The Future of Breast Cancer Organized Screening Program Through Artificial Intelligence: A Scoping Review
Abstract
:1. Introduction
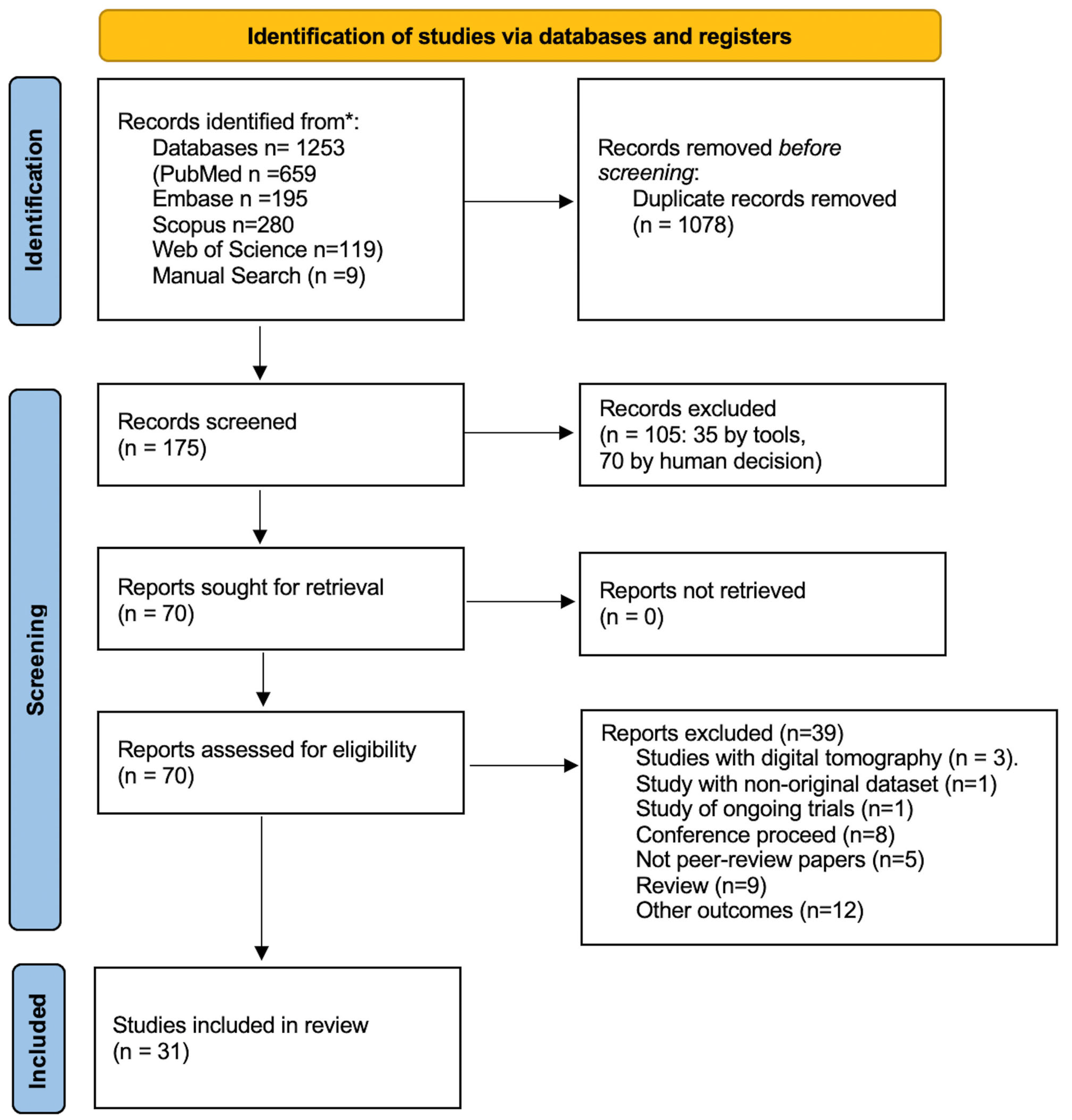
2. Methods
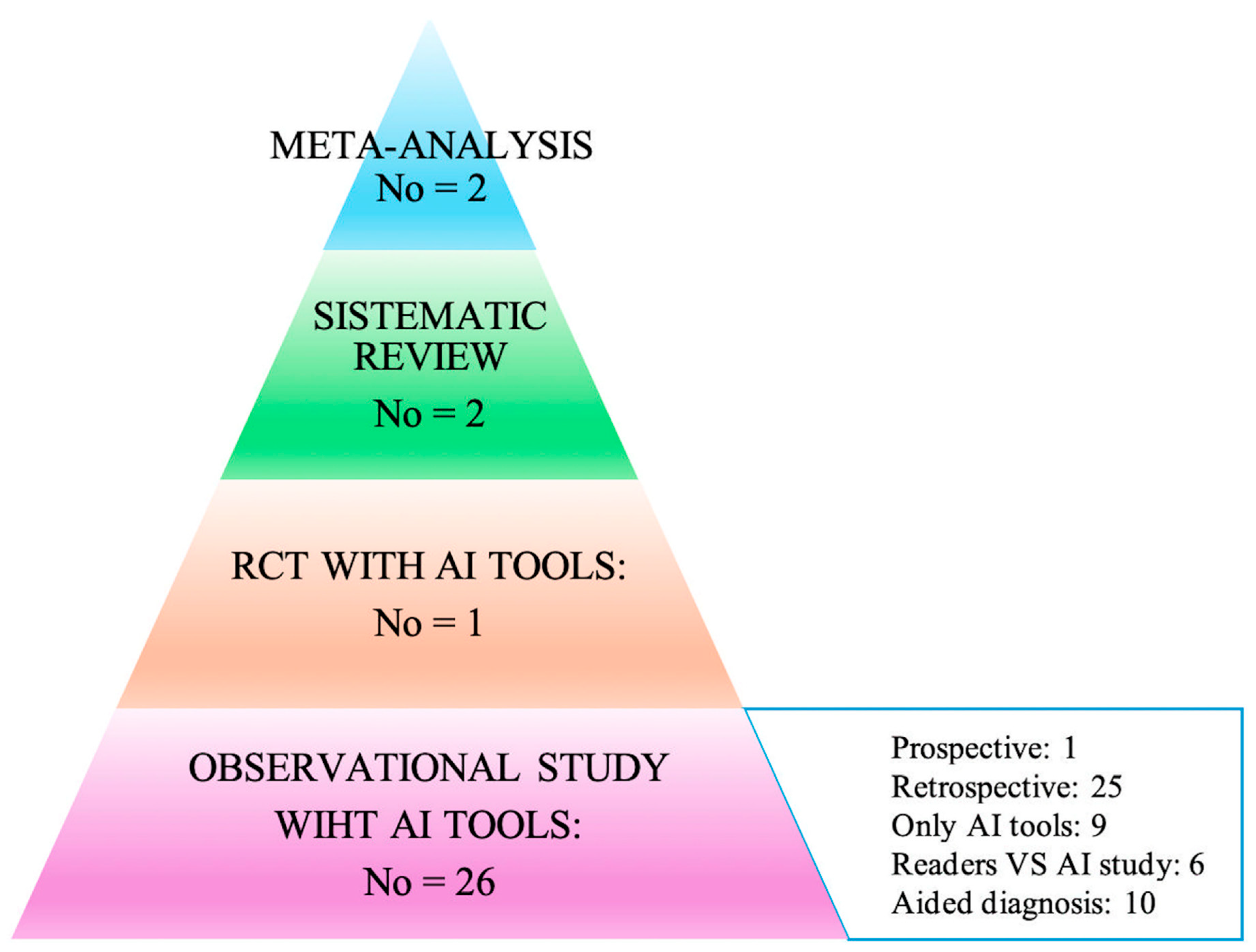
3. Results
3.1. Meta-Analyses
3.2. Systematic Reviews
3.3. Primary Studies
3.4. RTC and Prospective Studies
- Retrospective studies from organized screening programs
- 2.
