Clinical Application Research on Stroke Situational Intelligent Rehabilitation Training System Based on Wearable Devices: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
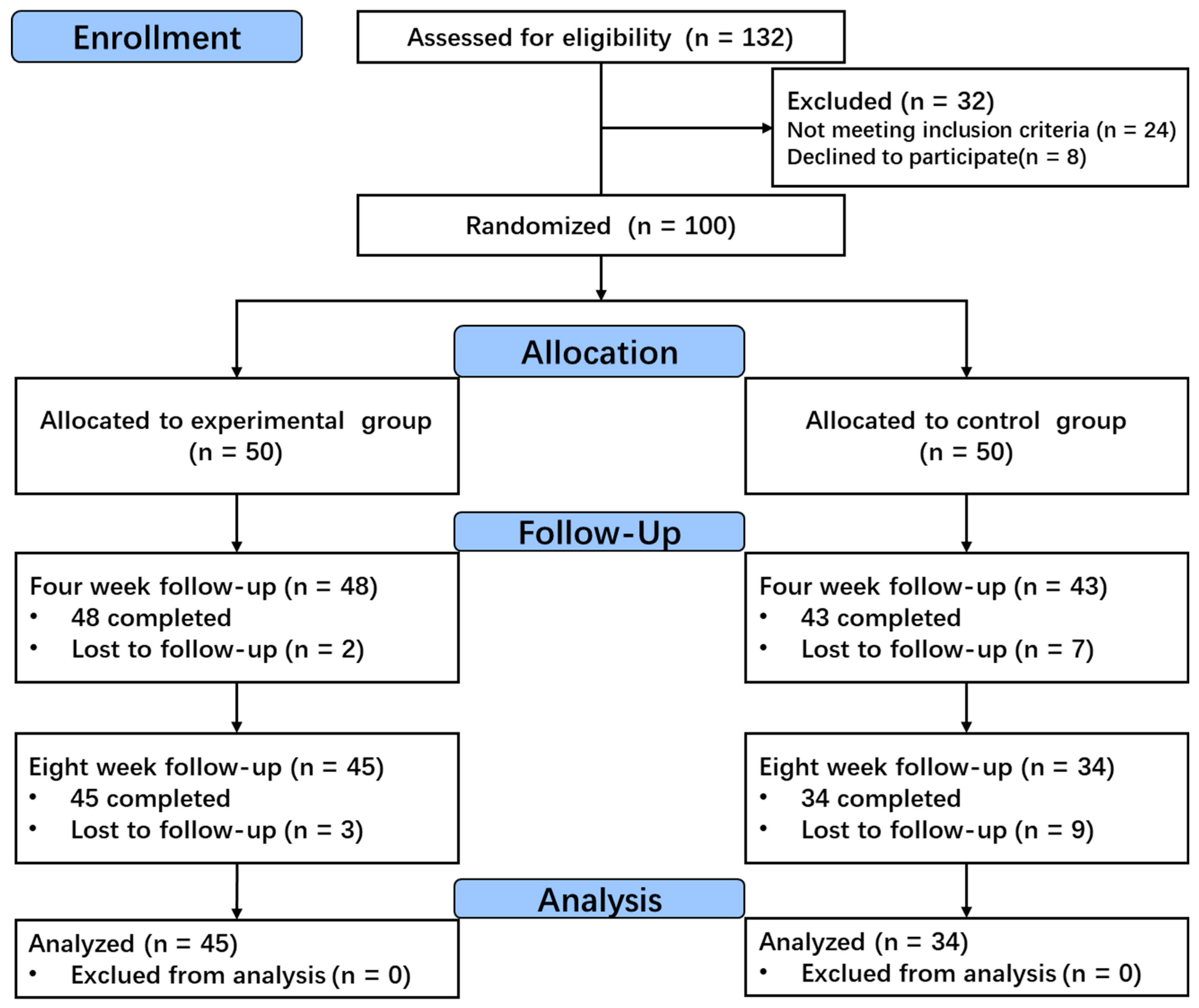
2.1. Participants
2.2. Study Design
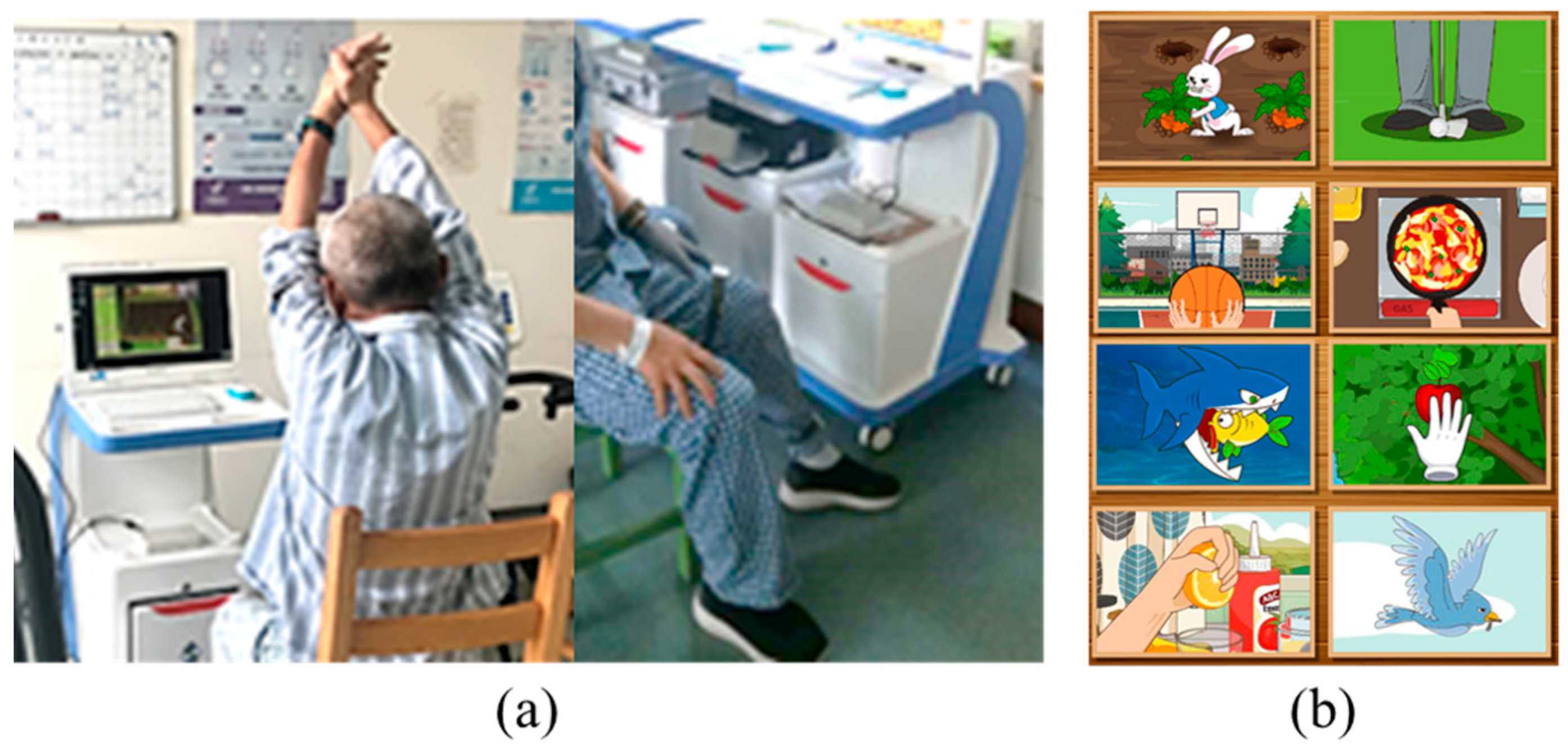
2.2.1. Stroke Situational Intelligent Rehabilitation Training System Based on Wearable Devices
2.2.2. Conventional Rehabilitation Training for Limb Motor Function
2.3. Outcome Measures
2.4. Data Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Comparison of FMA Scores Before and After Treatment Between the Two Groups
3.3. Comparison of Activities of Daily Living Before and After Treatment Between the Two Groups
3.4. Results of the Intention-to-Treat Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 397, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019 Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery a Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, E98–E169. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Chien, W.; Chong, Y.; Tse, M.; Chien, C.; Cheng, H. Robot-Assisted Therapy for Upper-Limb Rehabilitation in Subacute Stroke Patients: A Systematic Review and Meta-Analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef] [PubMed]
- van Mierlo, M.L.; Schroder, C.; van Heugten, C.M.; Post, M.W.M.; de Kort, P.L.M.; Visser-Meily, J.M.A. The Influence of Psychological Factors on Health-Related Quality of Life after Stroke: A Systematic Review. Int. J. Stroke 2014, 9, 341–348. [Google Scholar] [CrossRef]
- O’Flaherty, D.; Ali, K. Recommendations for Upper Limb Motor Recovery: An Overview of the UK and European Rehabilitation after Stroke Guidelines (2023). Healthcare 2024, 12, 1433. [Google Scholar] [CrossRef]
- Lee, J.-L.; Ko, S.-H.; Huh, S.; Jung, J.-C.; Kim, S.-Y.; Bae, D.-Y.; Shin, Y.-I.; Kim, Y. The Safety, Feasibility, and Efficacy of a Structured Individual Exercise Program for Community-Dwelling Stroke Patients. Healthcare 2024, 12, 2281. [Google Scholar] [CrossRef]
- Kasner, S.E. Clinical Interpretation and Use of Stroke Scales. Lancet Neurol. 2006, 5, 603–612. [Google Scholar] [CrossRef]
- Sengupta, N.; Rao, A.S.; Yan, B.; Palaniswami, M. A Survey of Wearable Sensors and Machine Learning Algorithms for Automated Stroke Rehabilitation. IEEE Access 2024, 12, 36026–36054. [Google Scholar] [CrossRef]
