The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases
Abstract
1. Introduction
1.1. Background
1.2. Role of Neuroinflammation in Psychiatric Disorders and Internal Disease
1.3. Objective
2. Depression and Cardiovascular Disease
2.1. Shared Pathophysiological Mechanisms
2.1.1. Systemic Inflammation and Endothelial Dysfunction
2.1.2. HPA Axis Dysregulation
2.1.3. Autonomic Nervous System Dysregulation
2.1.4. Clinical Implications
2.2. Bidirectional Relationship
2.2.1. Depression’s Effect on Cardiovascular Disease
Poor Treatment Adherence and Lifestyle Behaviors
Inflammatory Pathways
HPA Axis Activation
2.2.2. Cardiovascular Disease’s Effect on Depression
Chronic Illness Stress
Systemic and Neuroinflammation
Cerebral Perfusion Deficits
2.2.3. Integrated Clinical Implications
2.2.4. Future Directions
2.3. Diagnostic and Therapeutic Challenges
2.3.1. Overlapping Symptoms
2.3.2. Treatment Dilemmas
- Pharmacological Safety:
- 2.
- Efficacy in Dual Pathologies:
- 3.
- Polypharmacy Risks:
- 4.
- Emerging Interventions
Anti-Inflammatory Therapies
Vagus Nerve Stimulation (VNS)
Behavioral Interventions
2.3.3. Clinical Implications
2.3.4. Gaps and Future Directions
3. Anxiety Disorders
3.1. Neuroinflammatory Mechanisms
3.1.1. Immune Dysregulation in Anxiety
3.1.2. Brain–Immune Axis Dysfunction
3.2. Comorbidities with Internal Diseases
3.2.1. Hypertension and Gastrointestinal Disorders
3.2.2. Hypertension
3.2.3. Gastrointestinal Disorders
3.2.4. Clinical Implications
3.3. Treatment Perspectives
3.3.1. Psychological and Pharmacological Interventions
Cognitive-Behavioral Therapy (CBT)
Anxiolytic Medications
3.3.2. Emerging Immunomodulatory Therapies
Anti-Inflammatory Agents
Vagus Nerve Stimulation (VNS)
3.3.3. Integrated Approaches
3.3.4. Future Directions
4. Diabetes Mellitus
4.1. Inflammation and Insulin Resistance
4.1.1. Role of IL-1β and TNF-α
Pancreatic Beta-Cell Dysfunction
4.1.2. Impact of Hyperglycemia on Neuroinflammation
Systemic Inflammation and Neural Effects
Neuroinflammatory Pathways
Hypothalamic Dysfunction
4.1.3. Clinical Implications
4.1.4. Future Directions
4.2. Bidirectional Relationship with Depression
4.2.1. Diabetes Increasing Depression Risk
Chronic Inflammation as a Driver of Depression
Metabolic Dysfunction and Neurochemical Changes
Psychological Stress and Burden of Disease
4.2.2. Depression Worsening Diabetes Outcomes
Poor Self-Management
Heightened Systemic Inflammation
Autonomic Nervous System (ANS) Dysregulation
4.2.3. Clinical Implications
4.2.4. Future Directions
4.3. Emerging Treatment Strategies
4.3.1. Anti-Inflammatory Therapies
IL-1 Antagonists and Beta-Cell Preservation
Dual Benefits for Mental Health Outcomes
TNF-α Inhibitors and Insulin Sensitivity
4.3.2. Models of Integrated Care
Multidisciplinary Approaches
Collaborative Interventions
Technology-Driven Integration
Clinical Implications
Future Directions
5. Models of Integrated Care
5.1. Multidisciplinary Approaches
5.1.1. Collaborative Models in Depression and Cardiovascular Disease
Case Examples of Integration
- Co-located Care Models
- Integrated Screening Protocols
5.1.2. Comprehensive Care for Anxiety and Diabetes
Behavioral Interventions
Medical Interventions
Nutritional Support
Examples of Comprehensive Care
5.1.3. Clinical Implications
5.1.4. Future Directions
5.2. Emerging Technologies
5.2.1. Telemedicine for Monitoring Comorbidities
Remote Monitoring of Inflammation and Mental Health
Examples of Clinical Application
Impact on Outcomes
5.2.2. Wearable Devices
Tracking Heart Rate Variability (HRV)
Glucose Monitoring and Metabolic Health
Inflammatory Marker Detection
Examples of Wearable Applications
Clinical Benefits
5.2.3. Clinical Implications
5.2.4. Future Directions
6. Discussion
6.1. Current Gaps in Research and Literature
- Longitudinal Evidence and Causal Pathways
- 2.
