Associations of Dietary Zinc Supplementation and Sleep Patterns with Chronic Kidney Disease Risk: A Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Primary Exposure
2.3. Ascertainment of CKD
2.4. Assessment of Other Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
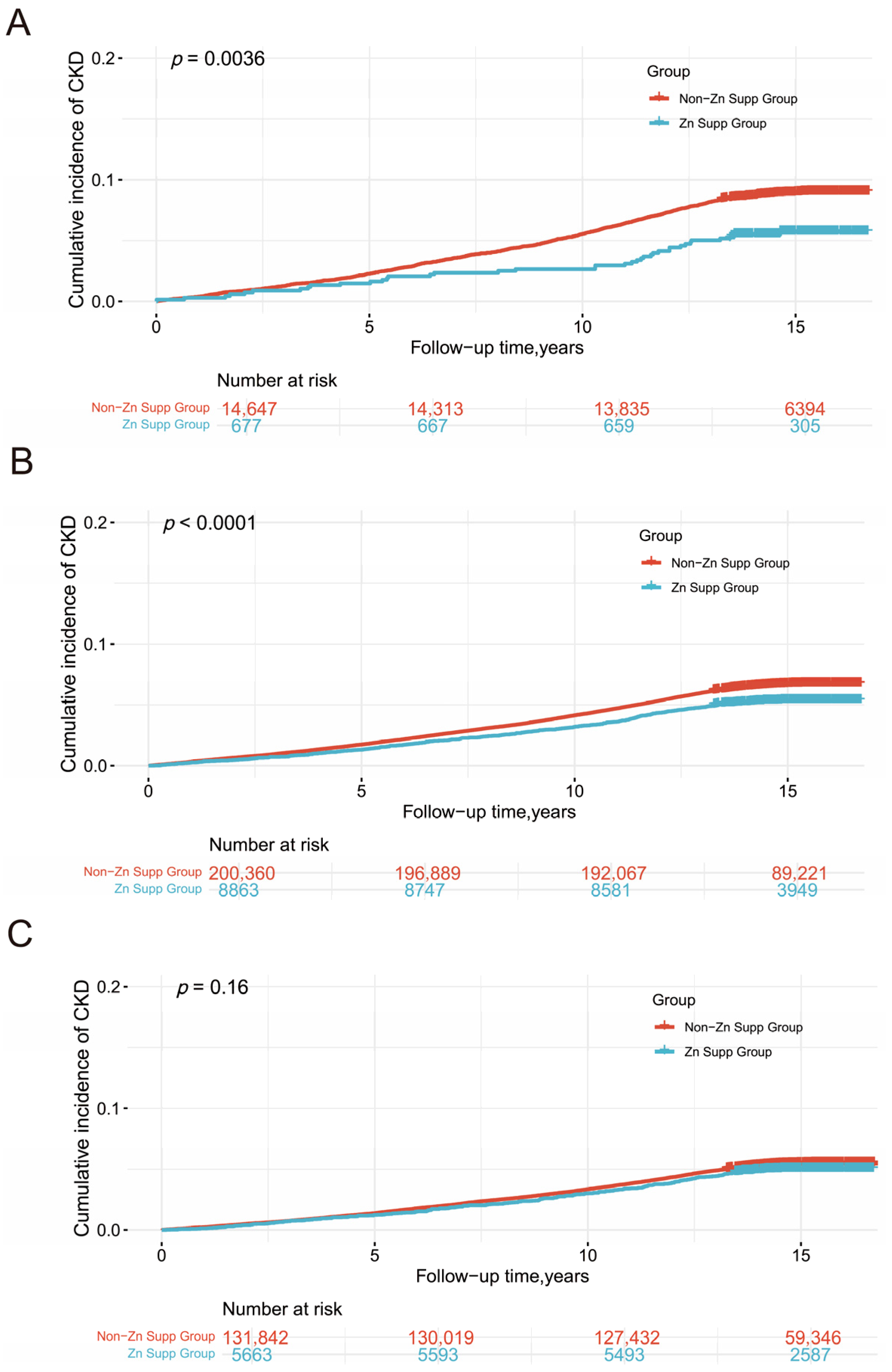
3.2. Dietary Zinc Supplementation, Sleep Patterns, and CKD
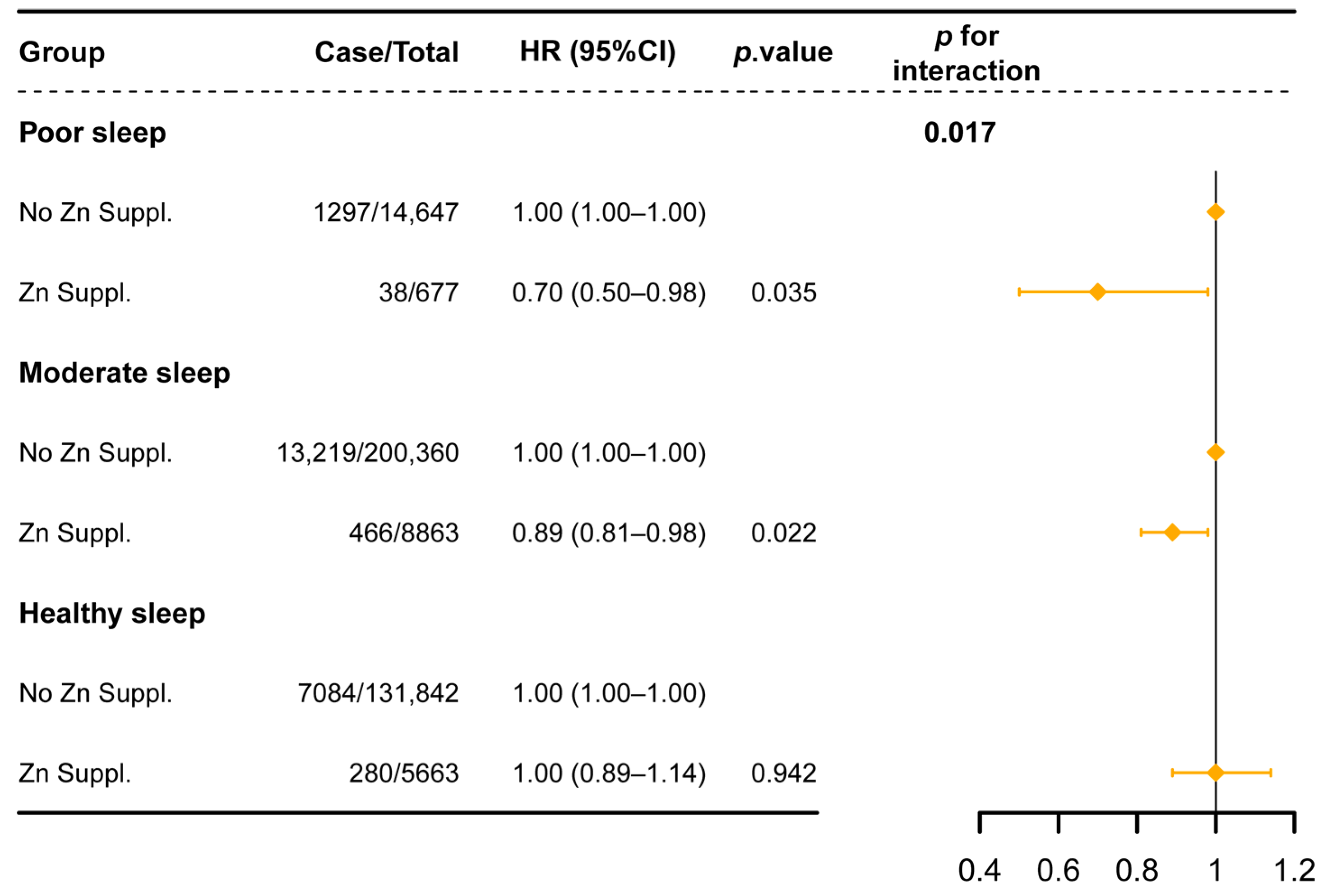
3.3. Subgroup and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| TDI | Townsend deprivation index |
| BMI | Body mass index |
| HR | Hazard ratio |
| CI | Confidence interval |
| eGFR | Estimated glomerular filtration rate |
| UACR | urinary albumin–creatinine ratio |
References
- Deng, L.; Guo, S.; Liu, Y.; Zhou, Y.; Liu, Y.; Zheng, X.; Yu, X.; Shuai, P. Global, Regional, and National Burden of Chronic Kidney Disease and Its Underlying Etiologies from 1990 to 2021: A Systematic Analysis for the Global Burden of Disease Study 2021. BMC Public Health 2025, 25, 636. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef]
- Guo, C.-H.; Wang, C.-L. Effects of Zinc Supplementation on Plasma Copper/Zinc Ratios, Oxidative Stress, and Immunological Status in Hemodialysis Patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.-R.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Dietary Zinc Intake and Incident Chronic Kidney Disease. Clin. Nutr. 2021, 40, 1039–1045. [Google Scholar] [CrossRef]
- Freitas, E.P.; Cunha, A.T.; Aquino, S.L.; Pedrosa, L.F.; Lima, S.C.; Lima, J.G.; Almeida, M.G.; Sena-Evangelista, K.C. Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study. Nutrients 2017, 9, 175. [Google Scholar] [CrossRef]
- Bandeira, V.d.S.; Pires, L.V.; Hashimoto, L.L.; de Alencar, L.L.; Almondes, K.G.S.; Lottenberg, S.A.; Cozzolino, S.M.F. Association of Reduced Zinc Status with Poor Glycemic Control in Individuals with Type 2 Diabetes Mellitus. J. Trace Elem. Med. Biol. 2017, 44, 132–136. [Google Scholar] [CrossRef]
- McMullan, C.J.; Curhan, G.C.; Forman, J.P. Association of Short Sleep Duration and Rapid Decline in Renal Function. Kidney Int. 2016, 89, 1324–1330. [Google Scholar] [CrossRef]
- Yamamoto, R.; Nagasawa, Y.; Iwatani, H.; Shinzawa, M.; Obi, Y.; Teranishi, J.; Ishigami, T.; Yamauchi-Takihara, K.; Nishida, M.; Rakugi, H.; et al. Self-Reported Sleep Duration and Prediction of Proteinuria: A Retrospective Cohort Study. Am. J. Kidney Dis. 2012, 59, 343–355. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Short or Long Sleep Duration and CKD: A Mendelian Randomization Study. J. Am. Soc. Nephrol. 