Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Phantom Models
2.2. Application of Contrast Agent
2.3. Imaging of the Phantom with Magnetic Resonance
- Implant Material: Specifies the material of the cranial implant used in the study, either titanium or PEEK, each selected for its unique interaction with MRI imaging.
- Implant Coverage: Indicates the proportion of the phantom skull covered by the implant, categorized as Type 1 (one-quarter), Type 2 (one-third), or Type 3 (one-half). This parameter allows for the evaluation of artifact formation and imaging quality relative to the size of the implant.
- Sequence: Lists the MRI imaging sequences employed during the study. Each sequence is tailored to optimize visualization of the phantom model and reduce artifacts, such as those caused by implants or surrounding structures.
- Slice Thickness [mm]: Refers to the thickness of the individual imaging slices captured during the sequence, measured in millimeters. This parameter affects the resolution and detail of the final image.
- Sequence Duration [min]: Specifies the time required to complete each imaging sequence, measured in minutes. The duration may vary depending on the field of view (FoV) or additional imaging adjustments.
- Contrast: Describes the type of contrast enhancement or suppression applied during the imaging sequence, such as fat suppression, water suppression, or SPAIR (Spectral Attenuated Inversion Recovery).
- Resolution Matrix [mm]: Denotes the resolution of the resulting images, represented as a pixel grid. Higher resolution matrices provide greater detail in the final image.
3. Results
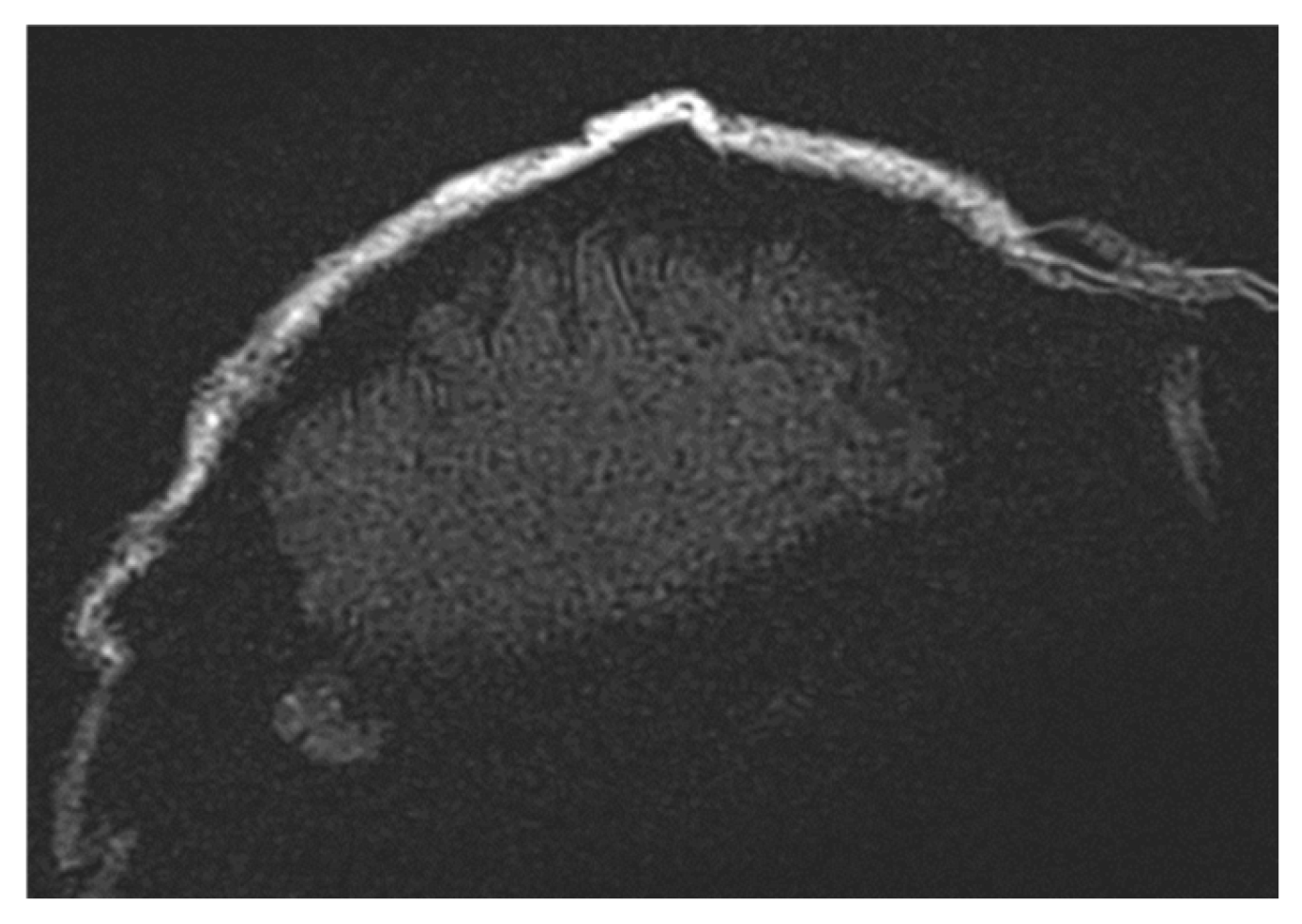
3.1. Types and Causes of MRI Artifacts
3.2. Artifact Suppression Techniques
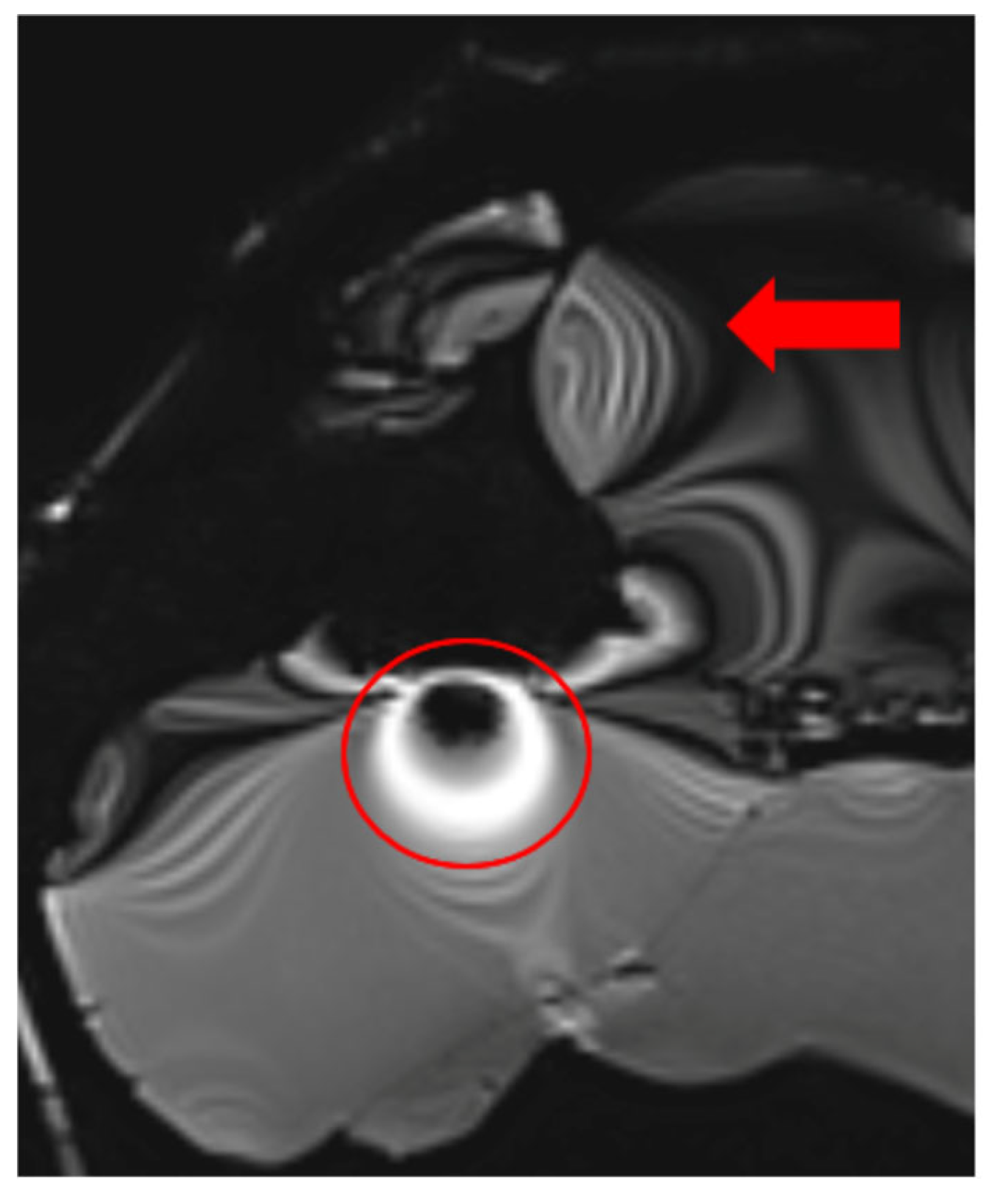
3.3. Radiofrequency (RF) Artifacts and Signal Homogeneity
3.4. Turbo Spin Echo (TSE) for Artifact Reduction
3.5. Fat Saturation Artifacts
3.6. Optimized Imaging Sequences for Tumor Detection Under Cranial Implants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krätzig, T.; Mende, K.; Mohme, M.; Kniep, H.; Dreimann, M.; Stangenberg, M.; Westphal, M.; Gauer, T.; Eicker, S. Carbon fiber–reinforced PEEK versus titanium implants: An in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg. Rev. 2020, 44, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Fierens, G.; Walraevens, J.; Peeters, R.; Glorieux, C.; Verhaert, N. Metal artefact reduction sequences for a piezoelectric bone conduction implant using a realistic head phantom in MRI. arXiv 2023, arXiv:2306.03767. [Google Scholar]
