The Role of the Discharge Planning Team on the Length of Hospital Stay and Readmission in Patients with Neurological Conditions: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Patient Selection
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
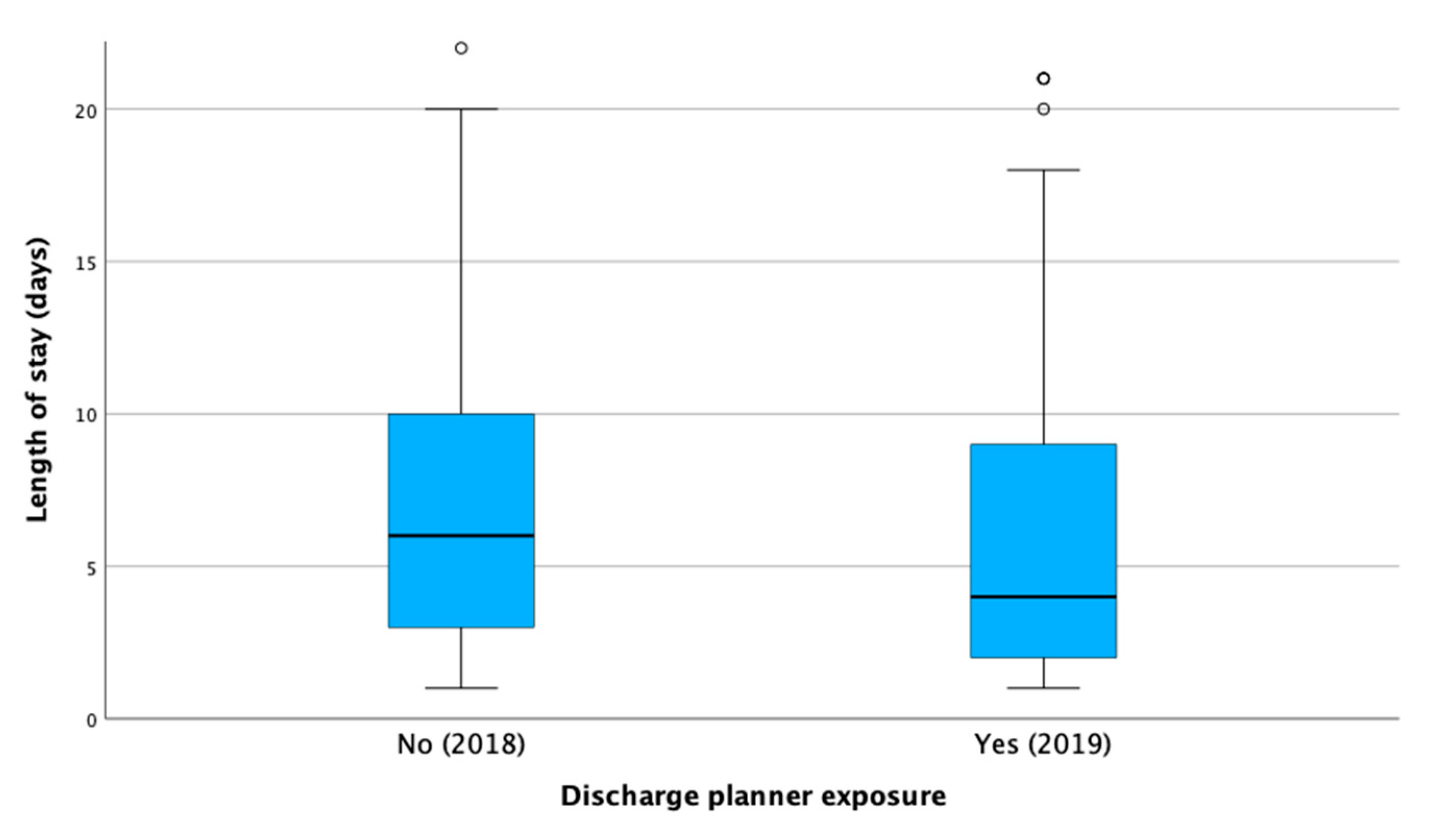
3.2. Length of Hospital Stay
3.3. Readmission Rates
3.4. Same-Admission Mortality
3.5. Overall Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ransmayr, G. Challenges of Caregiving to Neurological Patients. Wien. Med. Wochenschr. 2021, 171, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Specogna, A.V.; Turin, T.C.; Patten, S.B.; Hill, M.D. Hospital Treatment Costs and Length of Stay Associated with Hypertension and Multimorbidity after Hemorrhagic Stroke. BMC Neurol. 2017, 17, 158. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J. The Role of Multidisciplinary Team Care in Stroke Rehabilitation. Prog. Neurol. Psychiatry 2013, 17, 5–8. [Google Scholar] [CrossRef]
- Ibrahim, H.; Harhara, T.; Athar, S.; Nair, S.C.; Kamour, A.M. Multi-Disciplinary Discharge Coordination Team to Overcome Discharge Barriers and Address the Risk of Delayed Discharges. Risk Manag. Healthc. Policy 2022, 15, 141–149. [Google Scholar] [CrossRef]
- Abuzied, Y.; Maymani, H.; AlMatouq, B.; AlDosary, O. Reducing the Length of Stay by Enhancing the Patient Discharge Process: Using Quality Improvement Tools to Optimize Hospital Efficiency. Glob. J. Qual. Saf. Healthc. 2021, 4, 44–49. [Google Scholar] [CrossRef]
- Gonçalves-Bradley, D.C.; Lannin, N.A.; Clemson, L.M.; Cameron, I.D.; Shepperd, S. Discharge Planning from Hospital. Cochrane Database Syst. Rev. 2016, 1, CD000313. [Google Scholar] [CrossRef]
- Lainscak, M.; Kadivec, S.; Kosnik, M.; Benedik, B.; Bratkovic, M.; Jakhel, T.; Marcun, R.; Miklosa, P.; Stalc, B.; Farkas, J. Discharge Coordinator Intervention Prevents Hospitalizations in Patients With COPD: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2013, 14, 450.e1–450.e6. [Google Scholar] [CrossRef]
- McDonagh, M.S.; Smith, D.H.; Goddard, M. Measuring Appropriate Use of Acute Beds. Health Policy 2000, 53, 157–184. [Google Scholar] [CrossRef]
- Challis, D.; Hughes, J.; Xie, C.; Jolley, D. An Examination of Factors Influencing Delayed Discharge of Older People from Hospital. Int. J. Geriat Psychiatry 2014, 29, 160–168. [Google Scholar] [CrossRef]
- Langhorne, P.; Ramachandra, S. Stroke Unit Trialists’ Collaboration Organised Inpatient (Stroke Unit) Care for Stroke: Network Meta-Analysis. Cochrane Database Syst. Rev. 2020, 4, CD000197. [Google Scholar] [CrossRef]
- Mitchell, G.K.; Brown, R.M.; Erikssen, L.; Tieman, J.J. Multidisciplinary Care Planning in the Primary Care Management of Completed Stroke: A Systematic Review. BMC Fam. Pract. 2008, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Braet, A.; Weltens, C.; Sermeus, W. Effectiveness of Discharge Interventions from Hospital to Home on Hospital Readmissions: A Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 106–173. [Google Scholar] [CrossRef] [PubMed]