2020, 31, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yoshioka, E.; Saijo, Y.; Bannai, A.; Kita, T.; Tamakoshi, A.; Kishi, R. A Prospective Cohort Study of Insomnia and Chronic Kidney Disease in Japanese Workers. Sleep. Breath. 2018, 22, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Joo, Y.S.; Lee, S.; Kang, S.; Kim, J.; Nam, K.H.; Yun, H.-R.; Jhee, J.H.; Kim, H.; Han, S.H.; et al. Snoring and Incident Chronic Kidney Disease: A Community-Based Prospective Cohort Study. BMJ Open 2019, 9, e030671. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, C.; Ma, A.; Zheng, H.; Zheng, W.; Hou, X.; Hu, C.; Chen, L.; Jia, W. Self-Reported Snoring Is Associated with Chronic Kidney Disease Independent of Metabolic Syndrome in Middle-Aged and Elderly Chinese. J. Diabetes Investig. 2019, 10, 124–130. [Google Scholar] [CrossRef]
- Yamamoto, R.; Shinzawa, M.; Isaka, Y.; Yamakoshi, E.; Imai, E.; Ohashi, Y.; Hishida, A.; CKD-JAC Investigators. Sleep Quality and Sleep Duration with CKD Are Associated with Progression to ESKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 1825–1832. [Google Scholar] [CrossRef]
- Cope, E.C.; Levenson, C.W. Role of Zinc in the Development and Treatment of Mood Disorders. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 685–689. [Google Scholar] [CrossRef]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Siegel, E.H.; Olney, D.K.; Katz, J.; Tielsch, J.M.; Kariger, P.K.; Khalfan, S.S.; LeClerq, S.C.; Khatry, S.K.; Stoltzfus, R.J. The Effects of Iron and/or Zinc Supplementation on Maternal Reports of Sleep in Infants from Nepal and Zanzibar. J. Dev. Behav. Pediatr. 2009, 30, 131–139. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Monteferrario, F.; Antoniello, N.; Manni, R.; Klersy, C. The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: A double-blind, placebo-controlled clinical trial. J. Am. Geriatr. Soc. 2011, 59, 82–90. [Google Scholar] [CrossRef]
- Saito, H.; Cherasse, Y.; Suzuki, R.; Mitarai, M.; Ueda, F.; Urade, Y. Zinc-rich oysters as well as zinc-yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol. Nutr. Food Res. 2017, 61, 1600882. [Google Scholar] [CrossRef]
- Geng, T.; Li, X.; Ma, H.; Heianza, Y.; Qi, L. Adherence to a Healthy Sleep Pattern and Risk of Chronic Kidney Disease: The UK Biobank Study. Mayo Clin. Proc. 2022, 97, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Stephens, M.A. EDF Statistics for Goodness of Fit and Some Comparisons. J. Am. Stat. Assoc. 1974, 69, 730–737. [Google Scholar] [CrossRef]