- Biomarker Validation and Clinical Application
- 3.
- Integrated Therapeutic Interventions
- Vagus nerve stimulation (VNS): Clinical trials have shown that VNS reduces depressive symptoms in treatment-resistant depression while modulating systemic inflammation, with documented reductions in TNF-α and IL-6 levels [69].
- Mindfulness-Based Stress Reduction (MBSR): Studies have linked MBSR to reductions in systemic inflammation (CRP) and improvements in psychological resilience, yet its integration into multimodal treatment frameworks remains underexplored [181].
- Immunotherapy approaches: IL-6 inhibitors, such as tocilizumab, have demonstrated efficacy in inflammatory conditions, but their role in treating psychiatric comorbidities remains under investigation. Future research should assess their long-term safety and efficacy in neuroinflammation-driven psychiatric disorders [182].
6.2. Challenges in Screening and Diagnosis
6.3. Technological Integration
Future Outlook and Directions
- Validating the clinical accuracy and reliability of biosensors through large-scale, multi-center trials.
- Developing regulatory frameworks that standardize the integration of these tools into clinical practice.
- Improving accessibility by reducing production costs and expanding reimbursement policies for telemedicine and digital health interventions.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGrath, J.J.; Lim, C.C.W.; Plana-Ripoll, O.; Holtz, Y.; Agerbo, E.; Momen, N.C.; Mortensen, P.B.; Pedersen, C.B.; Abdulmalik, J.; Aguilar-Gaxiola, S.; et al. Comorbidity within mental disorders: A comprehensive analysis based on 145 990 survey respondents from 27 countries. Epidemiol. Psychiatr. Sci. 2020, 29, e153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sariaslan, A.; Sharpe, M.; Larsson, H.; Wolf, A.; Lichtenstein, P.; Fazel, S. Psychiatric comorbidity and risk of premature mortality and suicide among those with chronic respiratory diseases, cardiovascular diseases, and diabetes in Sweden: A nationwide matched cohort study of over 1 million patients and their unaffected siblings. PLoS Med. 2022, 19, e1003864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luciano, M.; Pompili, M.; Sartorius, N.; Fiorillo, A. Editorial: Mortality of people with severe mental illness: Causes and ways of its reduction. Front. Psychiatry 2022, 13, 1009772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerrero Fernández de Alba, I.; Gimeno-Miguel, A.; Poblador-Plou, B.; Gimeno-Feliu, L.A.; Ioakeim-Skoufa, I.; Rojo-Martínez, G.; Forjaz, M.J.; Prados-Torres, A. Association between mental health comorbidity and health outcomes in type 2 diabetes mellitus patients. Sci. Rep. 2020, 10, 19583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Sig. Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martínez-González, M.B.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Beltran-Velasco, A.I.; Ruisoto, P.; Diaz Arroyo, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 10041. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Kenwood, M.M.; Kalin, N.H.; Barbas, H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 2022, 47, 260–275, Erratum in Neuropsychopharmacology 2022, 47, 1141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaccarino, V.; Badimon, L.; Bremner, J.D.; Cenko, E.; Cubedo, J.; Dorobantu, M.; Duncker, D.J.; Koller, A.; Manfrini, O.; Milicic, D.; et al. ESC Scientific Document Group Reviewers. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur. Heart J. 2020, 41, 1687–1696, Erratum in Eur. Heart J. 2020, 41, 1696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuomisto, K.; Jousilahti, P.; Sundvall, J.; Pajunen, P.; Salomaa, V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb. Haemost. 2006, 95, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, A.; Abunaser, R.; Khassawneh, A.; Alfaqih, M.; Khasawneh, A.; Abdo, N. The Bidirectional Relationship between Diabetes and Depression: A Literature Review. Korean J. Fam. Med. 2018, 39, 137–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Depressive Disorder (Depression). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 20 January 2025).
- World Health Organization. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 January 2025).