- Studies from non-organized screening programs or a sample extracted from organized screening
- 3.
- Screening from multicenter studies: US, EU, UK, and SWEDEN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Bite, S. Lifetime Probability Among Females of Dying of Cancer. JNCI J. Natl. Cancer Inst. 2004, 96, 1311–1321. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 33818764. [Google Scholar] [CrossRef]
- Kreier, F. Cancer will cost the world $25 trillion over next 30 years. Nature 2023. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol 2023, 9, 465–472. [Google Scholar] [CrossRef]
- Altobelli, E.; Rapacchietta, L.; Angeletti, P.M.; Barbante, L.; Profeta, F.V.; Fagnano, R. Breast Cancer Screening Programmes across the WHO European Region: Differences among Countries Based on National Income Level. Int. J. Environ. Res. Public Health 2017, 14, 452. [Google Scholar] [CrossRef]
- Shieh, Y.; Eklund, M.; Sawaya, G.F.; Black, W.C.; Kramer, B.S.; Esserman, L.J. Population-based screening for cancer: Hope and hype. Nat. Rev. Clin. Oncol. 2016, 13, 550–565. [Google Scholar] [CrossRef]
- Bitkina, O.V.; Park, J.; Kim, H.K. Application of artificial intelligence in medical technologies: A systematic review of main trends. Digit. Health 2023, 9, 20552076231189331. [Google Scholar] [CrossRef]
- Pashkov, V.M.; Harkusha, A.O.; Harkusha, Y.O. Artificial intelligence in medical practice: Regulative issues and perspectives. Wiad. Lek. 2020, 73, 2722–2727. [Google Scholar] [CrossRef]
- Amisha, M.P.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Family Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef]
- Lee, C.I.; Elmore, J.G. Cancer Risk Prediction Paradigm Shift: Using Artificial Intelligence to Improve Performance and Health Equity. J. Natl. Cancer Inst. 2022, 114, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef]
- Bellini, V.; Coccolini, F.; Forfori, F.; Bignami, E. The artificial intelligence evidence-based medicine pyramid. World J. Crit. Care Med. 2023, 12, 89–91. [Google Scholar] [CrossRef]
- Hickman, S.E.; Woitek, R.; Le, E.P.V.; Im, Y.R.; Mouritsen Luxhøj, C.; Aviles-Rivero, A.I.; Baxter, G.C.; MacKay, J.W.; Gilbert, F.J. Machine Learning for Workflow Applications in Screening Mammography: Systematic Review and Meta-Analysis. Radiology 2022, 302, 88–104. [Google Scholar] [CrossRef]
- Yoon, J.H.; Strand, F.; Baltzer, P.A.T.; Conant, E.F.; Gilbert, F.J.; Lehman, C.D.; Morris, E.A.; Mullen, L.A.; Nishikawa, R.M.; Sharma, N.; et al. Standalone AI for Breast Cancer Detection at Screening Digital Mammography and Digital Breast Tomosynthesis: A Systematic Review and Meta-Analysis. Radiology 2023, 307, e222639. [Google Scholar] [CrossRef]
- Schopf, C.M.; Ramwala, O.A.; Lowry, K.P.; Hofvind, S.; Marinovich, M.L.; Houssami, N.; Elmore, J.G.; Dontchos, B.N.; Lee, J.M.; Lee, C.I. Artificial Intelligence-Driven Mammography-Based Future Breast Cancer Risk Prediction: A Systematic Review. J. Am. Coll. Radiol. 2024, 21, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Díaz, O.; Rodríguez-Ruíz, A.; Sechopoulos, I. Artificial Intelligence for breast cancer detection: Technology, challenges, and prospects. Eur. J. Radiol. 2024, 175, 111457. [Google Scholar] [CrossRef] [PubMed]
- Lång, K.; Josefsson, V.; Larsson, A.M.; Larsson, S.; Högberg, C.; Sartor, H.; Hofvind, S.; Andersson, I.; Rosso, A. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): A clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol. 2023, 24, 936–944. [Google Scholar]
- Dembrower, K.; Crippa, A.; Colón, E.; Eklund, M.; Strand, F. ScreenTrustCAD Trial Consortium. Artificial intelligence for breast cancer detection in screening mammography in Sweden: A prospective, population-based, paired-reader, non-inferiority study. Lancet Digit. Health 2023, 5, e703–e711, Erratum in Lancet Digit. Health 2023, 5, e646.. [Google Scholar] [CrossRef]
- Sharma, N.; Ng, A.Y.; James, J.J.; Khara, G.; Ambrózay, É.; Austin, C.C.; Forrai, G.; Fox, G.; Glocker, B.; Heindl, A.; et al. Multi-vendor evaluation of artificial intelligence as an independent reader for double reading in breast cancer screening on 275,900 mammograms. BMC Cancer 2023, 23, 460. [Google Scholar] [CrossRef]
- Hickman, S.E.; Payne, N.R.; Black, R.T.; Huang, Y.; Priest, A.N.; Hudson, S.; Kasmai, B.; Juette, A.; Nanaa, M.; Aniq, M.I.; et al. Mammography Breast Cancer Screening Triage Using Deep Learning: A UK Retrospective Study. Radiology 2023, 309, e231173. [Google Scholar] [CrossRef]
- Seker, M.E.; Koyluoglu, Y.O.; Ozaydin, A.N.; Gurdal, S.O.; Ozcinar, B.; Cabioglu, N.; Ozmen, V.; Aribal, E. Diagnostic capabilities of artificial intelligence as an additional reader in a breast cancer screening program. Eur. Radiol. 2024, 34, 6145–6157. [Google Scholar] [CrossRef]
- Larsen, M.; Olstad, C.F.; Lee, C.I.; Hovda, T.; Hoff, S.R.; Martiniussen, M.A.; Mikalsen, K.Ø.; Lund-Hanssen, H.; Solli, H.S.; Silberhorn, M.; et al. Performance of an Artificial Intelligence System for Breast Cancer Detection on Screening Mammograms from Breast Screen Norway. Radiol. Artif. Intell. 2024, 6, e230375. [Google Scholar] [CrossRef]