- Germann, C.; Falkowski, A.; von Deuster, C.; Nanz, D.; Sutter, R. Basic and Advanced Metal-Artifact Reduction Techniques at Ultra-High Field 7-T Magnetic Resonance Imaging—Phantom Study Investigating Feasibility and Efficacy. Investig. Radiol. 2022, 57, 387–398. [Google Scholar] [CrossRef]
- Vasilev, Y.; Panina, O.Y.; Semenov, D.S.; Akhmad, E.S.; Sergunova, K.; Kivasev, S.; Petraikin, A. Prostate magnetic resonance imaging (MRI) in patients with hip implants—Presetting a protocol using a phantom. Quant. Imaging Med. Surg. 2024, 14, 7128–7137. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Ulloa, P.; Jansen, O.; Hoevener, J. Metallic Implants in MRI—Hazards and Imaging Artifacts. RöFo–Fortschritte Auf Dem Geb. Der Röntgenstrahlen Und Der Bildgeb. Verfahr. 2021, 193, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, B.; Wostrack, M.; Ringel, F.; Ryang, Y.; Förschler, A.; Waldt, S.; Zimmer, C.; Nittka, M.; Preibisch, C. Novel Metal Artifact Reduction Techniques with Use of Slice-Encoding Metal Artifact Correction and View-Angle Tilting MR Imaging for Improved Visualization of Brain Tissue near Intracranial Aneurysm Clips. Clin. Neuroradiol. 2016, 26, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, L.; Park, S.W.; Rhim, J.; Kang, Y.; Chung, Y.; Son, Y.; Kim, S.C. Metal Artifact Reduction for Orthopedic Implants: Brain CT Angiography in Patients with Intracranial Metallic Implants. J. Korean Med. Sci. 2018, 33, e158. [Google Scholar] [PubMed]
- Wimmer, W.; Hakim, A.; Kiefer, C.; Pastore-Wapp, M.; Anschuetz, L.; Caversaccio, M.; Wagner, F. MRI Metal Artifact Reduction Sequence for Auditory Implants: First Results with a Transcutaneous Bone Conduction Implant. Laryngo-Rhino-Otol. 2019, 99, S49. [Google Scholar]
- Lerch, K.; Morgenstern, F.; Lau, K.; Hoffmann, G. Rivet-like titanium clamps for refixation of bone covers after craniotomy—Radiologic identification, safety and image quality in CT and MR studies. RoFo Fortschritte Auf Dem Geb. Der Rontgenstrahlen Und Der Nukl. 1998, 169, 601–604. [Google Scholar]
