The Impact of Polycystic Ovary Syndrome on Gestational Diabetes Mellitus, Disease Knowledge, and Health Behaviors
Abstract
1. Introduction
2. Materials and Methods
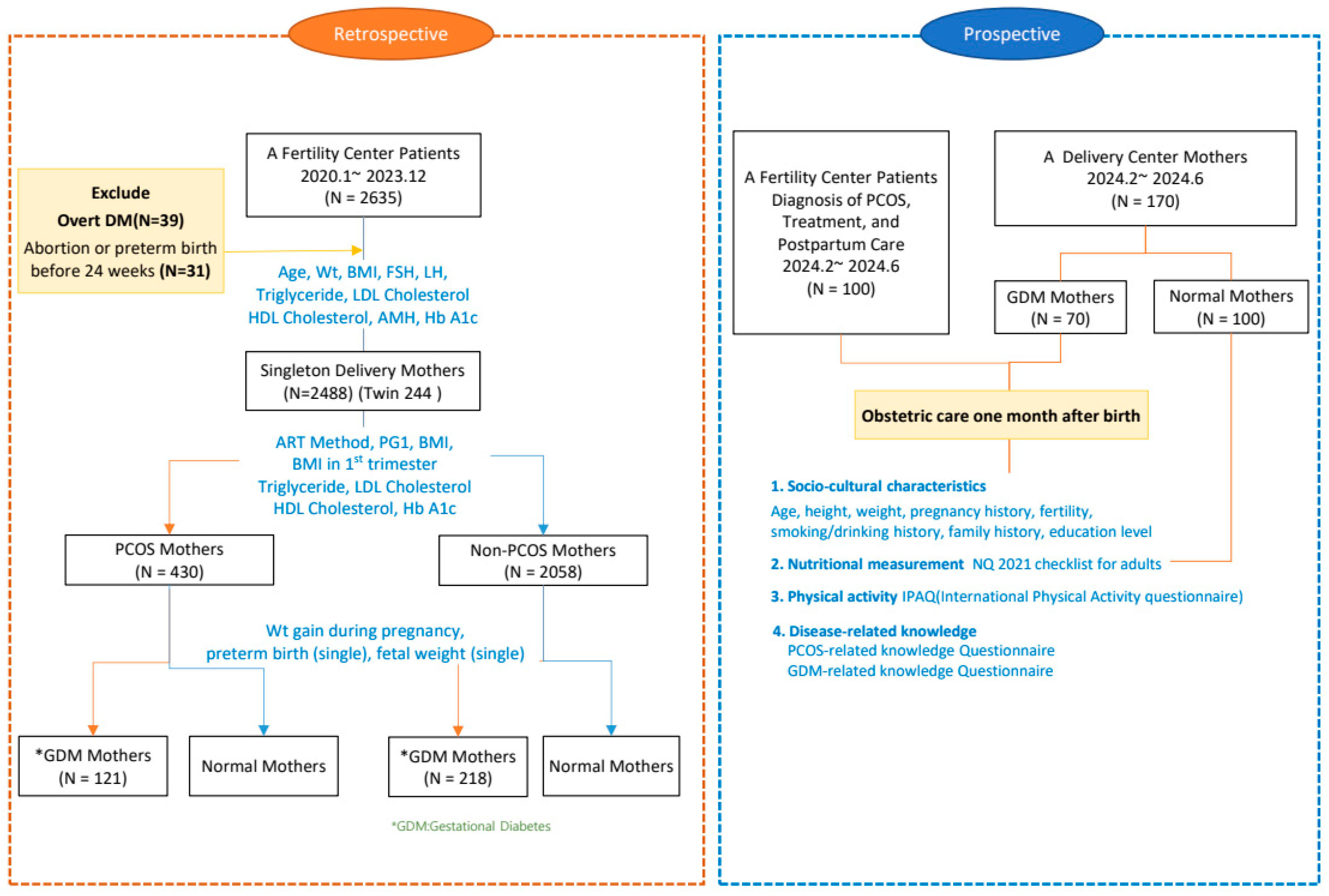
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Study Questionnaires
2.4.1. Dietary Habits
2.4.2. Physical Activity Levels
2.4.3. Knowledge Related to PCOS
2.4.4. Knowledge Related to GDM
2.5. Data Analysis
3. Results
4. Discussion
4.1. Metabolic Outcomes
4.2. Negative Birth Outcomes
4.3. The Relationship Between PCOS and GDM
4.4. PCOS Knowledge and Lifestyle-Related Factors
4.5. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Infertility Prevalence Estimates: 1990–2021; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Ebrahimzadeh Zagami, S.; Latifnejad Roudsari, R.; Janghorban, R.; Mousavi Bazaz, S.M.; Amirian, M.; Allan, H.T. Infertile Couples’ Needs after Unsuccessful Fertility Treatment: A Qualitative Study. J. Caring Sci. 2019, 8, 95–104. [Google Scholar] [CrossRef]
- Health Insurance Review and Assessment Service. Analysis of Infertility and Assisted Reproductive Technology Treatment; Seoul, Korea, 25 May 2023. Available online: https://www.hira.or.kr/bbsDummy.do?brdBltNo=10880&brdScnBltNo=4&pageIndex=1&pageIndex2=1&pgmid=HIRAA020041000100&utm_source=chatgpt.com (accessed on 10 January 2025).
