Pumping up the Fight against Multiple Sclerosis: The Effects of High-Intensity Resistance Training on Functional Capacity, Muscle Mass, and Axonal Damage
Abstract
:1. Introduction
2. Materials and Methods
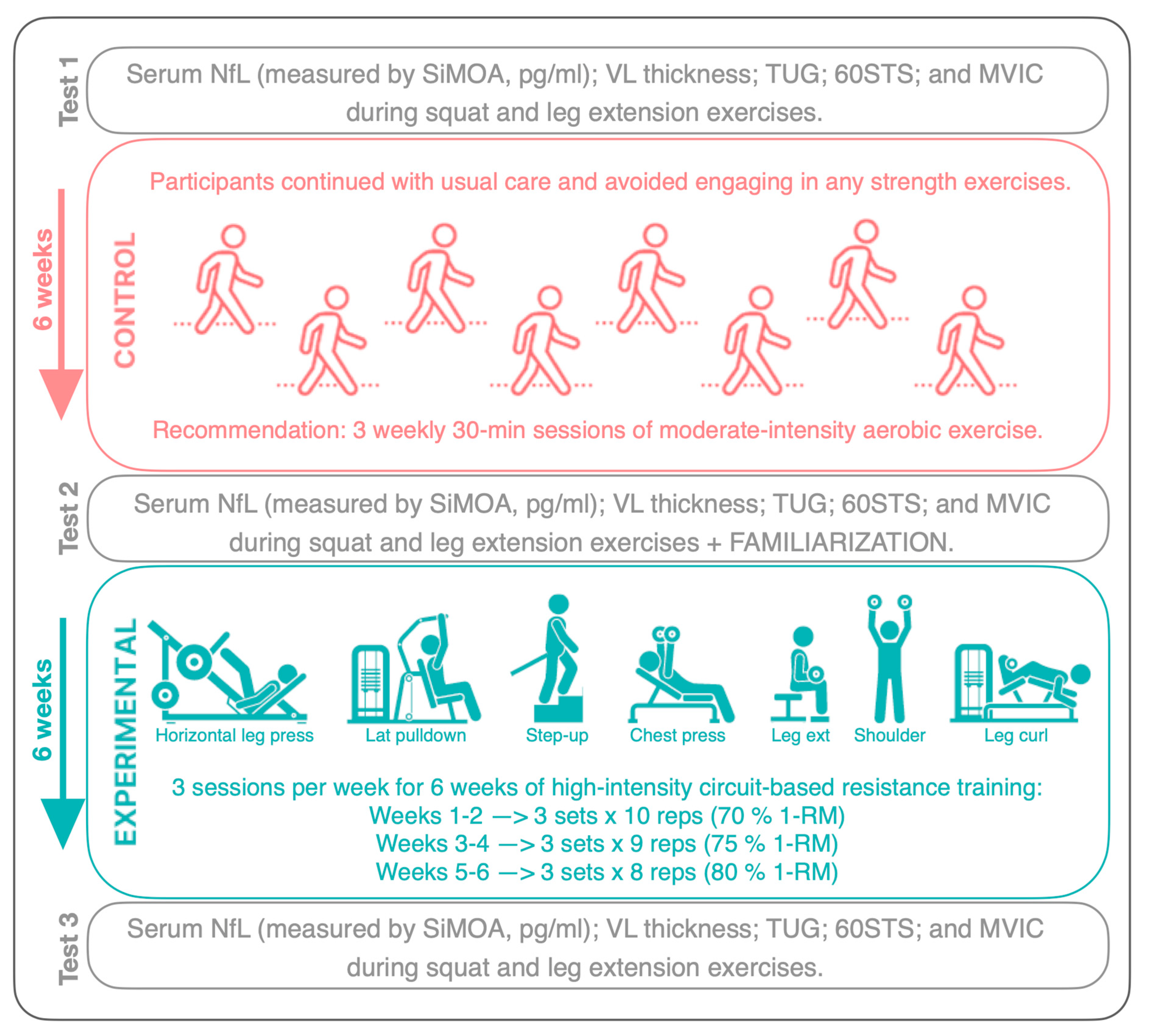
2.1. Study Design
2.2. Participants
2.3. Procedures
2.3.1. NfL Measurement
2.3.2. Muscle Thickness
2.3.3. Functional Capacity
2.3.4. Neuromuscular Capacity
2.3.5. Training Intervention
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Dvir, Z.; Deriu, F. Meta-analytic and scoping study on strength training in people with multiple sclerosis. J. Strength Cond. Res. 2019, 33, 874–889. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828.e3. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Jakobsen, J.; Petersen, T.; Hansen, H.J.; Knudsen, C.; Overgaard, K.; Ingemann-Hansen, T. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009, 73, 1478–1484. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.; Shen, J.; Yang, H.; Xu, W.; Shao, M.; Pan, F. Effect of exercise on fatigue in multiple sclerosis patients: A network meta-analysis. Int. J. Sports Med. 2021, 42, 1250–1259. [Google Scholar] [CrossRef]
- Prosperini, L.; Di Filippo, M. Beyond Clinical Changes: Rehabilitation-induced neuroplasticity in MS. Mult. Scler. Houndmills Basingstoke Engl. 2019, 25, 1348–1362. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Byrne, L.M.; Rodrigues, F.B.; Blennow, K.; Durr, A.; Leavitt, B.R.; Roos, R.A.C.; Scahill, R.I.; Tabrizi, S.J.; Zetterberg, H.; Langbehn, D.; et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: A retrospective cohort analysis. Lancet Neurol. 2017, 16, 601–609. [Google Scholar] [CrossRef]
- Joisten, N.; Rademacher, A.; Warnke, C.; Proschinger, S.; Schenk, A.; Walzik, D.; Knoop, A.; Thevis, M.; Steffen, F.; Bittner, S.; et al. Exercise diminishes plasma neurofilament light chain and reroutes the kynurenine pathway in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e982. [Google Scholar] [CrossRef]
- Scherling, C.S.; Hall, T.; Berisha, F.; Klepac, K.; Karydas, A.; Coppola, G.; Kramer, J.H.; Rabinovici, G.; Ahlijanian, M.; Miller, B.L.; et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration: Neurofilament in ftD. Ann. Neurol. 2014, 75, 116–126. [Google Scholar] [CrossRef]
- Bacioglu, M.; Maia, L.F.; Preische, O.; Schelle, J.; Apel, A.; Kaeser, S.A.; Schweighauser, M.; Eninger, T.; Lambert, M.; Pilotto, A.; et al. Neurofilament light chain in blood and csf as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016, 91, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, Ö.; et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Brureau, A.; Blanchard-Bregeon, V.; Pech, C.; Hamon, S.; Chaillou, P.; Guillemot, J.-C.; Barneoud, P.; Bertrand, P.; Pradier, L.; Rooney, T.; et al. NF-L in cerebrospinal fluid and serum is a biomarker of neuronal damage in an inducible mouse model of neurodegeneration. Neurobiol. Dis. 2017, 104, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Barro, C.; Disanto, G.; Mathias, A.; Soneson, C.; Bonnier, G.; Yaldizli, Ö.; Regeniter, A.; Derfuss, T.; Canales, M.; et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult. Scler. J. 2016, 22, 1550–1559. [Google Scholar] [CrossRef]
