Effects of Hypopressive Abdominal Training on Ventilatory Capacity and Quality of Life: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization and Masking
2.4. Intervention
2.5. Methods
2.6. Data Analysis
3. Results
3.1. Selection Process
3.2. Respiratory Muscle Function
3.3. Quality of Life (QoL)
4. Discussion
5. Conclusions
6. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Pardiñas, M.A.; Torres-Lacomba, M.; Navarro-Brazález, B. Función Muscular Del Suelo Pélvico En Mujeres Sanas, Puérperas y Con Disfunciones Del Suelo Pélvico. Actas Urol. Esp. 2017, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Grimes, W.R.; Stratton, M. Pelvic Floor Dysfunction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lacima, G.; Espuña, M. Patología Del Suelo Pélvico. Gastroenterol. Hepatol. 2008, 31, 587–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hage-Fransen, M.A.H.; Wiezer, M.; Otto, A.; Wieffer-Platvoet, M.S.; Slotman, M.H.; Nijhuis-van der Sanden, M.W.G.; Pool-Goudzwaard, A.L. Pregnancy- and Obstetric-Related Risk Factors for Urinary Incontinence, Fecal Incontinence, or Pelvic Organ Prolapse Later in Life: A Systematic Review and Meta-Analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Musibay, E.R.; Borges Sandrino, R.S. Cuestionarios de Calidad de Vida En Las Mujeres Con Disfunciones Del Suelo Pélvico. Rev. Cuba. Obstet. Ginecol. 2016, 42, 372–385. [Google Scholar]
- Pelier, B.Y.N.; García, J.M.V. Rehabilitación física del suelo pélvico: Ejercicios de Kegel y gimnasia abdominal hipopresiva. Investig. Medicoquirúrgicas 2020, 12. [Google Scholar]
- Adams, E.M.R.; Raya, D.A.A.; Elías, N.L. Calidad de vida en la Incontinencia urinaria femenina. Investig. Medicoquirúrgicas 2020, 12. [Google Scholar]
- Riss, P.; Kargl, J. Quality of Life and Urinary Incontinence in Women. Maturitas 2011, 68, 137–142. [Google Scholar] [CrossRef]
- Aljuraifani, R.; Stafford, R.E.; Hall, L.M.; van den Hoorn, W.; Hodges, P.W. Task-Specific Differences in Respiration-Related Activation of Deep and Superficial Pelvic Floor Muscles. J. Appl. Physiol. 2019, 126, 1343–1351. [Google Scholar] [CrossRef]
- Bø, K.; Nygaard, I.E. Is Physical Activity Good or Bad for the Female Pelvic Floor? A Narrative Review. Sports Med. 2020, 50, 471–484. [Google Scholar] [CrossRef]
- Carrière, B. The influence of intra-abdominal pressure on pelvic floor and sexual health. J. Sex. Med. 2007, 4, 1468–1473. [Google Scholar]
- Sapsford, R. The pelvic floor: A clinical model for function and rehabilitation. Physiotherapy 2001, 87, 620–630. [Google Scholar] [CrossRef]