- Jeong, K.; Yoon, J.; Cho, H.J.; Kim, S.; Jang, J. The Relationship between Changes in the Korean Fertility Rate and Policies to Encourage Fertility. BMC Public Health 2022, 22, 2298. [Google Scholar] [CrossRef]
- Cha, W.; Yun, I.; Nam, C.M.; Nam, J.Y.; Park, E.C. Evaluation of Assisted Reproductive Technology Health Insurance Coverage for Multiple Pregnancies and Births in Korea. JAMA Netw. Open 2023, 6, e2316696. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility Evaluation of Infertile Women: A Committee Opinion. Fertil. Steril. 2021, 116, 1255–1265. [Google Scholar] [CrossRef]
- Wolf, W.; Wattick, R.; Kinkade, O.; Olfert, M. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Lujan, M.E.; Chizen, D.R.; Pierson, R.A. Diagnostic Criteria for Polycystic Ovary Syndrome: Pitfalls and Controversies. J. Obstet. Gynaecol. Can. 2008, 30, 671–679. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Jiang, J.; Yang, N.; Liu, Y.; Cai, L.; Cui, Y.; Diao, F.; Han, X.; Liu, J.; et al. Follicular Hyperandrogenism and Insulin Resistance in Polycystic Ovary Syndrome Patients with Normal Circulating Testosterone Levels. J. Biomed. Res. 2017, 32, 208–214. [Google Scholar]
- Yang, S.W.; Yoon, S.H.; Kim, M.; Seo, Y.S.; Yuk, J.S. Risk of Gestational Diabetes and Pregnancy-Induced Hypertension with a History of Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2023, 12, 1738. [Google Scholar] [CrossRef]
- Ni, Z.; Mei, S.; You, S.; Lin, Y.; Cheng, W.; Zhou, L.; Kuang, Y.; Yu, C. Adverse Effects of Polycystic Ovarian Syndrome on Pregnancy Outcomes in Women with Frozen-Thawed Embryo Transfer: Propensity Score-Matched Study. Front. Endocrinol. 2022, 13, 878853. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38.
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Dunne, F.; Lawlor, D.A. Hyperglycemia and Risk of Adverse Perinatal Outcomes: Systematic Review and Meta-Analysis. BMJ 2016, 354, i4694. [Google Scholar] [PubMed]
- Patten, R.K.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K. Exercise Interventions in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Le, D.C.; Vu, T.B.; Tran, T.N.; Nguyen, T.L.; Nguyen, T.B.; Nguyen, D.C.; Hoang, V.T. The Effectiveness of Lifestyle Changes in Glycemic Control among Pregnant Women with Gestational Diabetes Mellitus. Medicina 2023, 59, 1587. [Google Scholar] [CrossRef]
- Yook, S.-M.; Lim, Y.-S.; Lee, J.-S.; Kim, K.-N.; Hwang, H.-J.; Kwon, S.; Hwang, J.Y.; Kim, H.Y. Revision of Nutrition Quotient for Korean Adults: NQ-2021. J. Nutr. Health 2022, 55, 198–210. [Google Scholar]
- Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms. 2007. Available online: http://physio-pedia.com/images/c/c7/Quidelines_for_interpreting_the_IPAQ.pdf (accessed on 10 January 2025).