- Sen, M.K.; Hossain, M.J.; Mahns, D.A.; Brew, B.J. Validity of serum neurofilament light chain as a prognostic biomarker of disease activity in multiple sclerosis. J. Neurol. 2023, 270, 1908–1930. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Briken, S.; Rosenkranz, S.C.; Keminer, O.; Patra, S.; Ketels, G.; Heesen, C.; Hellweg, R.; Pless, O.; Schulz, K.-H.; Gold, S.M. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J. Neuroimmunol. 2016, 299, 53–58. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Ingemann-Hansen, T. Multiple sclerosis and physical exercise: Recommendations for the application of resistance, endurance and combined training. Mult. Scler. Houndmills Basingstoke Engl. 2008, 14, 35–53. [Google Scholar] [CrossRef]
- Mulero, P.; Maroto-Izquierdo, S.; Redondo, N.; Gonzalo-Benito, H.; Chavarría-Miranda, A.; Calvo, H.; Cabero, M.I.; Hernandez, M.; Nieto, M.L.; Tellez, N. Effect of resistance exercise training on plasma neurofilaments in multiple sclerosis: A proof of concept for future designs. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2023, 44, 3997–4000. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Jakobsen, J.; Petersen, T.; Overgaard, K.; Ingemann-Hansen, T. Muscle fiber size increases following resistance training in multiple sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2010, 16, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Lund, C.; Rasmussen, C.; Petersen, T.; Sørensen, H.; Ingemann-Hansen, T.; Overgaard, K. Neural drive increases following resistance training in patients with multiple sclerosis. J. Neurol. 2013, 260, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Englund, S.; Piehl, F.; Kierkegaard, M. High-intensity resistance training in people with multiple sclerosis experiencing fatigue: A randomised controlled trial. Mult. Scler. Relat. Disord. 2022, 68, 104106. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Dalgas, U.; Keytsman, C.; Eijnde, B.O.; Wens, I. High intensity training may reverse the fiber type specific decline in myogenic stem cells in multiple sclerosis patients. Front. Physiol. 2016, 7, 193. [Google Scholar] [CrossRef]
- Kierkegaard, M.; Lundberg, I.E.; Olsson, T.; Johansson, S.; Ygberg, S.; Opava, C.; Holmqvist, L.W.; Piehl, F. High-Intensity resistance training in multiple sclerosis—An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J. Neurol. Sci. 2016, 362, 251–257. [Google Scholar] [CrossRef]