- Lauritzen, A.D.; Rodríguez-Ruiz, A.; von Euler-Chelpin, M.C.; Lynge, E.; Vejborg, I.; Nielsen, M.; Karssemeijer, N.; Lillholm, M. An Artificial Intelligence-based Mammography Screening Protocol for Breast Cancer: Outcome and Radiologist Workload. Radiology 2022, 304, 41–49. [Google Scholar] [CrossRef]
- Leibig, C.; Brehmer, M.; Bunk, S.; Byng, D.; Pinker, K.; Umutlu, L. Combining the strengths of radiologists and AI for breast cancer screening: A retrospective analysis. Lancet Digit. Health 2022, 4, e507–e519. [Google Scholar] [CrossRef]
- Romero-Martín, S.; Elías-Cabot, E.; Raya-Povedano, J.L.; Broeders, M.; Gennaro, G.; Clauser, P.; Helbich, T.H.; Chevalier, M.; Tan, T.; Mertelmeier, T.; et al. Stand-Alone Use of Artificial Intelligence for Digital Mammography and Digital Breast Tomosynthesis Screening: A Retrospective Evaluation. Radiology 2022, 302, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.; Wåhlin, E.; Dembrower, K.; Azavedo, E.; Foukakis, T.; Liu, Y.; Smith, K.; Eklund, M.; Strand, F. External Evaluation of 3 Commercial Artificial Intelligence Algorithms for Independent Assessment of Screening Mammograms. JAMA Oncol. 2020, 6, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.T.; Mees, W.; Bun, P.A.M.; Janssen, N.; Rodríguez-Ruiz, A.; Dalmış, M.U.; Karssemeijer, N.; van Gils, C.H.; Sechopoulos, I.; Mann, R.M.; et al. Interval Cancer Detection Using a Neural Network and Breast Density in Women with Negative Screening Mammograms. Radiology 2022, 303, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.S.; Marcon, M.; Ghafoor, S.; Wurnig, M.C.; Frauenfelder, T.; Boss, A. Deep Learning in Mammography: Diagnostic Accuracy of a Multipurpose Image Analysis Software in the Detection of Breast Cancer. Investig. Radiol. 2017, 52, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Arasu, V.A.; Habel, L.A.; Achacoso, N.S.; Buist, D.S.M.; Cord, J.B.; Esserman, L.J.; Hylton, N.M.; Glymour, M.M.; Kornak, J.; Kushi, L.H.; et al. Comparison of Mammography AI Algorithms with a Clinical Risk Model for 5-year Breast Cancer Risk Prediction: An Observational Study. Radiology 2023, 307, e222733. [Google Scholar] [CrossRef]
- Lehman, C.D.; Mercaldo, S.; Lamb, L.R.; King, T.A.; Ellisen, L.W.; Specht, M.; Tamimi, R.M. Deep Learning vs. Traditional Breast Cancer Risk Models to Support Risk-Based Mammography Screening. J. Natl. Cancer Inst. 2022, 114, 1355–1363. [Google Scholar] [CrossRef]
- Yala, A.; Mikhael, P.G.; Strand, F.; Satuluru, S.; Kim, T.; Banerjee, I.; Gichoya, J.; Trivedi, H.; Lehman, C.D.; Hughes, K.; et al. Multi-Institutional Validation of a Mammography-Based Breast Cancer Risk Model. J. Clin. Oncol. 2022, 40, 1732–1740. [Google Scholar] [CrossRef]
- Arefan, D.; Mohamed, A.A.; Berg, W.A.; Zuley, M.L.; Sumkin, J.H.; Wu, S. Deep learning modeling using normal mammograms for predicting breast cancer risk. Med. Phys. 2020, 47, 110–118. [Google Scholar] [CrossRef]
- Lång, K.; Hofvind, S.; Rodríguez-Ruiz, A.; Andersson, I. Can artificial intelligence reduce the interval cancer rate in mammography screening? Eur. Radiol. 2021, 31, 5940–5947. [Google Scholar] [CrossRef]
- Gastounioti, A.; Eriksson, M.; Cohen, E.A.; Mankowski, W.; Pantalone, L.; Ehsan, S.; McCarthy, A.M.; Kontos, D.; Hall, P.; Conant, E.F. External Validation of a Mammography-Derived AI-Based Risk Model in a U.S. Breast Cancer Screening Cohort of White and Black Women. Cancers 2022, 14, 4803. [Google Scholar] [CrossRef]
- Ha, R.; Chang, P.; Karcich, J.; Mankowski, W.; Pantalone, L.; Ehsan, S.; McCarthy, A.M.; Kontos, D.; Hall, P.; Conant, E.F. Convolutional Neural Network Based Breast Cancer Risk Stratification Using a Mammographic Dataset. Acad. Radiol. 2019, 26, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Hinton, B.; Ma, L.; Mahmoudzadeh, A.P.; Malkov, S.; Fan, B.; Greenwood, H.; Joe, B.; Lee, V.; Kerlikowske, K.; Shepherd, J. Deep learning networks find unique mammographic differences in previous negative mammograms between interval and screen-detected cancers: A case-case study. Cancer Imaging 2019, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wolfgruber, T.K.; Leong, L.; Jensen, M.; Scott, C.; Winham, S.; Sadowski, P.; Vachon, C.; Kerlikowske, K.; Shepherd, J.A. Deep Learning Predicts Interval and Screening-detected Cancer from Screening Mammograms: A Case-Case-Control Study in 6369 Women. Radiology 2021, 301, 550–558. [Google Scholar] [CrossRef]
- Sasaki, M.; Tozaki, M.; Rodríguez-Ruiz, A.; Yotsumoto, D.; Ichiki, Y.; Terawaki, A.; Oosako, S.; Sagara, Y. Artificial intelligence for breast cancer detection in mammography: Experience of use of the ScreenPoint Medical Transpara system in 310 Japanese women. Breast Cancer 2020, 27, 642–651. [Google Scholar] [CrossRef]