- Mistiaen, P.; Francke, A.L.; Poot, E. Interventions Aimed at Reducing Problems in Adult Patients Discharged from Hospital to Home: A Systematic Meta-Review. BMC Health Serv. Res. 2007, 7, 47. [Google Scholar] [CrossRef]
- Hunt-O’Connor, C.; Moore, Z.; Patton, D.; Nugent, L.; Avsar, P.; O’Connor, T. The Effect of Discharge Planning on Length of Stay and Readmission Rates of Older Adults in Acute Hospitals: A Systematic Review and Meta-analysis of Systematic Reviews. J. Nurs. Manag. 2021, 29, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.D.; Brooten, D.A.; Campbell, R.L.; Maislin, G.; McCauley, K.M.; Schwartz, J.S. Transitional Care of Older Adults Hospitalized with Heart Failure: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2004, 52, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Shepperd, S.; Lannin, N.A.; Clemson, L.M.; McCluskey, A.; Cameron, I.D.; Barras, S.L. Discharge Planning from Hospital to Home. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Burgess, J.F.; Hockenberry, J.M. Can All Cause Readmission Policy Improve Quality or Lower Expenditures? A Historical Perspective on Current Initiatives. Health Econ. Policy Law 2014, 9, 193–213. [Google Scholar] [CrossRef]
- Parker, S.G.; Peet, S.M.; McPherson, A.; Cannaby, A.M.; Abrams, K.; Baker, R.; Wilson, A.; Lindesay, J.; Parker, G.; Jones, D.R. A Systematic Review of Discharge Arrangements for Older People. Health Technol. Assess. 2002, 6. [Google Scholar] [CrossRef]
- Patel, P.R.; Bechmann, S. Discharge Planning. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weinberger, M.; Oddone, E.Z.; Henderson, W.G. Does Increased Access to Primary Care Reduce Hospital Readmissions? N. Engl. J. Med. 1996, 334, 1441–1447. [Google Scholar] [CrossRef]
- Jack, B.W. A Reengineered Hospital Discharge Program to Decrease Rehospitalization: A Randomized Trial. Ann. Intern. Med. 2009, 150, 178. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Guo, R.; Prochazka, A.V.; Misky, G.J. Identifying Keys to Success in Reducing Readmissions Using the Ideal Transitions in Care Framework. BMC Health Serv. Res. 2014, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Kripalani, S.; Theobald, C.N.; Anctil, B.; Vasilevskis, E.E. Reducing Hospital Readmission Rates: Current Strategies and Future Directions. Annu. Rev. Med. 2014, 65, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Fjærtoft, H.; Indredavik, B.; Lydersen, S. Stroke Unit Care Combined With Early Supported Discharge: Long-Term Follow-Up of a Randomized Controlled Trial. Stroke 2003, 34, 2687–2691. [Google Scholar] [CrossRef]
- Katikireddi, S.V.; Cloud, G.C. Planning a Patient’s Discharge from Hospital. BMJ 2008, 337, a2694. [Google Scholar] [CrossRef]