- Medina-Perez, C.; de Souza-Teixeira, F.; Fernandez-Gonzalo, R.; Hernandez-Murua, J.A.; Antonio de Paz-Fernandez, J. Effects of high-speed power training on muscle strength and power in patients with multiple sclerosis. J. Rehabil. Res. Dev. 2016, 53, 359–368. [Google Scholar] [CrossRef]
- Moradi, M.; Sahraian, M.A.; Aghsaie, A.; Kordi, M.R.; Meysamie, A.; Abolhasani, M.; Sobhani, V. Effects of eight-week resistance training program in men with multiple sclerosis. Asian J. Sports Med. 2015, 6, e22838. [Google Scholar] [CrossRef]
- Patrocinio de Oliveira, C.E.; Moreira, O.C.; Carrion-Yagual, Z.M.; Medina-Perez, C.; de Paz, J.A. Effects of classic progressive resistance training versus eccentric-enhanced resistance training in people with multiple sclerosis. Arch. Phys. Med. Rehabil. 2018, 99, 819–825. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Andreu Caravaca, L.; Martínez-Rodríguez, A.; Rubio-Arias, J.Á. Effects of resistance circuit-based training on body composition, strength and cardiorespiratory fitness: A systematic review and meta-analysis. Biology 2021, 10, 377. [Google Scholar] [CrossRef]
- Youssef, H.; Gönül, M.N.; Sobeeh, M.G.; Akar, K.; Feys, P.; Cuypers, K.; Vural, A. Is high-intensity interval training more effective than moderate continuous training in rehabilitation of multiple sclerosis: A comprehensive systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2024, S000399932400025X. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Izquierdo, S.; Garcia-Lopez, D.; de Paz, J.A. Functional and muscle-size effects of flywheel resistance training with eccentric-overload in professional handball players. J. Hum. Kinet. 2017, 60, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Dolev, M.; Givon, U. Further construct validity of the timed up-and-go test as a measure of ambulation in multiple sclerosis patients. Eur. J. Phys. Rehabil. Med. 2017, 53, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Measurement of sit-to-stand among older adults. Top. Geriatr. Rehabil. 2012, 28, 11–16. [Google Scholar] [CrossRef]
- Courel-Ibáñez, J.; Hernández-Belmonte, A.; Cava-Martínez, A.; Pallarés, J.G. Familiarization and reliability of the isometric knee extension test for rapid force production assessment. Appl. Sci. 2020, 10, 4499. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Razavi, S.; Nazem, G.; Mardani, M.; Esfandiari, E.; Esfahani, S.; Salehi, H. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv. Biomed. Res. 2015, 4, 53. [Google Scholar] [CrossRef]
- Amin, N.S.; El Tayebi, H.M. More Gain, Less Pain: How Resistance training affects immune system functioning in multiple sclerosis patients: A review. Mult. Scler. Relat. Disord. 2023, 69, 104401. [Google Scholar] [CrossRef]
- Najafi, P.; Hadizadeh, M.; Cheong, J.P.G.; Mohafez, H.; Abdullah, S. Cytokine profile in patients with multiple sclerosis following exercise: A systematic review of randomized clinical trials. Int. J. Environ. Res. Public Health 2022, 19, 8151. [Google Scholar] [CrossRef]