- Dang, L.A.; Chazard, E.; Poncelet, E.; Serb, T.; Rusu, A.; Pauwels, X.; Parsy, C.; Poclet, T.; Cauliez, H.; Engelaere, C.; et al. Impact of artificial intelligence in breast cancer screening with mammography. Breast Cancer 2022, 29, 967–977. [Google Scholar] [CrossRef]
- Lee, S.E.; Han, K.; Yoon, J.H.; Youk, J.H.; Kim, E.K. Depiction of breast cancers on digital mammograms by artificial intelligence-based computer-assisted diagnosis according to cancer characteristics. Eur. Radiol. 2022, 32, 7400–7408. [Google Scholar] [CrossRef]
- Schaffter, T.; Buist, D.S.M.; Lee, C.I.; Nikulin, Y.; Ribli, D.; Guan, Y.; Lotter, W.; Jie, Z.; Du, H.; Wang, S.; et al. Evaluation of Combined Artificial Intelligence and Radiologist Assessment to Interpret Screening Mammograms. JAMA Netw. Open 2020, 3, e200265, Erratum in JAMA Netw. Open 2020, 3, e204429.. [Google Scholar] [CrossRef]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H.; Back, T.; Chesus, M.; Corrado, G.S.; Darzi, A.; et al. International evaluation of an AI system for breast cancer screening. Nature 2020, 577, 89–94, Erratum in Nature 2020, 586, E19.. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Kim, H.H.; Han, B.K.; Kim, K.H.; Han, K.; Nam, H.; Lee, E.H.; Kim, E.K. Changes in cancer detection and false-positive recall in mammography using artificial intelligence: A retrospective, multireader study. Lancet Digit. Health 2020, 2, e138–e148. [Google Scholar] [CrossRef]
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I.; Precise4Q consortium. Explainability for artificial intelligence in healthcare: A multidisciplinary perspective. BMC Med. Inform. Decis. Mak. 2020, 20, 310. [Google Scholar] [CrossRef]
- Higgins, D.; Madai, V.I. From bit to bedside: A practical framework for artificial intelligence product development in healthcare. Adv. Intell. Syst. 2020, 2, 2000052. [Google Scholar] [CrossRef]
- Hong, L.; Cheng, X.; Zheng, D. Application of Artificial Intelligence in Emergency Nursing of Patients with Chronic Obstructive Pulmonary Disease. Contrast Media Mol. Imaging 2021, 2021, 6423398. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Topalovic, M.; Janssens, W. Artificial intelligence in diagnosis of obstructive lung disease: Current status and future potential. Curr. Opin. Pulm. Med. 2018, 24, 117–123. [Google Scholar] [CrossRef]
- Kotanko, P.; Nadkarni, G.N. Advances in Chronic Kidney Disease Lead Editorial Outlining the Future of Artificial Intelligence/Machine Learning in Nephrology. Adv. Kidney Dis. Health 2023, 30, 2–3. [Google Scholar] [CrossRef]
- Khodve, G.B.; Banerjee, S. Artificial Intelligence in Efficient Diabetes Care. Curr. Diabetes Rev. 2023, 19, e050922208561. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, N.; Liu, S.; Yan, Z.; Qian, H.; Zhu, S.; Zhang, J.; Wang, M.; Jiang, Q.; Yang, W. Research Progress of Artificial Intelligence Image Analysis in Systemic Disease-Related Ophthalmopathy. Dis. Markers 2022, 2022, 3406890. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakayama, K.I. Artificial intelligence in oncology. Cancer Sci. 2020, 111, 1452–1460. [Google Scholar] [CrossRef]
- Syed, A.B.; Zoga, A.C. Artificial Intelligence in Radiology: Current Technology and Future Directions. Semin. Musculoskelet. Radiol. 2018, 22, 540–545. [Google Scholar]
- Taylor, C.R.; Monga, N.; Johnson, C.; Hawley, J.R.; Patel, M. Artificial Intelligence Applications in Breast Imaging: Current Status and Future Directions. Diagnostics 2023, 13, 2041. [Google Scholar] [CrossRef]
- Jayasekera, J.; Mandelblatt, J.S. Systematic Review of the Cost Effectiveness of Breast Cancer Prevention, Screening, and Treatment Interventions. J. Clin. Oncol. 2020, 38, 332–350. [Google Scholar] [CrossRef]
- Van Nijnatten, T.J.A.; Payne, N.R.; Hickman, S.E.; Ashrafian, H.; Gilbert, F.J. Overview of trials on artificial intelligence algorithms in breast cancer screening—A roadmap for international evaluation and implementation. Eur. J. Radiol. 2023, 67, 111087, Erratum in Eur. J. Radiol. 2024, 170, 111202.. [Google Scholar] [CrossRef]
- Combi, C.; Amico, B.; Bellazzi, R.; Holzinger, A.; Moore, J.H.; Zitnik, M.; Holmes, J.H. A manifesto on explainability for artificial intelligence in medicine. Artif. Intell. Med. 2022, 133, 102423. [Google Scholar] [CrossRef] [PubMed]
| Author | Aims | Patients Population | Results |
|---|---|---|---|
| META-ANALYSIS | |||
| Hickman [19] | Evaluate machine learning (ML) accuracy in detecting breast cancer in screening mammography. | 14 eligible studies,
| Triage studies: AI could be used to reduce the number of mammography examinations read by radiologists by 17–91% while “missing” 0–7% of cancers AI vs. readers’ studies AI/ML algorithm
Readers
|
| Jung Hyun Yoon [20] | A random effects meta-analysis and meta-regression analysis were performed for overall studies and for different study types (reader studies vs. historic cohort studies) and imaging techniques (digital mammography vs. DBT). Conclusions: Standalone AI for screening digital mammography performed as well as or better than the radiologists. | 16 studies.