- Chun, M.Y. Validity and Reliability of Korean Version of International Physical Activity Questionnaire Short Form in the Elderly. Korean J. Fam. Med. 2012, 33, 144–151. [Google Scholar]
- Abu-Taha, M.; Daghash, A.; Daghash, R.; Abu Farha, R. Evaluation of Women Knowledge and Perception about Polycystic Ovary Syndrome and Its Management in Jordan: A Survey-Based Study. Int. J. Clin. Pract. 2020, 74, e13552. [Google Scholar] [CrossRef]
- Choi, E.S.; Oh, J.A.; Hur, M.H.; Lee, I.S.; Choi, S.Y. The Knowledge and Learning Needs about Gestational Diabetes in Pregnant Women. J. Korean Acad. Women’s Health Nurs. 2000, 6, 12. [Google Scholar]
- Park, S.; Lee, J.L.; In Sun, J.; Kim, Y. Knowledge and Health Beliefs about Gestational Diabetes and Healthy Pregnancy’s Breastfeeding Intention. J. Clin. Nurs. 2018, 27, 4058–4065. [Google Scholar]
- Chudzicka-Strugala, I.; Gołębiewska, I.; Banaszewska, B.; Brudecki, G.; Zwoździak, B. The Role of Individually Selected Diets in Obese Women with PCOS: A Review. Nutrients 2022, 14, 4555. [Google Scholar] [CrossRef]
- Amiri, M.; Hatoum, S.; Hopkins, D.; Buyalos, R.P.; Ezeh, U.; Pace, L.A.; Bril, F.; Sheidaei, A.; Azziz, R. The Association Between Obesity and Polycystic Ovary Syndrome: An Epidemiologic Study of Observational Data. J. Clin. Endocrinol. Metab. 2024, 109, 2640–2657. [Google Scholar]
- Mansour, A.; Noori, M.; Hakemi, M.S.; Haghgooyan, Z.; Mohajeri-Tehrani, M.R.; Mirahmad, M.; Sajjadi-Jazi, S.M. Hyperandrogenism and Anthropometric Parameters in Women with Polycystic Ovary Syndrome. BMC Endocr. Disord. 2024, 24, 201. [Google Scholar]
- Tal, R.; Seifer, C.M.; Khanimov, M.; Seifer, D.B.; Tal, O. High Serum Antimullerian Hormone Levels Are Associated with Lower Live Birth Rates in Women with Polycystic Ovarian Syndrome Undergoing Assisted Reproductive Technology. Reprod. Biol. Endocrinol. 2020, 18, 20. [Google Scholar]
- Tan, C.; Huang, S.; Xu, L.; Zhang, T.; Yuan, X.; Li, Z.; Chen, M.; Chen, C.; Yan, Q. Cross-Talk between Oxidative Stress and Lipid Metabolism Regulators Reveals Molecular Clusters and Immunological Characterization in Polycystic Ovarian Syndrome. Lipids Health Dis. 2024, 23, 248. [Google Scholar]
- Liu, S.; Mo, M.; Xiao, S.; Li, L.; Hu, X.; Hong, L.; Wang, L.; Lian, R.; Huang, C.; Zeng, Y.; et al. Pregnancy Outcomes of Women with Polycystic Ovary Syndrome for the First In Vitro Fertilization Treatment: A Retrospective Cohort Study with 7678 Patients. Front. Endocrinol. 2020, 11, 575337. [Google Scholar]
- Bahri Khomami, M.; Joham, A.E.; Boyle, J.A.; Piltonen, T.; Silagy, M.; Arora, C.; Misso, M.L.; Teede, H.J.; Moran, L.J. Increased Maternal Pregnancy Complications in Polycystic Ovary Syndrome Appear to Be Independent of Obesity: A Systematic Review, Meta-Analysis, and Meta-Regression. Obes. Rev. 2019, 20, 659–674. [Google Scholar]