- Filipi, M.; Jack, S. Interferons in the treatment of multiple sclerosis. Int. J. MS Care 2020, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, R.; Biswas, S.; Zhu, Y.; Qin, X.; Zhang, M.; Zhai, L.; Luo, Y.; He, X.; Mao, C.; et al. Neural stem cell-based regenerative approaches for the treatment of multiple sclerosis. Mol. Neurobiol. 2018, 55, 3152–3171. [Google Scholar] [CrossRef]
- Ercan, Z.; Bilek, F.; Demir, C.F. The Effect of aerobic exercise on neurofilament light chain and glial fibrillary acidic protein level in patients with relapsing remitting type multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 55, 103219. [Google Scholar] [CrossRef]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The neuroprotective effects of exercise: Maintaining a healthy brain throughout aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef]
- Yuksel, H.; Balaban, M.; Tan, O.O.; Mungan, S. Sarcopenia in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 58, 103471. [Google Scholar] [CrossRef] [PubMed]
- Willingham, T.B.; McCully, K.; Backus, D. Skeletal muscle dysfunction in people with multiple sclerosis: A physiological target for improving physical function and mobility. Arch. Phys. Med. Rehabil. 2023, 104, 694–706. [Google Scholar] [CrossRef]
- Broekmans, T.; Roelants, M.; Feys, P.; Alders, G.; Gijbels, D.; Hanssen, I.; Stinissen, P.; Eijnde, B.O. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult. Scler. J. 2011, 17, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kjølhede, T.; Vissing, K.; De Place, L.; Pedersen, B.G.; Ringgaard, S.; Stenager, E.; Petersen, T.; Dalgas, U. Neuromuscular adaptations to long-term progressive resistance training translates to improved functional capacity for people with multiple sclerosis and is maintained at follow-up. Mult. Scler. J. 2015, 21, 599–611. [Google Scholar] [CrossRef]
- Suchomel, T.J.; Nimphius, S.; Stone, M.H. The importance of muscular strength in athletic performance. Sports Med. 2016, 46, 1419–1449. [Google Scholar] [CrossRef]
- Allgöwer, K.; Kern, C.; Hermsdörfer, J. Predictive and reactive grip force responses to rapid load increases in people with multiple sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kjølhede, T.; Vissing, K.; Dalgas, U. Multiple sclerosis and progressive resistance training: A systematic review. Mult. Scler. J. 2012, 18, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Dalgas, U.; Wens, I.; Hvid, L. Muscle strength and power in persons with multiple sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 376, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.A.; Gappmaier, E.; LaStayo, P.C. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: A randomized controlled trial. J. Neurol. Phys. Ther. 2011, 35, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Manonelles, P.; Boas, J.P.V.; Rubio-Arias, J.