497,091 women (six reader studies, seven historic cohort studies on digital mammography, and four studies on digital breast tomography (DBT). | AI standalone AUCs were significantly higher for standalone AI than radiologists in the six reader studies on digital mammography (0.87 vs. 0.81, p = 0.002), but not for historic cohort studies (0.89 vs. 0.96, p = 0.152). Four studies on DBT showed significantly higher AUCs in AI compared with radiologists (0.90 vs. 0.79, p < 0.001). Higher sensitivity and lower specificity were seen for standalone AI compared with radiologists. |
| REVIEW AND SYSTEMATIC REVIEW | |||
| Schopf [21] | Summarize the literature regarding the performance of mammography-image based artificial intelligence (AI) algorithms, with and without additional clinical data, for future breast cancer risk prediction. | 16 studies | The median AUC performance of AI image-only models was 0.72 (range 0.62–0.90) compared with 0.61 for breast density or clinical risk factor-based tools (range 0.54–0.69). Of the seven studies that compared AI image-only performance directly to combined image + clinical risk factor performance, six demonstrated no significant improvement, and one study demonstrated increased improvement. |
| Diaz [22] | Overview of the current state of artificial intelligence (AI) technology for automated detection of breast cancer in digital mammography (DM) and digital breast tomosynthesis (DBT). Aimed to discuss the technology, available AI systems, and the challenges faced by AI in breast cancer screening. | DL-based AI systems have shown significant improvements in breast cancer detection. They have the potential to enhance screening outcomes, reduce false negatives and positives, and detect subtle abnormalities missed by human observers. However, challenges like the lack of standardized datasets, potential bias in training data, and regulatory approval hinder their widespread adoption. | |
| RECRUIMENT: ORGANIZED SCREENING | |||||||
|---|---|---|---|---|---|---|---|
| Author, Country, Study Publication Year | Study Design | Outcomes | Patient Population | Outcome Results | |||
| Period of Collection Mammograms and Woman’s Age | No. of Mammograms/Patients | Mammography Reading Protocol and AI Reading Protocol | Diagnosis Confirmations | ||||
| Lång Sweden [23] | RCT Comparing study | Cancer detection rate Recall rates Workload reduction | 12 April 2021 28 July 2022 40–74 years | 80,033 AI-supported screening (n = 40,003) or double reading without AI (n = 40,030) | DR Transpara | Cancer detection rates were 6.1 (95% CI 5.4–6.9) per 1000 screened participants in the intervention group, and 5.1 (4.4–5.8) per 1000 in the control group-a ratio of 1.2 (95% CI 1.0–1.5; p = 0·052). Recall rates were 2.2% (95% CI 2.0–2.3) in the intervention group and 2.0% (1.9–2.2) in the control group. The false-positive rate was 1.5% (95% CI 1.4–1.7) in both groups. The PPV of recall was 28.3% (95% CI 25.3–31.5) in the intervention group and 24.8% (21.9–28.0) in the control group. The screen-reading workload was reduced by 44.3% using AI. | |
| Dembrower Sweden [24] | Prospective study Comparing study | Cancer detection rate in single reading with AI, double reading with AI, and triple reading with AI | 1 April 2021 9 June 2022 40–74 years | 58,344 | Double reading by one radiologist plus AI was non-inferior for cancer detection compared with double reading by two radiologists (261 [0.5%] vs. 250 [0.4%] detected cases relative proportion 1.04 [95% CI 1.00–1.09]). Single reading by AI (246 [0.4%] vs. 250 [0.4%] detected cases; relative proportion 0.98 [0.93–1.04]) Triple reading by two radiologists plus AI (269 [0.5%] vs. 250 [0.4%] detected cases; relative proportion 1.08 [1.04–1.11]) were also non-inferior to double reading by two radiologists. | ||
| Sharma Hungary and UK 2023 [25] | Retrospective Comparing study | SE, SP, PPV Recall rate workload for each mammography equipment | 2009–2012 50–70 years | 304,360/- | Double reading Mia version 2.0 | Histopathology (Hungarian data) Cancer Registry (UK data) | DR with AI, compared with human DR, showed at least a non-inferior recall rate, cancer detection rate, sensitivity, specificity and positive predictive value (PPV) for each mammography vendor and site, and superior recall rate, specificity, and PPV for two systems. The simulation indicates that using AI would have increased the arbitration rate (3.3% to 12.3%) but could have reduced the human workload by 30.0% to 44.8%. |
| Hickman UK 2023 [26] | Retrospective AI tool efficacy study | Three different DL models as triage and in interval cancers in a possible second automatized second reading | January 2017 to December 2018 | 78,849 | CAD, Deep learning | Histopathology | Rule-out triage: Models DL-1, DL-2, and DL-3 triaged 35.0% (27,565 of 78,849), 53.2% (41,937 of 78,849), and 55.6% (43,869 of 78,849) of mammograms, respectively, with 0.0% (0 of 887) to 0.1% (one of 887) of the screening-detected cancers undetected. Interval cancers: DL algorithms triaged in 4.6% (20 of 439) to 8.2% (36 of 439) of interval and 5.2% (36 of 688) to 6.1% (42 of 688) of subsequent-round cancers when applied after the routine double-reading workflow. Both approaches: overall noninferior specificity (difference, −0.9%; p < 0.001) and superior sensitivity (difference, 2.7%; p < 0.001) for the adaptive workflow compared with routine double reading for all three algorithm |
| Seker Turkey 2024 [27] | Retrospective AI tool efficacy study | SE, SP, AUC | 2009 to 2019 | 22,621/8758 Woman’s age: Not reported | Double reading Lunit INSIGHT MMG version 1.1.7.1 Positive BIRADS 0, 3, 4, 5 Negative 1–2 | Not reported | AUC: 89.6 (86.1–93.2%) SE 72.38 SP 92.86 |