- Gubbi, J.; Rao, A.S.; Fang, K.; Yan, B.; Palaniswami, M. Motor Recovery Monitoring Using Acceleration Measurements in Post Acute Stroke Patients. Biomed. Eng. Online 2013, 12, 33. [Google Scholar] [CrossRef]
- Friedman, N.; Rowe, J.B.; Reinkensmeyer, D.J.; Bachman, M. The Manumeter: A Wearable Device for Monitoring Daily Use of the Wrist and Fingers. IEEE J. Biomed. Health Inform. 2014, 18, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Kattan, A. Physical Activities Monitoring Using Wearable Acceleration Sensors Attached to the Body. PLoS ONE 2015, 10, e0130851. [Google Scholar] [CrossRef]
- Lee, S.I.; Adans-Dester, C.P.; Grimaldi, M.; Dowling, A.V.; Horak, P.C.; Black-Schaffer, R.M.; Bonato, P.; Gwin, J.T. Enabling Stroke Rehabilitation in Home and Community Settings: A Wearable Sensor-Based Approach for Upper-Limb Motor Training. IEEE J. Transl. Eng. Health Med. 2018, 6, 2100411. [Google Scholar] [CrossRef]
- Oubre, B.; Daneault, J.-F.; Jung, H.-T.; Whritenour, K.; Miranda, J.G.V.; Park, J.; Ryu, T.; Kim, Y.; Lee, S.I. Estimating Upper-Limb Impairment Level in Stroke Survivors Using Wearable Inertial Sensors and a Minimally-Burdensome Motor Task. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 601–611. [Google Scholar] [CrossRef]
- Zhang, Z.; Liparulo, L.; Panella, M.; Gu, X.; Fang, Q. A Fuzzy Kernel Motion Classifier for Autonomous Stroke Rehabilitation. IEEE J. Biomed. Health Inform. 2016, 20, 893–901. [Google Scholar] [CrossRef]
- Yu, L.; Xiong, D.; Guo, L.; Wang, J. A Compressed Sensing-Based Wearable Sensor Network for Quantitative Assessment of Stroke Patients. Sensors 2016, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Markopoulos, P.; Yu, B.; Chen, W.; Timmermans, A. Interactive Wearable Systems for Upper Body Rehabilitation: A Systematic Review. J. Neuroeng. Rehabil. 2017, 14, 1–21. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.E.H.; Meskers, C.G.M.; Kwakkel, G. Effects of Robot-Assisted Therapy for the Upper Limb After Stroke: A Systematic Review and Meta-Analysis. Neurorehabilit. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.-C.; Hummel, F.C. Wearable Technology in Stroke Rehabilitation: Towards Improved Diagnosis and Treatment of Upper-Limb Motor Impairment. J. Neuroeng. Rehabil. 2019, 16, 142. [Google Scholar] [CrossRef]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Alali, S.I.I.I.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-Stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef]