- Smith, M.D.; Russell, A.; Hodges, P.W. Disorders of breathing and continence have a stronger association with back pain than obesity and physical activity. Aust. J. Physiother. 2014, 51, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; O’Sullivan, P.; Low, C. The impact of respiratory and pelvic floor muscle training on pelvic floor dysfunction after childbirth. Int. J. Obstet. Gynaecol. 2018, 125, 715–722. [Google Scholar]
- Caufriez, M.; Fernández, J.C.; Guignel, G.; Heimann, A. Comparación de Las Variaciones de Presión Abdominal En Medio Acuático y Aéreo Durante La Realización de Cuatro Ejercicios Abdominales Hipopresivos. Rev. Iberoam. Fisioter. Kinesiol. 2007, 10, 12–23. [Google Scholar] [CrossRef]
- Navarro Brazález, B.; Sánchez Sánchez, B.; Prieto Gómez, V.; De La Villa Polo, P.; McLean, L.; Torres Lacomba, M. Pelvic Floor and Abdominal Muscle Responses during Hypopressive Exercises in Women with Pelvic Floor Dysfunction. Neurourol. Urodyn. 2020, 39, 793–803. [Google Scholar] [CrossRef] [PubMed]
- del Mar Moreno-Muñoz, M.; Hita-Contreras, F.; Estudillo-Martínez, M.D.; Aibar-Almazán, A.; Castellote-Caballero, Y.; Bergamin, M.; Gobbo, S.; Cruz-Díaz, D. The Effects of Abdominal Hypopressive Training on Postural Control and Deep Trunk Muscle Activation: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 2741. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, B.; Torres-Lacomba, M.; Yuste-Sánchez, M.J.; Navarro-Brazález, B.; Pacheco-Da-Costa, S.; Gutiérrez-Ortega, C.; Zapico-Goni, A. Cultural adaptation and validation of the Pelvic Floor Distress Inventory Short Form (PFDI-20) and Pelvic Floor Impact Questionnaire Short Form (PFIQ-7) Spanish versions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 281–285. [Google Scholar] [CrossRef]
- Guillaumes, S.; O’Callaghan, C.A. Versión en español del software gratuito OxMaR para minimización y aleatorización de estudios clínicos. Gac. Sanit. 2019, 33, 395–397. [Google Scholar] [CrossRef]
- Rial, T.; Pinsach, P. Practical Manual Low Pressure Fitness Level 1R; International Hypopressive & Physical Therapy Institute: Vigo, Spain, 2017. [Google Scholar]
- Gutiérrez, C.M.; Beroiza, W.T.; Borzone, T.G.; Caviedes, S.I.; Céspedes, G.J.; Gutiérrez, N.M.; Oyarzún, G.M.; Palacios, M.S.; Cartagena, S.C.; Corrales, V.R.; et al. Espirometría: Manual de Procedimientos. SERChile. Rev. Chil. Enfermedades Respir. 2018, 34, 171–188. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Corchete, L.A.; García, J.F.; Jerves Donoso, D.; Lantarón-Caeiro, E.; Cobreros Mielgo, R.; Mielgo-Ayuso, J.; Gallego-Gallego, D.; Seco-Calvo, J. Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis. Biology 2022, 12, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffman, M.; Augusto, V.M.; Eduardo, D.S.; Silveira, B.M.F.; Lemos, M.D.; Parreira, V.F. Inspiratory muscle training reduces dyspnea during activities of daily living and improves inspiratory muscle function and quality of life in patients with advanced lung disease. Physiother. Theory Pract. 2021, 37, 895–905. [Google Scholar] [CrossRef] [PubMed]