| Larsen Norway 2022 [28] | Retrospective AI tool efficacy study | Rate of cancer detection | 2009–2018 Woman’s age: 50–69 years | 22,969/ 478,772 | Transpara 1.7 1–5 to indicate suspicion of malignancy—1–2 negative, probably benign; 3–5 from suspicion of malignancy to malignancy | Cancer Registry | A total of 653/752 screen-detected cancers (86.8%) and 92/205 interval cancers (44.9%) were given a score of 10 by the AI system (threshold 1). Using a threshold of 3, 80.1% of the screen-detected cancers (602/752) and 30.7% of the interval cancers (63/205) were selected. Screen-detected cancers with AI scores not selected using the thresholds had favorable histopathologic characteristics compared with those selected; contrasting results were observed for interval cancer. |
| Lauritzen Denmark 2022 [29] | Retrospective Comparing study | AUC, SE, SP of AI versus readers’ performance for AI performance according to BI-RADS for all cancers, screen detected, interval cancers, and long-term cancers | 2012–2013 Woman’s age: 50–69 years | 54,977 | Transpara 1.7 BI-RADS | Histopathology | AI AUC 0.97 (0.97–0.98) SE 69.7 (66.9–72.4) SP 98.6 (98.5–98.7) Readers AUC not rep. SE 70.8 (68.0–73.5) SP 98.6 (98.5–98.7) |
| Leibig Germany 2022 [30] | Retrospective Comparing study | AUC, SE, SP of AI versus readers’ performance | 2007–2020 Woman’s age: 50–70 years | 1,193,197 | Histopathology | AI AUC 0.94 (0.939–0.950) SE 84.6 (83.5–85.9) SP 91.3 (91.1–91.5) Readers AUC not rep. SE87.2 (58.2–75.2) SP 93.4 (93.2–93.6) | |
| Romero Martin Spain 2022 [31] | Retrospective Comparing study | AUC, SE, SP of AI versus | 2015–2016 Woman’s age: 50–69 | 15,999 | Transpara 1.7 BI-RADS | Histopathology | AI AUC 0.94 (0.91–0.97) SE 70.8 (61.8–78.4) SP NR Readers AUC not rep. SE 67.3 (58.2–75.2) SP NR |
| Salim Sweden 2020 [32] | Retrospective Comparing study | AUC, SE, SP of 3 different AI; Overall, SE, SP of AI; readers’ SE, SP | 2008/2015 Woman’s age: 50–69 | 8805 | 2 readers; 25 different; first radiologist for first reading and 20 for second reading CAD based | Cancer Registry | AUC AI-1 0.956 (95% CI, 0.948–0.965), SE 81.9; sp: 96.1 AI-2 0.922 (95% CI, 0.910–0.934) SE 67.0; SP: 96.6 AI-3 0.920 (95% CI, 0.909–0.931) SE 67.4; SP: 96.7 Overall AI: SE 86.7% (95% CI, 84.2–89.2%) and specificity of 92.5% (95% CI, 92.3–92.7%) Overall readers: SP was 98.5 (98.4–98.6) and SE 85.0 (82.2–87.5) |
| Wanders The Netherlands 2022 [33] | Retrospective nested case–control AI tool efficacy study | Interval cancer (IC) risk prediction | January 2011 January 2015 Woman’s age: according to organized screening | 1,163,147 | Neural network (NN)-based model | Cancer Registry | AUC of the NN model was 0.79 (95% CI: 0.77, 0.81), which was higher than the AUC of the AI cancer detection system or breast density alone (AUC, 0.73 [95% CI: 0.71, 0.76] and 0.69 [95% CI: 0.67, 0.71], respectively; p < 0.001 for both). At 90% specificity, the NN model had a sensitivity of 50.9% (339 of 666 women; 95% CI: 45.2, 56.3) for the prediction of IC, which was higher than that of the AI system (37.5%; 250 of 666 women; 95% CI: 33.0, 43.7; p < 0.001) or breast density percentage alone (22.4%; 149 of 666 women; 95% CI: 17.9, 28.5; p < 0.001). |
| Beker Switzerland 2017 [34] | Retrospective Comparing study | AUC, SE, SP of AI versus AUC, SE, SP of 3 different readers | 2012 | -/3228 | ViDi Suite Version 2.0; ViDi Systems Inc, Villaz-Saint-Pierre, Switzerland) to 3 radiologist s 7, 10, and 3 years of experience in breast imaging BI-RADS | Histopathology | AUC of AI of 0.82 (95% CI, 0.75–0.89) with an optimal sensitivity/specificity of 73.7/72.0%. Diagnostic accuracy measured by AUC was not significantly different between the readers (AUC = 0.79, 0.77, and 0.87; p = 0.18, 0.32, and 0.83) or the AI (p = 0.45, 0.56, and 0.62). However, all readers exhibited a higher specificity but lower sensitivity when compared with the ANN, with a sensitivity/specificity of 60.0%/94.4% for reader 1, 60.0%/93.6% for reader 2, and 80.0%/90.2% for reader 3. |
| RECRUIMENT: Studies from Non-Organized Screening Programs or a Sample Came from Organized Screening | |||||||
| Author, Country, Study Publication Year | Study Design | Outcomes | Patient Population | Outcome Results | |||
| Period of Collection Mammograms and Woman Age | No. of Mammograms/Patients | Mammography Reading Protocol and AI Reading Protocol | Diagnosis Confirmations | ||||
| Arasu USA 2023 [35] | Retrospective, case–control AI tools study | Prediction of 5-year risk between AI tools and AI tool and Breast Cancer Surveillance Consortium (BCSC) | 2016 and 2021 | 13,628 | Mirai Globally-Aware Multiple Instance Classifier MammoScreen ProFound AI and Mia | Kaiser Permanente Northern California Breast Cancer Tracking System |
AI predicted incident cancers at 0 to 5 years better than the Breast Cancer Surveillance Consortium (BCSC) clinical risk model (AI time-dependent area under the receiver operating characteristic curve [AUC] range, 0.63–0.67; BCSC time-dependent AUC, 0.61; Bonferroni-adjusted p < 0.0016). Combining AI algorithms with BCSC slightly improved the time-dependent AUC versus AI alone (AI with BCSC time-dependent AUC range, 0.66–0.68; Bonferroni-adjusted p < 0.0016). |
| Lehman USA 2022 [36] | Retrospective, case–control AI tools study | AI detecting cancer vs. a NCI BCRAT risk model from 18 September 2017 to 1 February 2021 | From 18 September 2017 to 1 February 2021 | 57,635 consecutive patients with a prior mammogram underwent 119,179 bilateral screening mammograms. | Deep learning | Not reported | Cancers detected per thousand patients screened were higher in patients at increased risk by the deep learning model (8.6, 95% confidence interval [CI] = 7.9 to 9.4) compared with the Tyrer-Cuzick (4.4, 95% CI = 3.9 to 4.9) and NCI BCRAT (3.8, 95% CI = 3.3 to 4.3) models (p < 0.001). Area under the receiver operating characteristic curves of the deep learning model (0.68, 95% CI = 0.66 to 0.70) was higher compared with the Tyrer-Cuzick (0.57, 95% CI = 0.54 to 0.60) and NCI BCRAT (0.57, 95% CI = 0.54 to 0.60) models. Simulated screening of the top 50th percentile risk by the deep learning model captured statistically significantly more patients with cancer compared with Tyrer-Cuzick and NCI BCRAT models (p < 0.001). |