- Boukhennoufa, I.; Zhai, X.; Utti, V.; Jackson, J.; McDonald-Maier, K.D. Wearable Sensors and Machine Learning in Post-Stroke Rehabilitation Assessment: A Systematic Review. Biomed. Signal Process. Control 2022, 71, 103197. [Google Scholar] [CrossRef]
- Nikolaev, V.A.; Safonicheva, O.G.; Nikolaev, A.A. Telerehabilitation of Post-Stroke Patients with Motor Function Disorders: A Review. Adv. Gerontol. 2022, 12, 339–346. [Google Scholar] [CrossRef]
- Studnicki, R.; Studzińska, K.; Adamczewski, T.; Hansdorfer-Korzon, R.; Krawczyk, M. Analyzing the Impact of Rehabilitation Utilizing Neurofunctional Exercises on the Functional Status of Stroke Patients. J. Clin. Med. 2024, 13, 6271. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, J.; Wu, Q.; Li, X.; Zhang, B.; Zhou, L.; Xiong, D. Clinical Study of a Wearable Remote Rehabilitation Training System for Patients with Stroke: Randomized Controlled Pilot Trial. JMIR Mhealth Uhealth 2023, 11, e40416. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, D.; Hamaguchi, T.; Nakayama, Y.; Hada, T.; Abo, M. Upper-Limb Functional Recovery in Chronic Stroke Patients after COVID-19-Interrupted Rehabilitation: An Observational Study. J. Clin. Med. 2024, 13, 2212. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The Post-Stroke Hemiplegic Patient. 1. A Method for Evaluation of Physical Performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.H.; di Bella, P.; Johnson, G. Reliability and Validity of Arm Function Assessment with Standardized Guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A Multicentre Study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Leung, S.O.C.; Chan, C.C.H.; Shah, S. Development of a Chinese Version of the Modified Barthel Index—Validity and Reliability. Clin. Rehabil. 2007, 21, 912–922. [Google Scholar] [CrossRef]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and Reliability of a Performance Evaluation Tool Based on the Modified Barthel Index for Stroke Patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef]