- Popova, P.V.; Klyushina, A.A.; Vasilyeva, L.B.; Tkachuk, A.S.; Bolotko, Y.A.; Gerasimov, A.S.; Pustozerov, E.A.; Kravchuk, E.N.; Predeus, A.; Kostareva, A.A.; et al. Effect of Gene-Lifestyle Interaction on Gestational Diabetes Risk. Oncotarget 2017, 8, 67. [Google Scholar]
- Mustaniemi, S.; Vääräsmäki, M.; Eriksson, J.G.; Gissler, M.; Laivuori, H.; Ijäs, H.; Bloigu, A.; Kajantie, E.; Morin-Papunen, L. Polycystic Ovary Syndrome and Risk Factors for Gestational Diabetes. Endocr. Connect 2018, 7, 859–869. [Google Scholar]
- Yan, Q.; Qiu, D.; Liu, X.; Xing, Q.; Liu, R.; Hu, Y. The Incidence of Gestational Diabetes Mellitus among Women with Polycystic Ovary Syndrome: A Meta-Analysis of Longitudinal Studies. BMC Pregnancy Childbirth 2022, 22, 370. [Google Scholar]
- Song, Z.; Cheng, Y.; Li, T.; Fan, Y.; Zhang, Q.; Cheng, H. Prediction of Gestational Diabetes Mellitus by Different Obesity Indices. BMC Pregnancy Childbirth 2022, 22, 288. [Google Scholar]
| Variable | PCOS (n = 430) | Normal (n = 2058) | χ2 or T | p-Value |
|---|---|---|---|---|
| Mean ± SD or n (%) | ||||
| Age in infertility | 33.5 ± 3.9 | 33.7 ± 3.6 | 0.718 | 0.473 |
| BMI 1 in infertility; kg/m2 | 27.3 ± 3.8 | 21.2 ± 2.4 | −42.762 | <0.001 |
| BMI > 25; % | 3.6 | 87.4 | 1682.904 | <0.001 |
| LH 2/FSH 3; mIU/mL | 1.3 ± 1.2 | 1.0 ± 0.5 | −9.312 | <0.001 |
| Triglyceride; mg/dL | 129 ± 84 | 82 ± 42 | −5.559 | <0.001 |
| LDL 4; mg/dL | 133 ± 31 | 116 ± 26 | −4.235 | <0.001 |
| HDL 5; mg/dL | 56 ± 14 | 69 ± 14 | 6.528 | <0.001 |
| AMH 6; pmol/L | 4.4 ± 3.6 | 3.8 ± 2.8 | −3.497 | < 0.001 |
| Hb A1c in infertility; % | 5.4 ± 0.3 | 5.1 ± 0.3 | −7.362 | <0.001 |
| Parity (Primipara) | 351 (81.6) | 1732(84.2) | 1.672 | 0.196 |
| ART 7 (IVF-TET 8) | 287(66.7) | 1140(55.4) | −4.343 | <0.001 |
| Twin | 56(13.0) | 188(9.1) | 6.079 | 0.014 |
| PG1; mg/dL | 137 ± 27 | 125 ± 24 | −8.783 | <0.001 |
| Age in pregnancy | 34.4 ± 3.9 | 34.5 ± 3.7 | 0.230 | 0.046 |
| BMI in 1st trimester; kg/m2 | 27.4 ± 4.2 | 21.4 ± 2.5 | −6.072 | <0.001 |
| BMI at term; kg/m2 | 30.0 ± 4.6 | 26.0 ± 3.1 | −5.058 | <0.001 |
| GDM 9 | 121(28.1) | 218(10.6) | 93.051 | <0.001 |
| Hb A1c in pregnancy; % | 5.2 ± 0.4 | 5.0 ± 0.3 | −6.049 | <0.001 |
| Triglyceride in pregnancy; mg/dL | 326 ± 204 | 272 ± 130 | −2.992 | 0.011 |
| LDL in pregnancy; mg/dL | 144 ± 43 | 151 ± 40 | 0.685 | <0.001 |
| HDL in pregnancy; mg/dL | 70 ± 17 | 77 ± 17 | 3.495 | 0.001 |
| Preterm birth (single) | 44/375 (16.7) | 147/1873 (8.2) | 4.796 | 0.029 |
| Weight gain during pregnancy; kg | 9.8 ± 5.7 | 12.3 ± 4.3 | 9.746 | <0.001 |
| Fetal weight (single) | 3207 ± 499 | 3160 ± 408 | −1.152 | 0.053 |
| Variable | PCOS (n = 99) | GDM (n = 70) | Normal (n = 100) | Total (n = 269) | |
|---|---|---|---|---|---|
| M ± SD or n (%) | |||||
| Age | 33.23 (3.68) | 35.67 (3.86) | 33.21 (3.58) | 33.86 (3.83) | |
| Height | 161.84 (4.93) | 162.55 (4.89) | 162.3 (5.01) | 162.20 (4.94) | |
| Weight | 63.25 (13.38) | 68.30 (9.62) | 68.28 (8.42) | 66.44 (11.02) | |