| Yala USA 2022 [37] | Retrospective, case–control AI tools study | Deep learning (DL) vs. cancer risk model | 1 January 2009, and 31 December 2012 | 88,994 consecutive screening mammograms in 39,571 women | Deep learning (DL) | Not reported | The test set included 3937 women, aged 56.20 years ± 10.04. Hybrid DL and image-only DL showed AUCs of 0.70 (95% confidence interval [CI]: 0.66, 0.75) and 0.68 (95% CI: 0.64, 0.73), respectively. RF-LR and TC showed AUCs of 0.67 (95% CI: 0.62, 0.72) and 0.62 (95% CI: 0.57, 0.66), respectively. Hybrid DL showed a significantly higher AUC (0.70) than TC (0.62; p < 0.001) and RF-LR (0.67; p = 0.01). |
| Arefan USA 2020 [38] | Retrospective, case–control AI tools study | AUC between different mammography projections | January 2007 and January 2012 | 226 | Deep learning model and a GoogLeNet-LDA | Not reported | AUC was 0.73 (95% Confidence Interval [CI]: 0.68–0.78; GoogLeNet-LDA model on CC view) when using the whole breast was 0.72 (95% CI: 0.67–0.76; GoogLeNet-LDA model on MLO + CC view) when using the dense tissue, respectively, as the model input. The GoogLeNet-LDA model significantly (all p < 0.05) outperformed the end-to-end GoogLeNet model in all experiments. CC view was consistently more predictive than MLO view in both deep learning models, regardless of the input sub-regions. Both models exhibited superior performance than the percent breast density (AUC = 0.54; 95% CI: 0.49–0.59). |
| Lang Sweden 2021 [39] | Retrospective, case–control AI tools study | Preceding screening mammograms of cancer in southern Sweden were analyzed with a deep learning-based AI system | 2013 and 2017 | 429 consecutive women diagnosed with interval | Not reported | Not reported | A statistically significant correlation between the interval cancer classification groups and AI risk score was observed (p < 0.0001). AI scored one in three (143/429) interval cancers with a risk score of 10, of which 67% (96/143) were either classified as minimal signs or false negatives. Of these, 58% (83/143) were correctly located by AI and could therefore potentially be detected at screening with the aid of AI, resulting in a 19.3% (95% CI 15.9–23.4) reduction of interval cancers. At the 4% and 1% recall thresholds, the reduction in interval cancers was 11.2% (95% CI 8.5–14.5) and 4.7% (95% CI 3.0–7.1). The corresponding reduction in interval cancers with grave outcomes (women who died or with stage IV disease) at a risk score of 10 was 23% (8/35; 95% CI 12–39). |
| Gastounioti USA 2018 [40] | Retrospective, case–control AI tools study | Breast density | 2002–2006 | 5139 176 cases and 4963 controls | Convolutional neural network | Not reported | Strong linear separability of cancer cases from the controls was demonstrated on the basis of the five meta-features generated by the proposed hybrid framework. The corresponding case–control classification performance was AUC = 0.90 (95% CI: 0.82–0.98), with a sensitivity and specificity equal to 0.81 and 0.98, respectively. |
| Ha USA 2019 [41] | Retrospective, case–control AI tools study | Breast density | January 2011 to January 2017 | 1474 mammographic images First, 210 patients with new incidence of breast cancer were identified. The control group consisted of 527 patients without breast cancer from the same time period | Convolutional neural network | Not reported | Breast density (BD) was significantly higher in the case group [2.39 (SD, 0.7)] than the control group [1.98 (SD, 0.75), p < 0.0001]. In multivariate logistic regression analysis, both the CNN pixel-wise mammographic risk model and BD were significant independent predictors of breast cancer risk (p < 0.0001). The CNN risk model showed greater predictive potential [OR = 4.42 (95% CI, 3.4–5.7)] compared with BD [OR = 1.67 (95% CI, 1.4–1.9)]. |
| Hinton USA 2019 [42] | Retrospective, case–control AI tools study | Interval cancer | 2006 and 2015 | A total of 316,001 examinations were performed in the screening population, leading to a total of 245 interval cancers of which 182 women were available for this study | ResNet-50 | Not reported | The optimized deep learning model achieved an AUC of 0.82. Contingency table analysis showed the network was correctly classifying 75.2% of the mammograms and that incorrect classifications were slightly more common for the interval cancer mammograms. |
| Zhu USA 2021 [43] | Retrospective, case–control AI tools study | Ability of DL models to estimate the risk of interval and screening-detected breast cancers with and without clinical risk factors | January 2006 December 2013. | 25,096 digital screening mammograms | Deep learning | Not reported | Cancer diagnosis DL model: The C statistics and odds ratios for comparing patients with screening-detected cancer versus the matched controls were 0.66 (95% CI: 0.63, 0.69) and 1.25 (95% CI: 1.17, 1.33). Clinical risk factors with the Breast Imaging Reporting and Data System (BI-RADS) density model: 0.62 (95% CI: 0.59, 0.65) and 2.14 (95% CI: 1.32, 3.45). Combined DL and clinical risk factors model 0.66 (95% CI: 0.63, 0.69) and 1.21 (95% CI: 1.13, 1.30) Interval cancer DL model: For comparing patients with interval cancer versus controls, the C statistics and odds ratios were 0.64 (95% CI: 0.58, 0.71) and 1.26 (95% CI: 1.10, 1.45), The risk factors with BI-RADS density: 0.71 (95% CI: 0.65, 0.77) and 7.25 (95% CI: 2.94, 17.9) Combined DL and clinical risk factors model: 0.72 (95% CI: 0.66, 0.78) and 1.10 (95% CI: 0.94, 1.29) for the The p values between the DL, BI-RADS, and combined model’s ability to detect screen and interval cancer were 0.99, 0.002, and 0.03, respectively. |