- Bishop, L.; Demers, M.; Rowe, J.; Zondervan, D.; Winstein, C.J. A Novel, Wearable Inertial Measurement Unit for Stroke Survivors: Validity, Acceptability, and Usability. Arch. Phys. Med. Rehabil. 2024, 105, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Bok, S.-K.; Lee, J.Y.; Ryoo, H.W.; Lee, H.Y.; Park, H.J.; Oh, H.M.; Kim, T.-W. Benefits of Robot-Assisted Upper-Limb Rehabilitation from the Subacute Stage after a Stroke of Varying Severity: A Multicenter Randomized Controlled Trial. J. Clin. Med. 2024, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, Z.; Gao, F.; Wu, J.; Tang, H. Clinical Effect Analysis of Wearable Sensor Technology-Based Gait Function Analysis in Post-Transcranial Magnetic Stimulation Stroke Patients. Sensors 2024, 24, 3051. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.X.J.; Fong, K.N.K.; Chung, R.C.K.; Cheung, H.K.Y.; Chow, E.S.L. “Remind-to-Move” for Promoting Upper Extremity Recovery Using Wearable Devices in Subacute Stroke: A Multi-Center Randomized Controlled Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 51–59. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, S.-H.; Seo, K.; Lee, M.; Chang, W.H.; Choi, B.-O.; Ryu, G.-H.; Kim, Y.-H. Training for Walking Efficiency with a Wearable Hip-Assist Robot in Patients with Stroke. Stroke 2019, 50, 3545–3552. [Google Scholar] [CrossRef]
- Lin, L.-F.; Lin, Y.-J.; Lin, Z.-H.; Chuang, L.-Y.; Hsu, W.-C.; Lin, Y.-H. Feasibility and Efficacy of Wearable Devices for Upper Limb Rehabilitation in Patients with Chronic Stroke: A Randomized Controlled Pilot Study. Eur. J. Phys. Rehabil. Med. 2018, 54, 388–396. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, B.; Wang, J.; Wu, Q.; Li, X.; Zhou, L.; Xiong, D. Wearable Intelligent Machine Learning Rehabilitation Assessment for Stroke Patients Compared with Clinician Assessment. J. Clin. Med. 2022, 11, 7467. [Google Scholar] [CrossRef]

| Control Group (n = 34) | Experimental Group (n = 45) | p-Value | |
|---|---|---|---|
| Age, mean ± SD, y | 63.33 ± 10.56 (38, 79) | 61.45 ± 11.21 (35, 78) | 0.45 |
| Sex | |||
| Male | 15 | 25 | 0.42 |
| Female | 19 | 20 | |
| Side of lesion | |||
| Left | 18 | 21 | 0.74 |
| Right | 16 | 24 | |
| Type of stroke | |||
| ICH | 21 | 27 | 0.41 |
| SAH | 13 | 18 | |
| Time of onset, mean ± SD, m | 1.45 ± 0.64 (0.5, 6) | 1.40 ± 0.75 (0.5, 6) | 0.34 |