- Schoenfeld, D. Partial Residuals for the Proportional Hazards Regression Model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Simera, I.; Moher, D.; Hirst, A.; Hoey, J.; Schulz, K.F.; Altman, D.G. Transparent and Accurate Reporting Increases Reliability, Utility, and Impact of Your Research: Reporting Guidelines and the EQUATOR Network. BMC Med. 2010, 8, 24. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Miao, X.; Wang, Y.; Zhang, L.; Xin, Y.; Zheng, S.; Epstein, P.N.; Fu, Y.; Cai, L. Renal Improvement by Zinc in Diabetic Mice Is Associated with Glucose Metabolism Signaling Mediated by Metallothionein and Akt, but Not Akt2. Free Radic. Biol. Med. 2014, 68, 22–34. [Google Scholar] [CrossRef]
- Barman, S.; Srinivasan, K. Diabetes and zinc dyshomeostasis: Can zinc supplementation mitigate diabetic complications? Crit. Rev. Food Sci. Nutr. 2022, 62, 1046–1061. [Google Scholar] [CrossRef]
- Chao, H.-C. Zinc Deficiency and Therapeutic Value of Zinc Supplementation in Pediatric Gastrointestinal Diseases. Nutrients 2023, 15, 4093. [Google Scholar] [CrossRef]
- Yang, C.; Yan, P.; Wu, X.; Zhang, W.; Cui, H.; Zhang, L.; Xu, Z.; Peng, S.; Tang, M.; Wang, Y.; et al. Associations of Sleep with Cardiometabolic Risk Factors and Cardiovascular Diseases: An Umbrella Review of Observational and Mendelian Randomization Studies. Sleep. Med. Rev. 2024, 77, 101965. [Google Scholar] [CrossRef]
- Nassan, M.; Videnovic, A. Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2022, 18, 7–24. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yun, H.-R.; Joo, Y.S.; Lee, S.; Shinchan, K.; Ryu, G.W.; Han, S.H.; Yoo, T.-H.; Park, J.T.; Kang, S.-W. SP302 low dietary zinc intake is associated with an increased risk of incident chronic kidney disease development. Nephrol. Dial. Transplant. 2019, 34, gfz103.SP302. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Yang, Y.; Cao, Y.; Liang, D. Association Between Dietary Zinc Intake and Increased Renal Function in US Adults. Biol. Trace Elem. Res. 2024, 202, 3871–3885. [Google Scholar] [CrossRef]
- Tang, R.; Wang, X.; Li, X.; Ma, H.; Liang, Z.; Heianza, Y.; Qi, L. Adherence to Life’s Essential 8 and Incident Chronic Kidney Disease: A Prospective Study of 147,988 UK Biobank Participants. Am. J. Clin. Nutr. 2023, 118, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-κB Signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef]
