |
| SBP (mmHg) | 120 ± 17 | 118 ± 20 | 124 ± 16 | 0.163 |
| DBP (mmHg) | 78.3 ± 9.5 | 78.5 ± 10.6 | 79.1 ± 10.3 | 0.894 |
| Habitual exercise (Yes, %) | 58.6 | 50.7 | 54.9 | 0.643 |
| Variable | Group 1 (n = 70) | Group 2 (n = 71) | Group 3 (n = 71) | p |
|---|---|---|---|---|
| Total calories | 2098 ± 650 | 2125 ± 780 | 2190 ± 840 | 0.610 |
| Proteins (%E/d) | 19.1 ± 4.9 | 18.6 ± 4.9 | 20.6 ± 3.8 | 0.421 |
| Fat (%E/d) | 36.4 ± 6.2 | 37.4 ± 5.6 | 35.8 ± 9.1 | 0.720 |
| Carbohydrates (%E/d) | 49.4 ± 6.3 | 51.6 ± 5.3 | 52.9 ± 7.6 | 0.456 |
| Fiber (g/d) | 23.3 ± 3.1 | 21.6 ± 4.2 | 22.8 ± 6.9 | 0.520 |
| Variable | Group 1 (n = 70) | Group 2 (n = 71) | Group 3 (n = 71) | p |
|---|---|---|---|---|
| Fasting glucose (mg/dL) | 92.4 ± 9.4 | 95.1 ± 10.6 | 94.6 ± 11.4 | 0.260 |
| Total cholesterol (mg/dL) | 202 ± 37 | 199 ± 38 | 180 ± 31 | 0.002 a |
| HDL-c (mg/dL) | 46.5 ± 12.9 | 44.5 ± 16.4 | 42.6 ± 13.8 | 0.322 |
| Non-HDL-c (mg/dL) | 154 ± 40 | 151 ± 37 | 137 ± 30 | 0.017 b |
| LDL-c (mg/dL) | 134 ± 35 | 129 ± 34 | 118 ± 32 | 0.028 c |
| Triglycerides (mg/dL) | 113 ± 76 | 108 ± 55 | 105 ± 52 | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Lopez, O.; Santuario-Loera, A. Low Dietary Betaine Intake Is Associated with Increased Blood Cholesterol in Mexican Subjects. Healthcare 2024, 12, 819. https://doi.org/10.3390/healthcare12080819
Ramos-Lopez O, Santuario-Loera A. Low Dietary Betaine Intake Is Associated with Increased Blood Cholesterol in Mexican Subjects. Healthcare. 2024; 12(8):819. https://doi.org/10.3390/healthcare12080819
Chicago/Turabian StyleRamos-Lopez, Omar, and Alma Santuario-Loera. 2024. "Low Dietary Betaine Intake Is Associated with Increased Blood Cholesterol in Mexican Subjects" Healthcare 12, no. 8: 819. https://doi.org/10.3390/healthcare12080819
APA StyleRamos-Lopez, O., & Santuario-Loera, A. (2024). Low Dietary Betaine Intake Is Associated with Increased Blood Cholesterol in Mexican Subjects. Healthcare, 12(8), 819. https://doi.org/10.3390/healthcare12080819