| Control Group (n = 34) | Experimental Group (n = 45) | p-Value 1 | |
|---|---|---|---|
| FMA scores before treatment | 36.34 ± 4.09 (26, 53) | 37.26 ± 4.46 (25, 55) | 0.344 |
| FMA scores after treatment | 52.57 ± 4.32 (40, 69) | 64.83 ± 3.41 (46, 83) | 0.001 * |
| p-Value 2 | 0.001 * | 0.001 * |
| Control Group (n = 34) | Experimental Group (n = 45) | p-Value 1 | |
|---|---|---|---|
| MBI scores before treatment | 54.53 ± 5.93 (40, 75) | 55.27 ± 5.71 (40, 75) | 0.58 |
| MBI scores after treatment | 65.47 ± 5.03 (50, 85) | 78.73 ± 4.92 (60, 90) | 0.001 * |
| p-Value 2 | 0.001 * | 0.001 * |
| Control Group (n = 50) | Experimental Group (n = 50) | p-Value | |
|---|---|---|---|
| FMA scores before treatment | 36.04 ± 4.35 (24, 55) | 37.55 ± 3.88 (25, 55) | 0.412 |
| FMA scores after treatment | 49.37 ± 4.16 (33, 69) | 64.45 ± 3.81 (39, 83) | 0.001 * |
| MBI scores before treatment | 54.46 ± 5.72 (37, 76) | 55.12 ± 5.59 (38, 75) | 0.562 |
| MBI scores after treatment | 63.82 ± 4.94 (43, 85) | 77.92 ± 4.83 (50, 90) | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Ding, K.; Dai, Y.; Yin, J.; Yao, J.; Guo, L.; Wang, J.; Wang, X. Clinical Application Research on Stroke Situational Intelligent Rehabilitation Training System Based on Wearable Devices: A Randomized Controlled Trial. Healthcare 2025, 13, 708. https://doi.org/10.3390/healthcare13070708
Lu Y, Ding K, Dai Y, Yin J, Yao J, Guo L, Wang J, Wang X. Clinical Application Research on Stroke Situational Intelligent Rehabilitation Training System Based on Wearable Devices: A Randomized Controlled Trial. Healthcare. 2025; 13(7):708. https://doi.org/10.3390/healthcare13070708
Chicago/Turabian StyleLu, Ying, Kangjia Ding, Yayuan Dai, Jie Yin, Jianjun Yao, Liquan Guo, Jiping Wang, and Xiaojun Wang. 2025. "Clinical Application Research on Stroke Situational Intelligent Rehabilitation Training System Based on Wearable Devices: A Randomized Controlled Trial" Healthcare 13, no. 7: 708. https://doi.org/10.3390/healthcare13070708
APA StyleLu, Y., Ding, K., Dai, Y., Yin, J., Yao, J., Guo, L., Wang, J., & Wang, X. (2025). Clinical Application Research on Stroke Situational Intelligent Rehabilitation Training System Based on Wearable Devices: A Randomized Controlled Trial. Healthcare, 13(7), 708. https://doi.org/10.3390/healthcare13070708