- Pan, J.; Huang, X.; Li, Y.; Li, M.; Yao, N.; Zhou, Z.; Li, X. Zinc Protects against Cadmium-Induced Toxicity by Regulating Oxidative Stress, Ions Homeostasis and Protein Synthesis. Chemosphere 2017, 188, 265–273. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Li, B.; Tan, Y.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. The Role of Zinc in the Prevention of Diabetic Cardiomyopathy and Nephropathy. Toxicol. Mech. Methods 2012, 23, 27–33. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Q.; Lu, J.; Zhang, X.; Suen, D.; Tan, Y.; Jin, L.; Xiao, J.; Xie, R.; Rane, M.; et al. Zinc Supplementation Partially Prevents Renal Pathological Changes in Diabetic Rats. J. Nutr. Biochem. 2010, 21, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Minami, A.; Seki, Y.; Oku, N. Differential Effects of Zinc on Glutamatergic and GABAergic Neurotransmitter Systems in the Hippocampus. J. Neurosci. Res. 2004, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Zhu, X.; Storfer-Isser, A.; Mehra, R.; Jenny, N.S.; Tracy, R.; Redline, S. Sleep Duration and Biomarkers of Inflammation. Sleep 2009, 32, 200–204. [Google Scholar] [CrossRef]
- Meier-Ewert, H.K.; Ridker, P.M.; Rifai, N.; Regan, M.M.; Price, N.J.; Dinges, D.F.; Mullington, J.M. Effect of Sleep Loss on C-Reactive Protein, an Inflammatory Marker of Cardiovascular Risk. J. Am. Coll. Cardiol. 2004, 43, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated Inflammatory Markers in Response to Prolonged Sleep Restriction Are Associated With Increased Pain Experience in Healthy Volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- Shan, Z.; Ma, H.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Mansukhani, M.P.; Covassin, N.; Somers, V.K. Apneic Sleep, Insufficient Sleep, and Hypertension. Hypertension 2019, 73, 744–756. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Bothwell, L.E.; Podolsky, S.H. The Emergence of the Randomized, Controlled Trial. N. Engl. J. Med. 2016, 375, 501–504. [Google Scholar] [CrossRef]
- Dong, C.; Ji, Y.; Fu, Z.; Qi, Y.; Yi, T.; Yang, Y.; Sun, Y.; Sun, H. Precision Management in Chronic Disease: An AI Empowered Perspective on Medicine-Engineering Crossover. iScience 2025, 28, 112044. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.A.; AbuAli, N.; Shuaib, K.; Awad, A.I. An Explainable Artificial Intelligence and Internet of Things Framework for Monitoring and Predicting Cardiovascular Disease. Eng. Appl. Artif. Intell. 2025, 144, 110138. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmad, S.; Wasid, M. Artificial Intelligence-Driven Intelligent Learning Models for Identification and Prediction of Cardioneurological Disorders: A Comprehensive Study. Comput. Biol. Med. 2025, 184, 109342. [Google Scholar] [CrossRef]

| Variable | Total | Non-Zn Supp | Zn Supp | p-Value |
|---|---|---|---|---|