- García-Río, F.; Calle, M.; Burgos, F.; Casan, P.; del Campo, F.; Galdiz, J.B.; Giner, J.; González-Mangado, N.; Ortega, F.; Puente Maestu, L. Espirometría. Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-Validation of Item Selection and Scoring for the SF-12 Health Survey in Nine Countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretation of SF-36 and SF-12 questionnaires in Spain: Physical and mental components. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Morán, M.T.C. Estudio Comparativo de Programas Para Cálculo Del Tamaño Muestral. Metodol. Encuestas 2009, 11, 121–129. [Google Scholar]
- Molina-Torres, G.; Moreno-Muñoz, M.; Rebullido, T.R.; Castellote-Caballero, Y.; Bergamin, M.; Gobbo, S.; Hita-Contreras, F.; Cruz-Diaz, D. The Effects of an 8-Week Hypopressive Exercise Training Program on Urinary Incontinence and Pelvic Floor Muscle Activation: A Randomized Controlled Trial. Neurourol. Urodyn. 2023, 42, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; de Andrade, A.D.; Rattes, C.; Gonçalves, M.E.; Fregonezi, G.; Filho, V.G.; Lemos, A. Effects of Abdominal Hypopressive Gymnastics in the Volume Distribution of Chest Wall and the Electromyographic Activity of the Respiratory Muscles. Physiotherapy 2015, 101, e322–e323. [Google Scholar] [CrossRef]
- Chung, Y.; Huang, T.-Y.; Liao, Y.-H.; Kuo, Y.-C. 12-Week Inspiratory Muscle Training Improves Respiratory Muscle Strength in Adult Patients with Stable Asthma: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3267. [Google Scholar] [CrossRef]
- Pinsach, P.; Rial, T.; Caufriez, M.; Fernandez, J.C.; Devroux, I.; Ruiz, K. Hipopresivos, un cambio de paradigma. Arch. Med. Deporte 2010, 16, 639–645. [Google Scholar]
- Juez, L.; Núñez-Córdoba, J.M.; Couso, N.; Aubá, M.; Alcázar, J.L.; Mínguez, J.Á. Hypopressive technique versus pelvic floor muscle training for postpartum pelvic floor rehabilitation: A prospective cohort study. Neurourol. Urodyn. 2019, 38, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, K.A.; Machado, L.T.P.; Bastos DEBrito, C.I.; Rebullido, T.R. Can 5-weeks of Hypopressive Exercise Influence Sagittal Lumbo-Pelvic Position in Athletic and Non-Athletic Females? Int. J. Exerc. Sci. 2023, 16, 550–562. [Google Scholar] [PubMed] [PubMed Central]
- Bernardes, B.T.; Resende, A.P.M.; Stüpp, L.; Oliveira, E.; Castro, R.A.; Jármy di Bella, Z.I.K.; Girão, M.J.B.C.; Sartori, M.G.F. Efficacy of Pelvic Floor Muscle Training and Hypopressive Exercises for Treating Pelvic Organ Prolapse in Women: Randomized Controlled Trial. Sao Paulo Med. J. 2012, 130, 5–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katz, C.M.S.; Barbosa, C.P. Effects of Hypopressive Exercises on Pelvic Floor and Abdominal Muscles in Adult Women: A Systematic Review of Randomized Clinical Trials. J. Bodyw. Mov. Ther. 2024, 37, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, N. Exercise and noninvasive ventilatory support. Monaldi Arch. Chest Dis. 2000, 55, 242–246. [Google Scholar] [PubMed]