| Sasaki Japan 2020 [44] | Retrospective Comparing study | AUC, SE, SP of 3 different AIs; Overall, SE, SP of AI; Readers SE, SP | January 2018 and October 2018 | 310 | Transpara | Not reported | The AUC was higher for human readers than with the standalone Transpara system (human readers 0.816; Transpara system 0.706; difference 0.11; p < 0.001). The sensitivity of the unaided HR for diagnosis was 89% and the specificity was 86%. The sensitivity of the standalone Transpara system for cutoff scores of 4 and 7 were 93% and 85%, and the specificities were 45% and 67%, respectively. |
| Dang France 2022 [45] | Retrospective Comparing study | AUC, SE, SP of 3 different AIs; Overall, SE, SP of AI; Readers SE, SP | June 2012 to March 2020 | 314 | Mammoscreen forced BI-RADS score of 1–5 per breast | Histopathology | AUC was significantly improved when using AI (0.74 vs. 0.77, p = 0.004). |
| Lee Korea 2022 [46] | Retrospective Comparing study | AUC, SE, SP of 3 different AIs; Overall, SE, SP of AI; Readers SE, SP, according to their experience in the field (breast radiology expertise vs. general radiologist) | March 2009 and September 2018 | 200 | Lunit INSIGHT MMG, version 1.1.1.0; Lunit Scale from 1 (normal) to 7 (highly suggestive of malignancy) | Histopathology | The AUROC of the AI alone, BSR (average across five readers), and GR (average across five readers) groups was 0.915 (95% c. i., 0.876–0.954), 0.813 (0.756–0.870), and 0.684 (0.616–0.752), respectively. With AI assistance, the AUROC significantly increased to 0.884 (0.840–0.928) and 0.833 (0.779–0.887) in the BSR and GR groups, respectively (p = 0.007 and p < 0.001, respectively). Sensitivity was improved by AI assistance in both groups (74.6% vs. 88.6% in BSR, p < 0.001; 52.1% vs. 79.4% in GR, p < 0.001), but the specificity did not differ significantly (66.6% vs. 66.4% in BSR, p = 0.238; 70.8% vs. 70.0% in GR, p = 0.689). |
| RECRUIMENT: Screening from Multicenter Studies: US, EU, UK, and SWEDEN | |||||||
| Author, Country, Study Publication Year | Study Design | Outcomes | Patient Population | Outcome Results | |||
| Period of Collection Mammograms and Woman Age | No. of Mammograms/Patients | Mammography Reading Protocol and AI Reading Protocol | Diagnosis Confirmations | ||||
| Schaffer Sweden and USA 2020 [47] | Retrospective Comparing study | Outcomes: AUC, SE, SP of AI versus AUC, SE, SP of AI of Swedish court and American readers | From April 2008 to December 2012 | 2 | Different protocols, according to aims of the study | Histopathology | The top-performing algorithm achieved an area under the curve of 0.858 (United States) and 0.903 (Sweden) and 66.2% (United States) and 81.2% (Sweden) specificity at the radiologists’ sensitivity, lower than the community-practice radiologists’ specificity of 90.5% (United States) and 98.5% (Sweden). Combining top-performing algorithms and U.S. radiologist assessments resulted in a higher area under the curve of 0.942 and achieved a significantly improved specificity (92.0%) at the same sensitivity. |
| McKinney, UK and USA 2020 [48] | Retrospective Comparing study | Outcomes: % improving of SE, SP between first and second readers with AI in UK; % improving of SE, SP with AI in USA | 2001 and 2018 | 25,856 | Two readers in UK, one reader in the USA In the UK, two readers, and in cases of disagreement, an arbitration process could invoke a third opinion. In the USA, each mammogram was interpreted by a single radiologist. BI-RADS | Biopsy | Compared with the first reader, the AI system demonstrated an improvement in specificity of 1.2% (95% C.I. 0.29%, 2.1%; p = 0.0096 for superiority) and an improvement in sensitivity of 2.7% (95% C.I. 3%, 8.5%; p = 0.004). Compared with the second reader, the AI system showed non-inferiority (at a 5% margin) for both specificity (p < 0.001) and sensitivity (p = 0.02). Likewise, the AI system showed non-inferiority (at a 5% margin) to the consensus judgment for the specificity (p < 0.001) and sensitivity (p = 0.0039). Compared with the typical reader, the AI system demonstrated an improvement in specificity of 5.7%. |
| Kim South Korea and USA 2020 [49] | Retrospective Comparing study | Outcomes: AUC of AI; versus AUC of readers even according to BI-RADS category | January 2004–December 2016, in South Korea; January 2000–December 2018, in the USA; and January 2010–December 2018, in the UK | 166,578/68,008 | ResNet BI-RADS (four category) | Histopathology | AI AUC 0.95 (0.93–0.96) SE NR SP NR Readers AUC 0.81 (0.77–0.85) SE NR SP NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altobelli, E.; Angeletti, P.M.; Ciancaglini, M.; Petrocelli, R. The Future of Breast Cancer Organized Screening Program Through Artificial Intelligence: A Scoping Review. Healthcare 2025, 13, 378. https://doi.org/10.3390/healthcare13040378
Altobelli E, Angeletti PM, Ciancaglini M, Petrocelli R. The Future of Breast Cancer Organized Screening Program Through Artificial Intelligence: A Scoping Review. Healthcare. 2025; 13(4):378. https://doi.org/10.3390/healthcare13040378
Chicago/Turabian StyleAltobelli, Emma, Paolo Matteo Angeletti, Marco Ciancaglini, and Reimondo Petrocelli. 2025. "The Future of Breast Cancer Organized Screening Program Through Artificial Intelligence: A Scoping Review" Healthcare 13, no. 4: 378. https://doi.org/10.3390/healthcare13040378
APA StyleAltobelli, E., Angeletti, P. M., Ciancaglini, M., & Petrocelli, R. (2025). The Future of Breast Cancer Organized Screening Program Through Artificial Intelligence: A Scoping Review. Healthcare, 13(4), 378. https://doi.org/10.3390/healthcare13040378