- Bartels, L.; Bakker, C.; Viergever, M. Improved lumen visualization in metallic vascular implants by reducing RF artifacts. Magn. Reson. Med. 2002, 47, 171–180. [Google Scholar] [PubMed]
- Ni, P. Clinical Value of PROPELLER Technique for Artifact Reduction in Cranial MRI. Chin. Med. Equip. J. 2010. [Google Scholar]
- Hilgenfeld, T.; Prager, M.; Schwindling, F.S.; Jende, J.; Rammelsberg, P.; Bendszus, M.; Heiland, S.; Juerchott, A. Protocol for the Evaluation of MRI Artifacts Caused by Metal Implants to Assess the Suitability of Implants and the Vulnerability of Pulse Sequences. J. Vis. Exp. JoVE 2018, 135, 57394. [Google Scholar]
- Aissa, J.; Boos, J.; Schleich, C.; Sedlmair, M.; Krzymyk, K.; Kröpil, P.; Antoch, G.; Thomas, C. Metal Artifact Reduction in Computed Tomography After Deep Brain Stimulation Electrode Placement Using Iterative Reconstructions. Investig. Radiol. 2017, 52, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.M.; Tansey, J.B.; Wu, L.; Choudhri, A.; Yawn, R.J.; MacDonald, C.B.; Richard, C. A Systematic Review of Cochlear Implant-Related Magnetic Resonance Imaging Artifact: Implications for Clinical Imaging. Otol. Neurotol. 2024, 45, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Carlotto, E.; Simoncelli, A.; Lafe, E.; Scribante, A.; Minervini, D.; Nardo, M.; Malpede, S.; Chiapparini, L.; Benazzo, M. The usefulness of the O-MAR algorithm in MRI skull base assessment to manage cochlear implant-related artifacts. Acta Otorhinolaryngol. Ital. 2023, 43, 273–282. [Google Scholar] [PubMed]





| Implant Material | Implant Coverage | Sequence | Slice Thickness [mm] | Sequence Duration [min] | Contrast | Resolution Matrix [mm] |
|---|---|---|---|---|---|---|
| Titanium | Type 1 | t2_tse_fs_tra | 4 | 2:11 | Strong fat suppression | 400 × 544 |
| t1_starvibe_tra | 5 | 1:45 | Fat suppression | 256 × 256 | ||
| t2_tse_fs_tra | 4 | 3:03 | Water suppression | 400 × 544 | ||
| Type 2 | t2_tse_fs_tra | 4 | 2:11 | Strong fat suppression | 400 × 544 | |
| t1_starvibe_tra | 5 | 2:09 | Fat suppression | 256 × 256 | ||
| t2_tse_fs_tra | 4 | 3:03 | Water suppression | 400 × 544 | ||
| Type 3 | t2_tse_fs_tra | 4 | 3:11 | Strong fat suppression | 400 × 544 | |