- Teijido, S.L.; Rial Rebullido, T.; Gómez-Tomás, C.; Alonso-Aubin, D.A.; Chulvi-Medrano, I. Effects of Hypopressive Exercise on Posterior Back Chain Kinematics and Pulmonary Function in Professional Female Basketball Players. J. Sport Rehabil. 2022, 31, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Viñaspre Hernández, R. Eficacia de La Gimnasia Abdominal Hipopresiva En La Rehabilitación Del Suelo Pélvico de Las Mujeres: Revisión Sistemática. Actas Urol. Esp. 2018, 42, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Ruys, C.L.; Lewis, C.R.H.; Wijeysuriya, N.S.; McBain, R.A.; Lee, B.B.; McKenzie, D.K.; Gandevia, S.C.; Butler, J.E. Impact of respiratory muscle training on respiratory muscle strength, respiratory function and quality of life in individuals with tetraplegia: A randomised clinical trial. Thorax 2020, 75, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Seixas, M.B.; Almeida, L.B.; Trevizan, P.F.; Martinez, D.G.; Laterza, M.C.; Vanderlei, L.C.M.; Silva, L.P. Effects of Inspiratory Muscle Training in Older Adults. Respir. Care 2020, 65, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ramli, M.I.; Hamzaid, N.A.; Engkasan, J.P.; Usman, J. Respiratory muscle training: A bibliometric analysis of 60 years’ multidisciplinary journey. Biomed. Eng. Online 2023, 22, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Technical Foundations | Definition |
|---|---|
| Autoelongation | Axial stretching of the spine, tensioning of the deep spine and back extensors |
| Double chin | Pulling the crown to the ceiling |
| Decoaptation of the glenohumeral joint | Scapula abduction and serratus activation |
| Neutral pelvis | Equal distance between anterior and posterior superior iliac spines |
| Dorsal ankle flexion | Parallel lower extremities with hip width, slight knee flexion, and dorsal ankle flexion |
| Gravity shaft overrun | Imbalance of the anteroposterior axis involving variation in the center of gravity |
| Diaphragmatic breathing | Nasal inspiration focusing on the lateral expansion of the basal lung area, emphasizing enlargement of the lower rib spaces. Slow and controlled exhalation through the mouth |
| Expiratory apnea | Total exhalation Exhalation with open rib cage maintained while the diaphragm is returned to a position of relaxation through the in-drawing of the abdominal muscles, thereby lowering the intra-abdominal pressure (involuntary lifting of the pelvic floor) |