| 362,052 | 346,849 | 15,203 | ||
| Age, years | 56.2 (8.1) | 56.2 (8.1) | 56.2 (8.1) | 0.995 |
| TDI | −1.49 (3.0) | −1.49 (3.0) | −1.29 (3.1) | <0.001 |
| Female, n (%) | 201,201 (55.6) | 191,564 (55.2) | 9637 (63.4) | <0.001 |
| Education, n (%) | <0.001 | |||
| Higher degree | 122,813 (33.9) | 116,880 (33.7) | 5933 (39.0) | |
| Any school degree | 139,381 (38.5) | 133,508 (38.5) | 5873 (38.6) | |
| Vocational qualifications | 42,109 (11.6) | 40,485 (11.7) | 1624 (10.7) | |
| None of the above | 55,271 (15.3) | 53,588 (15.4) | 1683 (11.1) | |
| Ethnic white, n (%) | 331,510 (91.6) | 318,106 (91.7) | 13,404 (88.2) | <0.001 |
| Diabetes, n (%) | 17,508 (4.8) | 16,881 (4.9) | 627 (4.1) | <0.001 |
| BMI, kg/m2, n (%) | <0.001 | |||
| underweight | 1681 (0.5) | 1587 (0.5) | 94 (0.6) | |
| normal weight | 119,846 (33.1) | 113,945 (32.9) | 5901 (38.8) | |
| overweight | 155,289 (42.9) | 149,155 (43.0) | 6134 (40.3) | |
| obese | 83,669 (23.1) | 80,671 (23.3) | 2998 (19.7) | |
| Hypertension, n (%) | 183,143 (50.6) | 176,266 (50.8) | 6877 (45.2) | <0.001 |
| Physical activity score, n (%) | <0.001 | |||
| Low | 85,155 (23.5) | 82,233 (23.7) | 2922 (19.2) | |
| Moderate | 136,922 (37.8) | 131,327 (37.9) | 5595 (36.8) | |
| High | 133,294 (36.8) | 126,872 (36.6) | 6422 (42.2) | |
| Smoking, n (%) | <0.001 | |||
| Current | 36,421 (10.1) | 35,032 (10.1) | 1389 (9.14) | |
| Never | 890 (0.3) | 850 (0.3) | 40 (0.3) | |
| Previous | 199,104 (55.0) | 190,926 (55.0) | 8178 (53.8) | |
| Alcohol, n (%) | <0.001 | |||
| Current | 336,307 (92.9) | 322,330 (92.9) | 13,977 (91.9) | |
| Never | 160 (0.04) | 152 (0.04) | 8 (0.05) | |
| Previous | 13,881 (3.8) | 13,319 (3.8) | 562 (3.7) | |
| Healthy diet score | 3.5 (1.5) | 3.5 (1.5) | 4.0 (1.5) | <0.001 |
| Chronotype, n (%) | <0.001 | |||
| “morning” person | 96,871 (26.8) | 92,932 (26.8) | 3939 (25.9) | |
| “evening” person | 31,899 (8.81) | 30,297 (8.73) | 1602 (10.5) | |
| More a “morning” than “evening” person | 130,208 (36.0) | 125,027 (36.0) | 5181 (34.1) | |
| More an “evening” than a “morning” person | 103,074 (28.5) | 98,593 (28.4) | 4481 (29.5) | |
| Snoring, n (%) | 133,004 (36.7) | 128,030 (36.9) | 4974 (32.7) | <0.001 |
| Daytime dozing, n (%) | <0.001 | |||
| All of the time | 21 (0.01) | 20 (0.01) | 1 (0.01) | |
| Never/rarely | 279,172 (77.1) | 267,475 (77.1) | 11,697 (76.9) | |
| Often | 9160 (2.53) | 8742 (2.52) | 418 (2.75) | |
| Sometimes | 73,699 (20.4) | 70,612 (20.4) | 3087 (20.3) | |
| Sleeplessness, n (%) | ||||
| Never/rarely | 89,644 (24.8) | 86,145 (24.8) | 3499 (23.0) | <0.001 |