| t1_starvibe_tra | 5 | 3:09 | Fat suppression | 256 × 256 | ||
| t1_vibe_fs_tra 1 | - | 2:59 | Strong fat suppression | - | ||
| PEEK | Type 1 | t2_tse_fs_tra | 4 | 2:11 | Strong fat suppression | 400 × 544 |
| t1_starvibe_tra | 5 | 2:09 | Fat suppression | 256 × 256 | ||
| t2_tse_fs_tra | 4 | 3:11 | Strong fat suppression | 400 × 544 | ||
| Type 2 | t1_starvibe_tra | 5 | 3:09 | Fat suppression | 256 × 256 | |
| t2_tse_fs_tra | 4 | 3:11 | Strong fat suppression | 400 × 544 | ||
| t1_starvibe_tra | 5 | 3:09 | Fat suppression | 256 × 256 | ||
| t1_space_sag | 1 | 3:03 | SPAIR | 259 × 259 |
| Sequence | Slice Thickness [mm] | Sequence Duration [min] | Contrast | TR (Repetition Time) [ms] | TE (Echo Time) [ms] |
|---|---|---|---|---|---|
| t2_tse_fs_tra | 4 | 3:11 | Strong fat suppression | 5190.0 | 105.00 |
| t1_starvibe_tra | 5 | 3:09 | Fat and water suppression | 4.4 | 2.20 |
| t1_space_sag | 1 | 3:03 | Fat suppression | 700.0 | 13.00 |
| Sequence | Slice Thickness [mm] | Sequence Duration [min] | Contrast | TR (Repetition Time) [ms] | TE (Echo Time) [ms] |
|---|---|---|---|---|---|
| t2_tse_fs_tra | 2 | 3:11 | Strong fat suppression | 12,480.0 | 87.00 |
| t1_vibe_fs_tra | 1.1 | 3:21 | Fat and water suppression | 6.1 | 3.57 |
| t1_tse_tra | 1 | 2:09 | Fat suppression | 12,480.0 | 87.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ondrejová, B.; Rajťúková, V.; Šavrtková, K.; Galajdová, A.; Živčák, J.; Hudák, R. Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models. Healthcare 2025, 13, 803. https://doi.org/10.3390/healthcare13070803
Ondrejová B, Rajťúková V, Šavrtková K, Galajdová A, Živčák J, Hudák R. Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models. Healthcare. 2025; 13(7):803. https://doi.org/10.3390/healthcare13070803
Chicago/Turabian StyleOndrejová, Bibiána, Viktória Rajťúková, Kristína Šavrtková, Alena Galajdová, Jozef Živčák, and Radovan Hudák. 2025. "Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models" Healthcare 13, no. 7: 803. https://doi.org/10.3390/healthcare13070803
APA StyleOndrejová, B., Rajťúková, V., Šavrtková, K., Galajdová, A., Živčák, J., & Hudák, R. (2025). Analysis of MRI Artifacts Induced by Cranial Implants in Phantom Models. Healthcare, 13(7), 803. https://doi.org/10.3390/healthcare13070803