| Demographic Characteristics | All Participants (n = 117) | CG (n = 55) | EG (n = 62) | p-Value | |
|---|---|---|---|---|---|
| Mean age ± SD | 45.65 ± 8.86 | 46.89 ± 6.59 | 44.54 ± 10.40 | 0.149 | |
| Mean weight ± SD (kg) | 63.59 ± 10.59 | 64.62 ± 10.04 | 62.67 ± 11.05 | 0.318 | |
| Mean height ± SD (cm) | 162.56 ± 5.95 | 163.45 ± 5.83 | 161.78 ± 5.99 | 0.128 | |
| Mean BMI ± SD | 24.03 ± 3.63 | 24.15 ± 3.31 | 23.93 ± 3.92 | 0.742 | |
| No. pregnant ± SD | 1.54 ± 1.07 | 1.71 ± 1.06 | 1.38 ± 1.07 | 0.090 | |
| No. delivery ± SD | 1.47 ± 1.05 | 1.63 ± 1.04 | 1.33 ± 1.05 | 0.130 | |
| Delivery type: n (%) | None | 28 (23.5) | 10 (35.7) | 18 (64.3) | 0.511 |
| Vaginal | 68 (57.1) | 34 (50) | 34 (50) | 0.824 | |
| Caesarean | 12 (10.1) | 7 (58.3) | 5 (41.7) | 0.619 | |
| Both | 10 (8.4) | 5 (45.5) | 6 (54.5) | 0.804 | |
| Smoking: n (%) | No | 100 (84) | 49 (49) | 51 (51) | 0.330 |
| Yes | 19 (16) | 7 (36.8) | 12 (63.2) | 0.563 | |
| Exercise: n (%) | No | 56 (47.1) | 27 (48.2) | 29 (51.8) | 0.812 |
| Yes | 63 (52.9) | 29 (46) | 34 (54) | 0.734 |
| Experimental Group (n = 62) | Control Group (n = 55) | Group | Time | Group × Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre Mean ± SD | Post Mean ± SD | Pre Mean ± SD | Post Mean ± SD | F(1,117) | p | η2 | F(1,117) | p | η2 | F(1,117) | p | η2 | |
| FVC | 2.52 ± 0.41 | 2.91 ± 0.38 | 2.80 ± 0.41 | 2.75 ± 0.39 | 3.22 | 0.076 | 0.027 | 284.90 | 0.000 | 0.709 | 0.63 | 0.430 | 0.005 |
| FEV1 | 1.98 ± 0.46 | 2.29 ± 0.45 | 2.16 ± 0.51 | 2.00 ± 0.44 | 0.37 | 0.546 | 0.003 | 3683.57 | 0.000 | 0.969 | 0.21 | 0.649 | 0.002 |
| PEF | 3.58 ± 1.15 | 4.02 ± 1.18 | 3.92 ± 1.42 | 3.55 ± 1.25 | 6.95 | 0.010 | 0.056 | 345.23 | 0.000 | 0.747 | 1.08 | 0.302 | 0.009 |
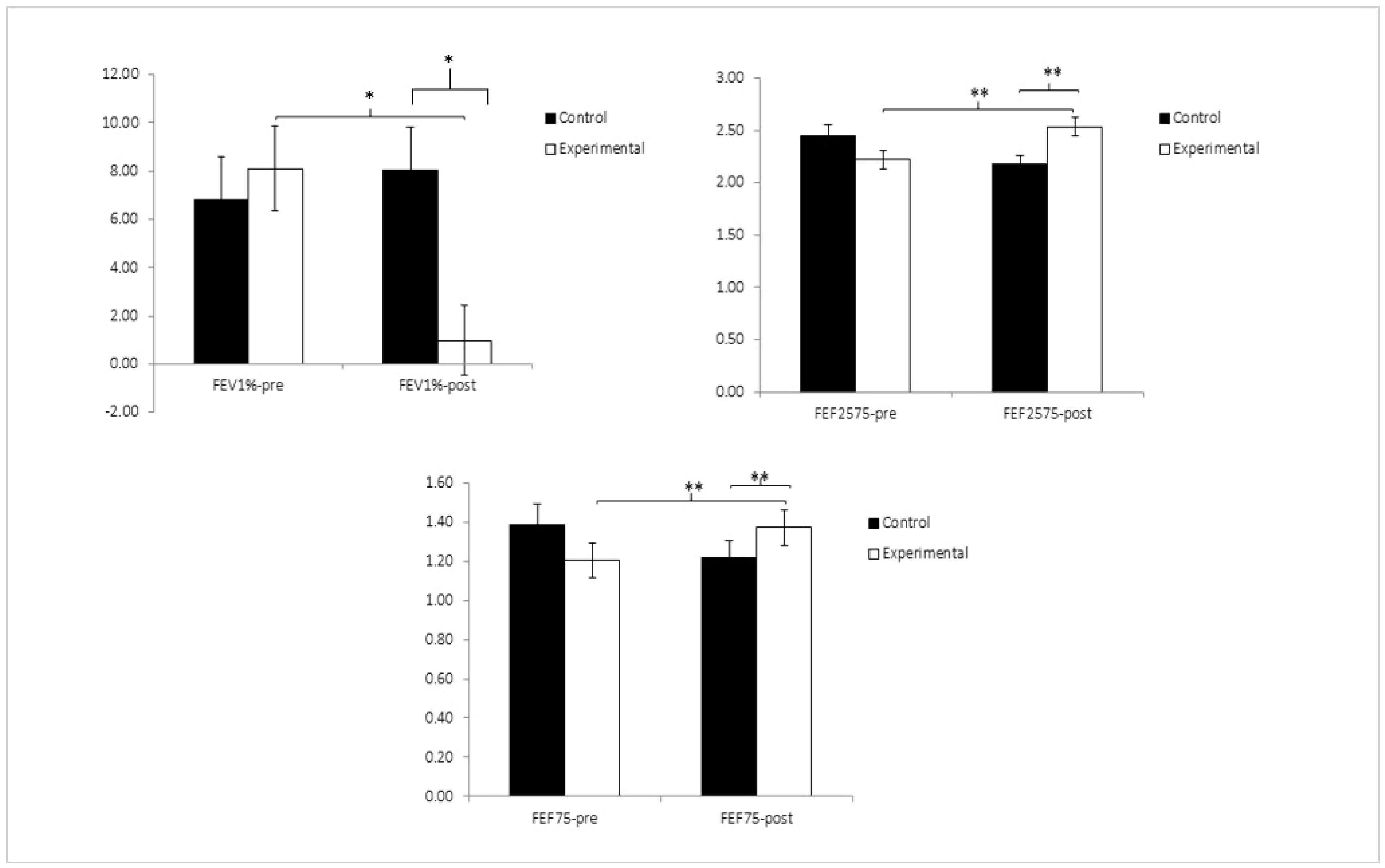
| FEV1% | 76.39 ± 14.01 | 79.11 ± 11.63 | 77.73 ± 13.41 | 73.48 ± 13.11 | 0.89 | 0.347 | 0.008 | 19.95 | 0.000 | 0.146 | 35.11 | 0.000 | 0.231 |
| FEF25% | 3.36 ± 1.07 | 3.72 ± 1.13 | 3.53 ± 1.35 | 3.18 ± 1.16 | 1.58 | 0.211 | 0.013 | 502.62 | 0.000 | 0.811 | 0.00 | 0.960 | 0.000 |
| FEF75% | 1.20 ± 0.45 | 1.37 ± 0.42 | 1.38 ± 0.48 | 1.22 ± 0.35 | 6.51 | 0.012 | 0.053 | 4696.97 | 0.000 | 0.976 | 5.73 | 0.018 | 0.047 |
| FEF25–75% | 2.22 ± 0.67 | 2.53 ± 0.71 | 2.44 ± 0.81 | 2.17 ± 0.65 | 7.54 | 0.007 | 0.061 | 601.86 | 0.000 | 0.837 | 5.62 | 0.019 | 0.046 |
| SF-12 | Experimental Group (n = 62) | Control Group (n = 55) | Group | Time | Group × Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre Mean ± SD | Post Mean ± SD | Pre Mean ± SD | Post Mean ± SD | F(1,117) | p | η2 | F(1,117) | p | η2 | F(1,117) | p | η2 | |
| GH | 66.67 ± 17.96 | 66.27 ± 16.29 | 62.95 ± 17.83 | 63.84 ± 17.14 | 1.18 | 0.279 | 0.010 | 0.03 | 0.865 | <0.001 | 0.20 | 0.658 | 0.002 |