| Sometimes | 173,289 (47.9) | 165,977 (47.9) | 7312 (48.1) | |
| Usually | 99,119 (27.4) | 94,727 (27.3) | 4392 (28.9) |
| Subgroup | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Zn Suppl. | ||||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 0.84 (0.78–0.90) | <0.001 | 0.92 (0.84–0.99) | 0.019 | 0.92 (0.85–0.99) | 0.026 |
| Sleep pattern | ||||||
| Poor | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Moderate | 0.73 (0.69–0.77) | <0.001 | 0.83 (0.79–0.88) | <0.001 | 0.86 (0.81–0.91) | <0.001 |
| Healthy | 0.61 (0.58–0.65) | <0.001 | 0.77 (0.72–0.81) | <0.001 | 0.80 (0.75–0.85) | <0.001 |
| p for trend | - | <0.001 | - | <0.001 | - | <0.001 |
| Sleep Behaviors | Case Subjects/N | HR (95% CI) | p for Interaction |
|---|---|---|---|
| Sleep duration | 0.068 | ||
| Low risk | 14,204/235,239 | 0.96 (0.88–1.05) | |
| High risk | 8180/104,429 | 0.86 (0.75–0.97) | |
| Chronotype | 0.467 | ||
| Low risk | 14,183/212,896 | 0.95 (0.86–1.04) | |
| High risk | 8201/126,772 | 0.88 (0.78–0.99) | |
| Insomnia | 0.753 | ||
| Low risk | 4804/84,840 | 0.88 (0.75–1.03) | |
| High risk | 17,580/254,828 | 0.93 (0.86–1.01) | |
| Snoring | 0.044 | ||
| Low risk | 13,258/215,790 | 0.98 (0.89–1.07) | |
| High risk | 9126/123,878 | 0.83 (0.73–0.94) | |
| Daytime sleepiness | 0.198 | ||
| Low risk | 21,525/331,346 | 0.93 (0.86–1.00) | |
| High risk | 859/8322 | 0.69 (0.46–1.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, S.; Lv, J.; Ma, X.; Lin, X.; Yang, L.; Li, S.; Zhang, T. Associations of Dietary Zinc Supplementation and Sleep Patterns with Chronic Kidney Disease Risk: A Prospective Cohort Study. Healthcare 2025, 13, 703. https://doi.org/10.3390/healthcare13070703
Zhang X, Zhang S, Lv J, Ma X, Lin X, Yang L, Li S, Zhang T. Associations of Dietary Zinc Supplementation and Sleep Patterns with Chronic Kidney Disease Risk: A Prospective Cohort Study. Healthcare. 2025; 13(7):703. https://doi.org/10.3390/healthcare13070703
Chicago/Turabian StyleZhang, Xiaofeng, Shuai Zhang, Jiali Lv, Xiaoyan Ma, Xia Lin, Lin Yang, Shengxu Li, and Tao Zhang. 2025. "Associations of Dietary Zinc Supplementation and Sleep Patterns with Chronic Kidney Disease Risk: A Prospective Cohort Study" Healthcare 13, no. 7: 703. https://doi.org/10.3390/healthcare13070703
APA StyleZhang, X., Zhang, S., Lv, J., Ma, X., Lin, X., Yang, L., Li, S., & Zhang, T. (2025). Associations of Dietary Zinc Supplementation and Sleep Patterns with Chronic Kidney Disease Risk: A Prospective Cohort Study. Healthcare, 13(7), 703. https://doi.org/10.3390/healthcare13070703