Á. Fast-velocity resistance training improves force development and mobility in multiple sclerosis. Int. J. Sports Med. 2022, 43, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.B.; Bibby, B.M.; Skjerbæk, A.G.; Jensen, E.; Sørensen, H.; Stenager, E.; Dalgas, U. Validity and variability of the 5-repetition sit-to-stand test in patients with multiple sclerosis. Disabil. Rehabil. 2012, 34, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.; Gnanapavan, S. Letter to the editor: Consensus statement on neurofilament proteins in multiple sclerosis under development by consortium of multiple sclerosis centers (CMSC) expert panel. Int. J. MS Care 2020, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Fimland, M.S.; Helgerud, J.; Gruber, M.; Leivseth, G.; Hoff, J. Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur. J. Appl. Physiol. 2010, 110, 435–443. [Google Scholar] [CrossRef]
- Sangelaji, B.; Kordi, M.; Banihashemi, F.; Nabavi, S.M.; Khodadadeh, S.; Dastoorpoor, M. A combined exercise model for improving muscle strength, balance, walking distance, and motor agility in multiple sclerosis patients: A randomized clinical trial. Iran. J. Neurol. 2016, 15, 111–120. [Google Scholar]

| Baseline Characteristics | n = 11 |
|---|---|
| Antropometric data | |
| Sex; F/M (% women) | 9/2 (81.8%) |
| Age (years) | 40.8 ± 7.8 |
| Weight (kg) | 66.1 ± 13.7 |
| Height (cm) | 160.5 ± 9.5 |
| Fat percentage (%) | 32.2 ± 7.1 |
| Disease characteristics | |
| Relapsing–remitting MS | 11 (100%) |
| Secondary progressive MS | 0 (0%) |
| Primary progressive MS | 0 (0%) |
| Progressive-relapsing MS | 0 (0%) |
| Last relapse | 8.3 ± 4.1 |
| Years since diagnosis | 12.1 ± 6.7 |
| EDSS | 0.5 ± 0.8 |
| Treatment | |
| None | 2 (18.2%) |
| Interferon beta | 3 (27.3%) |
| Dimethyl Fumarate | 2 (18.2%) |
| Teriflunomide | 1 (9.1%) |
| Fingolimod | 1 (9.1%) |
| Cladribine | 1 (9.1%) |
| Alemtuzumab | 1 (9.1%) |
| Habits | |
| Low physical activity level (IPAQ scale) | 3 (27.3%) |
| Moderate physical activity level (IPAQ scale) | 8 (72.7%) |
| Smoking | 1 (9.1%) |
| Weeks | Intensity | Volume | Resistance Exercises |
|---|---|---|---|
| Weeks 1–2 | 70% 1-RM | 3 sets × 10 reps |
|
| Weeks 3–4 | 75% 1-RM | 3 sets × 9 reps | |
| Weeks 5–6 | 80% 1-RM | 3 sets × 8 reps |
| Test 2 vs. Test 1 | Test 3 vs. Test 1 | Test 3 vs. Test 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | p | Mean (95CI) | ES | p | Mean (95CI) | ES | p | Mean (95CI) | ES | ||||
| Muscle mass | |||||||||||||||
| VL (mm) | 18.6 ± 3.8 | 18.5 ± 4.1 | 21.5 ± 3.1 | 0.506 | 0.1 (−0.6–0.2) | 0.21 | <0.001 | 2.8 (1.9–3.7) | 2.22 | <0.001 | 2.9 (2.0–3.9) | 2.15 | |||
| Functional capacity | |||||||||||||||
| 60STS (n) | 39.8 ± 8.9 | 43.5 ± 6.9 | 53.2 ± 7.1 | 0.089 | 3.6 (−0.7–7.9) | 0.57 | <0.001 | 13.4 (7.2–19.6) | 1.45 | <0.001 | 9.7 (6.4–13.0) | 1.98 | |||
| TUG (s) | 5.4 ± 0.4 | 5.4 ± 0.5 | 4.7 ± 0.4 | 0.953 | 0.0 (−0.2–0.2) | 0.02 | <0.001 | 0.7 (0.4–1.0) | 1.55 | <0.001 | 0.69 (0.4–0.9) | 1.70 | |||