| Pregnancy history | 0 | 59 (59.60) | 3 (4.29) | 3 (3.00) | 65 (24.16) |
| 1 | 29 (29.29) | 51 (72.86) | 64 (64.00) | 144 (53.53) | |
| 2 | 9 (9.09) | 12 (17.14) | 28 (28.00) | 49 (18.22) | |
| 3 | 1 (1.01) | 3 (4.29) | 3 (3.00) | 7 (2.60) | |
| 4 | 0 (0.00) | 1 (1.42) | 2 (2.00) | 3 (1.11) | |
| 5 | 1 (1.01) | 0 (0.00) | 0 (2.00) | 1 (0.37) | |
| Birth history | 0 | 89 (89.90) | 49 (70.00) | 49 (49.00) | 187 (69.52) |
| 1 | 9 (9.09) | 18 (25.71) | 43 (43.00) | 70 (26.02) | |
| 2 | 1 (1.01) | 3 (4.29) | 7 (7.00) | 11 (4.09) | |
| 3 | 0 (0.00) | 0 (0.00) | 1 (1.00) | 1 (0.37) | |
| Waist circumference | 80.35 (12.41) | 101.77 (9.91) | 100.38 (12.47) | 93.37 (15.44) | |
| Hip circumference | 98.27 (9.75) | 104.17 (6.94) | 105.56 (6.56) | 102.51 (8.60) | |
| Waist to hip ratio | 0.82 (0.07) | 0.96 (0.14) | 0.95 (0.11) | 0.90 (0.13) | |
| Smoking (Yes) | 8 (8.08) | 0 (0.00) | 1 (1.00) | 9 (3.35) | |
| Drinking (Yes) | 38 (38.38) | 12 (17.14) | 28 (28.00) | 78 (29.00) | |
| Paternal family history(Yes) | 37 (37.37) | 38 (54.28) | 36 (36.00) | 111 (41.26) | |
| Maternal family history(Yes) | 41 (41.41) | 40 (57.14) | 27 (27.00) | 108 (40.15) | |
| PCOS 1 | 4 (4.04) | 0 (0.00) | 0 (0.00) | 4 (1.49) | |
| Education | High school | 13 (13.13) | 8 (11.43) | 9 (9.00) | 30 (11.15) |
| Undergraduate school | 76 (76.76) | 56 (80.00) | 84 (84.00) | 216 (80.30) | |
| Graduate school | 10 (10.10) | 6 (8.57) | 7 (7.00) | 23 (8.55) | |
| PCOS 1 knowledge | 11.25 (2.81) | 11.4 (3.70) | 10.71 (3.31) | 11.09 (3.25) | |
| GDM 2 knowledge | 7.05 (2.87) | 10.37 (1.75) | 9.08 (3.09) | 8.67 (3.02) | |
| Nutritional status | 48.75 (12.59) | 58.84 (11.27) | 52.07 (11.20) | 52.61 (12.36) | |
| Physical activity | 1596.0(1871.71) | 647.48 (824.87) | 712.72 (959.57) | 1020.87(1410.81) | |
| B | S.E. | β | t | p | VIF | |
|---|---|---|---|---|---|---|
| Constant | 2.291 | 24.750 | 0.093 | 0.926 | ||
| Age | 0.509 | 0.194 | 0.158 | 2.618 | 0.009 | 1.207 |
| Height | 0.254 | 0.146 | 0.101 | 1.735 | 0.084 | 1.132 |
| Weight | −0.212 | 0.070 | −0.189 | −3.026 | 0.003 | 1.293 |
| Pregnancy history (Yes) | 1.553 | 2.183 | 0.054 | 0.712 | 0.477 | 1.906 |
| Birth history (Yes) | −1.955 | 1.658 | −0.073 | −1.179 | 0.240 | 1.272 |
| Waist to hip ratio | 7.916 | 6.686 | 0.081 | 1.184 | 0.238 | 1.551 |
| Smoking (Yes) | −0.519 | 4.250 | −0.008 | −0.122 | 0.903 | 1.274 |
| Drinking (Yes) | −6.395 | 1.592 | −0.235 | −4.016 | <0.001 | 1.139 |
| Paternal family history (Yes) | −0.846 | 1.460 | −0.034 | −0.579 | 0.563 | 1.127 |
| Maternal family history (Yes) | −1.002 | 1.487 | −0.040 | −0.674 | 0.501 | 1.160 |
| Maternal PCOS history (Yes) | 3.098 | 5.812 | 0.030 | 0.533 | 0.595 | 1.080 |
| Education (ref. = graduate school) | ||||||
| High school | −1.706 | 3.234 | −0.044 | −0.527 | 0.598 | 2.261 |
| Undergraduate school | −1.668 | 2.510 | −0.054 | −0.665 | 0.507 | 2.175 |