| PF | 89.29 ± 19.94 | 92.46 ± 16.58 | 87.50 ± 18.46 | 88.39 ± 19.05 | 1.01 | 0.317 | 0.009 | 1.33 | 0.251 | 0.011 | 0.42 | 0.519 | 0.004 |
| PR | 85.71 ± 30.36 | 91.27 ± 24.66 | 75.89 ± 39.30 | 86.61 ± 32.32 | 2.42 | 0.122 | 0.020 | 5.27 | 0.023 | 0.043 | 0.53 | 0.468 | 0.005 |
| ER | 67.46 ± 42.27 | 71.43 ± 43.73 | 78.57 ± 37.97 | 78.57 ± 40.29 | 1.84 | 0.178 | 0.015 | 0.33 | 0.568 | 0.003 | 0.33 | 0.568 | 0.003 |
| BP | 87.70 ± 18.98 | 92.06 ± 14.77 | 83.04 ± 25.27 | 91.52 ± 17.37 | 0.72 | 0.397 | 0.006 | 12.79 | 0.001 | 0.099 | 1.31 | 0.254 | 0.011 |
| MH | 66.03 ± 17.18 | 71.11 ± 17.14 | 69.11 ± 17.61 | 67.86 ± 14.98 | 0.00 | 0.972 | <0.001 | 1.27 | 0.263 | 0.011 | 3.46 | 0.065 | 0.029 |
| V | 59.05 ± 21.98 | 67.30 ± 22.80 | 61.07 ± 23.95 | 68.57 ± 20.13 | 0.23 | 0.635 | 0.002 | 13.09 | <0.001 | 0.101 | 0.03 | 0.863 | <0.001 |
| SF | 83.33 ± 21.53 | 83.73 ± 25.46 | 83.48 ± 23.49 | 86.61 ± 17.81 | 0.20 | 0.653 | 0.002 | 0.56 | 0.456 | 0.005 | 0.34 | 0.336 | 0.089 |
| MSC | 84.06 ± 17.46 | 87.63 ± 13.24 | 78.79 ± 22.32 | 84.23 ± 19.00 | 2.30 | 0.132 | 0.019 | 6.88 | 0.010 | 0.056 | 0.29 | 0.294 | 0.084 |
| PSC | 68.23 ± 21.96 | 72.69 ± 22.62 | 73.32 ± 21.33 | 74.67 ± 19.13 | 1.01 | 0.317 | 0.009 | 2.85 | 0.094 | 0.024 | 0.81 | 0.812 | 0.145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herena-Funes, M.d.C.; Correia de Alencar, C.; Velázquez-Torres, D.M.; Marrero García, E.; Castellote-Caballero, Y.; León-Morillas, F.; Infante-Guedes, A.; Cruz-Díaz, D. Effects of Hypopressive Abdominal Training on Ventilatory Capacity and Quality of Life: A Randomized Controlled Trial. Healthcare 2024, 12, 893. https://doi.org/10.3390/healthcare12090893
Herena-Funes MdC, Correia de Alencar C, Velázquez-Torres DM, Marrero García E, Castellote-Caballero Y, León-Morillas F, Infante-Guedes A, Cruz-Díaz D. Effects of Hypopressive Abdominal Training on Ventilatory Capacity and Quality of Life: A Randomized Controlled Trial. Healthcare. 2024; 12(9):893. https://doi.org/10.3390/healthcare12090893
Chicago/Turabian StyleHerena-Funes, Maria del Carmen, Caroline Correia de Alencar, Dara María Velázquez-Torres, Elisenda Marrero García, Yolanda Castellote-Caballero, Felipe León-Morillas, Aday Infante-Guedes, and David Cruz-Díaz. 2024. "Effects of Hypopressive Abdominal Training on Ventilatory Capacity and Quality of Life: A Randomized Controlled Trial" Healthcare 12, no. 9: 893. https://doi.org/10.3390/healthcare12090893
APA StyleHerena-Funes, M. d. C., Correia de Alencar, C., Velázquez-Torres, D. M., Marrero García, E., Castellote-Caballero, Y., León-Morillas, F., Infante-Guedes, A., & Cruz-Díaz, D. (2024). Effects of Hypopressive Abdominal Training on Ventilatory Capacity and Quality of Life: A Randomized Controlled Trial. Healthcare, 12(9), 893. https://doi.org/10.3390/healthcare12090893