| Muscle force | |||||||||||||||
| MVICLEXT (N) | 551 ± 263 | 529 ± 249 | 647 ± 314 | 0.163 | −22.2 (−55.0–10.6) | 0.45 | 0.017 | 96.0 (21.5–170.5) | 0.87 | 0.005 | 118.2 (45.7–190.6) | 1.10 | |||
| MVICLEXT NA (N) | 276 ± 136 | 285 ± 124 | 320 ± 159 | 0.619 | 9.3 (−31.1–49.7) | 0.16 | 0.022 | 44.9 (8.0–81.8) | 0.82 | 0.152 | 35.6 (−15.5–86.7) | 0.47 | |||
| MVICLEXT A (N) | 279 ± 128 | 266 ± 122 | 297 ± 140 | 0.544 | −12.7 (−57.7–32.3) | 0.19 | 0.409 | 18.6 (−29.5–66.6) | 0.26 | 0.267 | 31.3 (−28.0–90.5) | 0.36 | |||
| MVICSQ (N) | 1809 ± 723 | 1725 ± 690 | 2394 ± 630 | 0.274 | 84.0 (−247.0–79.1) | 0.37 | <0.001 | 585.7 (351.0–820.1) | 1.79 | <0.001 | 669.7 (401.0–938.6) | 1.78 | |||
| MVICSQ NA (N) | 829 ± 154 | 836 ± 137 | 1186 ± 239 | 0.889 | 6.6 (−102.0–115.0) | 0.05 | 0.002 | 293.1 (145.0–442.0) | 1.41 | 0.006 | 337.4 (135.0–540.0) | 1.39 | |||
| MVICSQ A (N) | 819 ± 188 | 736 ± 112 | 1168 ± 304 | 0.167 | −82.3 (−208.0–44.0) | 0.54 | 0.003 | 252.6 (114.0–390.9) | 1.31 | <0.001 | 386.8 (228.0–545.5) | 2.04 | |||
| RF (N·kg−1) | 280 ± 87 | 276 ± 67 | 381 ± 87 | 0.745 | −4.2 (−32.7–24.2) | 0.11 | <0.001 | 100.9 (58.5–143.3) | 1.70 | <0.001 | 105.1 (68.1–142.1) | 2.03 | |||
| RFD 0–100 ms (N·s−1) | 1323 ± 427 | 1640 ± 445 | 1790 ± 427 | 0.483 | 317.0 (−663.0–1297.0) | 0.23 | 0.172 | 467.0 (−245.0–1178.0) | 0.47 | 0.710 | 150.0 (−733.0–1033.0) | 0.12 | |||
| RFD 0–250 ms (N·s−1) | 1061 ± 236 | 1111 ± 364 | 1294 ± 410 | 0.877 | 49.5 (−678.0–777.0) | 0.06 | 0.529 | 235.6 (−578.0–1049.0) | 0.21 | 0.382 | 359.5 (−553.0–1272.0) | 0.21 | |||
| Neurodegeneration | |||||||||||||||
| NfL (pg·ml−1) | 5.3 ± 1.8 | 5.9 ± 2.0 | 4.2 ± 1.7 | 0.171 | 0.6 (−0.3–1.5) | 0.45 | <0.001 | 1.0 (−1.9–−0.1)) | 0.75 | <0.001 | 1.6 (−2.3–−0.8) | 1.43 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroto-Izquierdo, S.; Mulero, P.; Menéndez, H.; Pinto-Fraga, J.; Lista, S.; Santos-Lozano, A.; Téllez, N. Pumping up the Fight against Multiple Sclerosis: The Effects of High-Intensity Resistance Training on Functional Capacity, Muscle Mass, and Axonal Damage. Healthcare 2024, 12, 837. https://doi.org/10.3390/healthcare12080837
Maroto-Izquierdo S, Mulero P, Menéndez H, Pinto-Fraga J, Lista S, Santos-Lozano A, Téllez N. Pumping up the Fight against Multiple Sclerosis: The Effects of High-Intensity Resistance Training on Functional Capacity, Muscle Mass, and Axonal Damage. Healthcare. 2024; 12(8):837. https://doi.org/10.3390/healthcare12080837
Chicago/Turabian StyleMaroto-Izquierdo, Sergio, Patricia Mulero, Héctor Menéndez, José Pinto-Fraga, Simone Lista, Alejandro Santos-Lozano, and Nieves Téllez. 2024. "Pumping up the Fight against Multiple Sclerosis: The Effects of High-Intensity Resistance Training on Functional Capacity, Muscle Mass, and Axonal Damage" Healthcare 12, no. 8: 837. https://doi.org/10.3390/healthcare12080837