| Group (ref = normal) | ||||||
| PCOS | −2.794 | 2.340 | −0.109 | −1.194 | 0.234 | 2.779 |
| GDM | 4.630 | 1.919 | 0.165 | 2.412 | 0.017 | 1.547 |
| PCOS knowledge | 0.276 | 0.218 | 0.073 | 1.264 | 0.207 | 1.094 |
| GDM knowledge | −0.097 | 0.260 | −0.024 | −0.373 | 0.709 | 1.345 |
| R2/R2adj./D-W | 0.244/0.193/1.859 | |||||
| F(p) | 4.764 (<0.001) | |||||
| B | S.E. | β | t | p | VIF | |
|---|---|---|---|---|---|---|
| Constant | −608.923 | 2905.247 | −0.210 | 0.834 | ||
| Age | −16.249 | 22.829 | −0.044 | −0.712 | 0.477 | 1.207 |
| Height | 1.828 | 17.154 | 0.006 | 0.107 | 0.915 | 1.132 |
| Weight | 4.956 | 8.217 | 0.039 | 0.603 | 0.547 | 1.293 |
| Pregnancy history (Yes) | −341.334 | 256.252 | −0.104 | −1.332 | 0.184 | 1.906 |
| Birth history (Yes) | 73.959 | 194.673 | 0.024 | 0.380 | 0.704 | 1.272 |
| Waist to hip ratio | 852.999 | 784.855 | 0.076 | 1.087 | 0.278 | 1.551 |
| Smoking (Yes) | −1245.002 | 498.836 | −0.159 | −2.496 | 0.013 | 1.274 |
| Drinking (Yes) | −219.048 | 186.927 | −0.071 | −1.172 | 0.242 | 1.139 |
| Paternal family history (Yes) | −138.790 | 171.335 | −0.049 | −0.810 | 0.419 | 1.127 |
| Maternal family history (Yes) | −211.482 | 174.568 | −0.074 | −1.211 | 0.227 | 1.160 |
| Maternal PCOS history (Yes) | 1886.115 | 682.259 | 0.162 | 2.765 | 0.006 | 1.080 |
| Education (ref. = graduate school) | 2.261 | |||||
| High school | 555.789 | 379.615 | 0.124 | 1.464 | 0.144 | 2.175 |
| Undergraduate school | 153.868 | 294.641 | 0.043 | 0.522 | 0.602 | 2.779 |
| Group (ref = normal) | 1.547 | |||||
| PCOS | 1051.754 | 274.681 | 0.360 | 3.829 | <0.001 | 1.094 |
| GDM | −73.957 | 225.277 | −0.023 | −0.328 | 0.743 | 1.345 |
| PCOS knowledge | 8.203 | 25.640 | 0.019 | 0.320 | 0.749 | 1.207 |
| GDM knowledge | 68.528 | 30.578 | 0.147 | 2.241 | 0.026 | 1.132 |
| R2/R2adj./D-W | 0.201/0.147/2.013 | |||||
| F(p) | 3.707 (<0.001) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Kim, E.H.; Ko, E.; Park, S.; Lee, Y. The Impact of Polycystic Ovary Syndrome on Gestational Diabetes Mellitus, Disease Knowledge, and Health Behaviors. Healthcare 2025, 13, 717. https://doi.org/10.3390/healthcare13070717
Kim HJ, Kim EH, Ko E, Park S, Lee Y. The Impact of Polycystic Ovary Syndrome on Gestational Diabetes Mellitus, Disease Knowledge, and Health Behaviors. Healthcare. 2025; 13(7):717. https://doi.org/10.3390/healthcare13070717
Chicago/Turabian StyleKim, Hye Jin, Eui Hyeok Kim, Eungil Ko, Sojung Park, and Yaelim Lee. 2025. "The Impact of Polycystic Ovary Syndrome on Gestational Diabetes Mellitus, Disease Knowledge, and Health Behaviors" Healthcare 13, no. 7: 717. https://doi.org/10.3390/healthcare13070717
APA StyleKim, H. J., Kim, E. H., Ko, E., Park, S., & Lee, Y. (2025). The Impact of Polycystic Ovary Syndrome on Gestational Diabetes Mellitus, Disease Knowledge, and Health Behaviors. Healthcare, 13(7), 717. https://doi.org/10.3390/healthcare13070717