- Garland, A.; Olafson, K.; Ramsey, C.D.; Yogendran, M.; Fransoo, R. Epidemiology of Critically Ill Patients in Intensive Care Units: A Population-Based Observational Study. Crit. Care 2013, 17, R212. [Google Scholar] [CrossRef]
- Rich, M.W.; Beckham, V.; Wittenberg, C.; Leven, C.L.; Freedland, K.E.; Carney, R.M. A Multidisciplinary Intervention to Prevent the Readmission of Elderly Patients with Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 1190–1195. [Google Scholar] [CrossRef]
- Preen, D.B. Effects of a Multidisciplinary, Post-Discharge Continuance of Care Intervention on Quality of Life, Discharge Satisfaction, and Hospital Length of Stay: A Randomized Controlled Trial. Int. J. Qual. Health Care 2005, 17, 43–51. [Google Scholar] [CrossRef]
- Santana Baskar, P.; Cordato, D.; Wardman, D.; Bhaskar, S. In-hospital Acute Stroke Workflow in Acute Stroke—Systems-based Approaches. Acta Neurol. Scand. 2021, 143, 111–120. [Google Scholar] [CrossRef]
- Baxter, S.; Johnson, M.; Chambers, D.; Sutton, A.; Goyder, E.; Booth, A. The Effects of Integrated Care: A Systematic Review of UK and International Evidence. BMC Health Serv. Res. 2018, 18, 350. [Google Scholar] [CrossRef]
- Naylor, M.D.; Aiken, L.H.; Kurtzman, E.T.; Olds, D.M.; Hirschman, K.B. The Importance Of Transitional Care In Achieving Health Reform. Health Aff. 2011, 30, 746–754. [Google Scholar] [CrossRef]
- Kruse, C.S.; Beane, A. Health Information Technology Continues to Show Positive Effect on Medical Outcomes: Systematic Review. J. Med. Internet Res. 2018, 20, e41. [Google Scholar] [CrossRef]

| Variable | 2018 = 420 | 2019 = 436 | p-Value |
|---|---|---|---|
| Age, median (IQR) | 63 (47–75) | 62 (46–74) | 0.5512 |
| Gender, n (%) | |||
| Male | 251 (60%) | 234 (54%) | 0.0845 |
| Female | 169 (40%) | 202 (46%) | |
| Body mass index, n (%) | |||
| Underweight | 22 (5%) | 25 (6%) | 0.3423 |
| Normal | 120 (29%) | 121 (28%) | |
| Overweight | 132 (32%) | 133 (31%) | |
| Obese | 102 (24%) | 90 (21%) | |
| Extremely obese | 43 (10%) | 64 (15%) | |
| Diabetes, n (%) | 212 (51%) | 211 (48%) | 0.5844 |
| Hypertension, n (%) | 235 (56%) | 239 (55%) | 0.7833 |
| Dyslipidemia, n (%) | 70 (17%) | 90 (21%) | 0.1374 |
| Stroke, n (%) | 92 (22%) | 95 (22%) | 0.9673 |
| Transient ischemic attack, n (%) | 7 (2%) | 8 (2%) | 0.8512 |
| Atrial fibrillation, n (%) | 20 (5%) | 27 (6%) | 0.3723 |
| Heart failure, n (%) | 21 (5%) | 23 (5%) | 0.8781 |
| Chronic kidney disease, n (%) | 21 (5%) | 32 (7%) | 0.1596 |
| Coronary artery disease, n (%) | 41 (10%) | 57 (13%) | 0.134 |
| Liver cirrhosis, n (%) | 2 (1%) | 4 (1%) | 0.6867 |
| Hemodialysis, n (%) | 2 (1%) | 5 (1%) | 0.4518 |
| Peripheral vascular disease, n (%) | 5 (1%) | 4 (1%) | 0.7484 |
| Barthel index, median (IQR) | 12 (5–19) | 12 (4–18) | 0.8602 |
| Diagnosis | Discharge Planner Exposure | Median | IQR | p-Value | Adjusted β Coefficient (95% CI) * | p-Value |
|---|---|---|---|---|---|---|
| Ischemic Stroke | No | 6 | 4–10 | <0.001 | 1.22 (−1.69, 4.13) | 0.411 |
| Yes | 4 | 3–7 | ||||
| Hemorrhagic stroke | No | 6 | 3–18 | 0.7788 | −0.96 (−14.71, 12.79) | 0.8825 |
| Yes | 7 | 2–12 | ||||
| TIA | No | 3 | 2–4 | 0.3525 | 0.53 (−0.54, 1.66) | 0.3051 |
| Yes | 3 | 2–8 | ||||
| Seizure | No | 4 | 3–6 | 0.8249 | −4.64 (−18.45, 9.16) | 0.4993 |
| Yes | 4 | 2–10 | ||||
| Multiple sclerosis relapse | No | 5 | 3–13 | 0.0739 | −2.15 (−3.79, −0.51) | 0.0125 |
| Yes | 3 | 2–7 | ||||
| Myasthenia gravis crisis ** | No | 10 | 7–14 | 0.5731 | 3.51 (−126.09, 133.12) | 0.7888 |
| Yes | 10 | 4–15 | ||||
| Meningitis | No | 7 | 3–15 | 0.6044 | 2.89 (−1.22, 7) | 0.1406 |
| Yes | 6 | 3–13 |
| Outcome | 2018 Group | 2019 Group | Adjusted OR (95% CI) * | p-Value |
|---|---|---|---|---|
| Overall readmission rate, n (%) | 196 (47%) | 169 (39%) | 0.70 (0.49, 0.99) | 0.0193 |
| Readmission by months after discharge | ||||
| 1 month | 43 (21%) | 30 (18%) | - | 0.2819 |
| 2–12 months | 111 (57%) | 110 (65%) | - | |
| >12 months | 41 (21%) | 29 (17%) | - | |
| Outcome | 2018 Group | 2019 Group | Adjusted OR (95% CI) * | p-Value |
|---|---|---|---|---|
| Mortality during the same admission | 20 (5%) | 26 (6%) | 2.83 (0.72, 11.19) | 0.1370 |
| Overall mortality | 57 (14%) | 50 (12%) | 0.99 (0.55, 1.82) | 0.9978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatani, A.; Alzebaidi, S.; Alghaythee, H.K.; Alharbi, S.; Bogari, M.H.; Salamatullah, H.K.; Alghamdi, S.; Makkawi, S. The Role of the Discharge Planning Team on the Length of Hospital Stay and Readmission in Patients with Neurological Conditions: A Single-Center Retrospective Study. Healthcare 2025, 13, 143. https://doi.org/10.3390/healthcare13020143
Fatani A, Alzebaidi S, Alghaythee HK, Alharbi S, Bogari MH, Salamatullah HK, Alghamdi S, Makkawi S. The Role of the Discharge Planning Team on the Length of Hospital Stay and Readmission in Patients with Neurological Conditions: A Single-Center Retrospective Study. Healthcare. 2025; 13(2):143. https://doi.org/10.3390/healthcare13020143
Chicago/Turabian StyleFatani, Anmar, Sarah Alzebaidi, Himyan Kamel Alghaythee, Suzan Alharbi, Mohammed Hisham Bogari, Hassan K. Salamatullah, Saeed Alghamdi, and Seraj Makkawi. 2025. "The Role of the Discharge Planning Team on the Length of Hospital Stay and Readmission in Patients with Neurological Conditions: A Single-Center Retrospective Study" Healthcare 13, no. 2: 143. https://doi.org/10.3390/healthcare13020143
APA StyleFatani, A., Alzebaidi, S., Alghaythee, H. K., Alharbi, S., Bogari, M. H., Salamatullah, H. K., Alghamdi, S., & Makkawi, S. (2025). The Role of the Discharge Planning Team on the Length of Hospital Stay and Readmission in Patients with Neurological Conditions: A Single-Center Retrospective Study. Healthcare, 13(2), 143. https://doi.org/10.3390/healthcare13020143