- De Hert, M.; Detraux, J.; Vancampfort, D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2018, 20, 31–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berk, M.; Köhler-Forsberg, O.; Turner, M.; Penninx, B.W.J.H.; Wrobel, A.; Firth, J.; Loughman, A.; Reavley, N.J.; McGrath, J.J.; Momen, N.C.; et al. Comorbidity between major depressive disorder and physical diseases: A comprehensive review of epidemiology, mechanisms and management. World Psychiatry 2023, 22, 366–387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The association between Diabetes mellitus and Depression. J. Med. Life. 2016, 9, 120–125. [Google Scholar] [PubMed] [PubMed Central]
- Glassman, J.R.; Jauregui, A.; Milstein, A.; Kaplan, R.M. Caring for People with Depression: Costs Among 43 Million Commercially Insured Patients with or Without Comorbid Illnesses. Ann. Behav. Med. 2023, 57, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Nigussie, K.; Sertsu, A.; Ayana, G.M.; Dessie, Y.; Bete, T.; Abdisa, L.; Debele, G.R.; Wadaje, D.; Negash, A. Determinants of depression and anxiety among type 2 diabetes patients in governments’ hospitals at Harari regional state, Eastern Ethiopia: A multi-center cross-sectional study. BMC Psychiatry 2023, 23, 13. [Google Scholar] [CrossRef]
- Liu, X.; Haagsma, J.; Sijbrands, E.; Buijks, H.; Boogaard, L.; Mackenbach, J.P.; Erasmus, V.; Polinder, S. Anxiety and depression in diabetes care: Longitudinal associations with health-related quality of life. Sci. Rep. 2020, 10, 8307. [Google Scholar] [CrossRef]
- Pardhan, S.; Siddique, A.B.; Motahara, U.; Islam, M.S. Investigating the prevalence and associated factors of depression, anxiety, and loneliness among people with type-2 diabetes in Bangladesh: A community-based study. Sci. Rep. 2024, 14, 25129. [Google Scholar] [CrossRef]
- Mezick, E.J.; Hall, M.; Matthews, K.A. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep. Med. Rev. 2011, 15, 51–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deschênes, S.S.; Burns, R.J.; Graham, E.; Schmitz, N. Depressive symptoms and sleep problems as risk factors for heart disease: A prospective community study. Epidemiol. Psychiatr. Sci. 2019, 29, e50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gathright, E.C.; Goldstein, C.M.; Josephson, R.A.; Hughes, J.W. Depression increases the risk of mortality in patients with heart failure: A meta-analysis. J. Psychosom. Res. 2017, 94, 82–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunn, G.A.; Loftis, J.M.; Sullivan, E.L. Neuroinflammation in psychiatric disorders: An introductory primer. Pharmacol. Biochem. Behav. 2020, 196, 172981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orsolini, L.; Pompili, S.; Tempia Valenta, S.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23, 1616. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A. Inflammation, heart disease, and depression. Curr. Psychiatry Rep. 2013, 15, 400. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herman, J.P.; McKlveen, J.M.; Solomon, M.B.; Carvalho-Netto, E.; Myers, B. Neural regulation of the stress response: Glucocorticoid feedback mechanisms. Braz. J. Med. Biol. Res. 2012, 45, 292–298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katon, W.J. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin. Neurosci. 2011, 13, 7–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, D.P.; Koenig, J.; Carnevali, L.; Sgoifo, A.; Jarczok, M.N.; Sternberg, E.M.; Thayer, J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain Behav. Immun. 2019, 80, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, B.; Sabbah, H.N.; Hauptman, P.J.; Colucci, W.S. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008, 118, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Akosile, W.; Tiyatiye, B.; Colquhoun, D.; Young, R. Management of depression in patients with coronary artery disease: A systematic review. Asian J. Psychiatr. 2023, 83, 103534. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharan, P.; Vellapandian, C. Hypothalamic-Pituitary-Adrenal (HPA) Axis: Unveiling the Potential Mechanisms Involved in Stress-Induced Alzheimer’s Disease and Depression. Cureus 2024, 16, e67595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.T.; Nieman, L.K.; Feelders, R.A. Comorbidities in Cushing’s disease. Pituitary 2015, 18, 188–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abercrombie, H.C.; Jahn, A.L.; Davidson, R.J.; Kern, S.; Kirschbaum, C.; Halverson, J. Cortisol’s effects on hippocampal activation in depressed patients are related to alterations in memory formation. J. Psychiatr. Res. 2011, 45, 15–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaccarino, V.; Lampert, R.; Bremner, J.D.; Lee, F.; Su, S.; Maisano, C.; Murrah, N.V.; Jones, L.; Jawed, F.; Afzal, N.; et al. Depressive symptoms and heart rate variability: Evidence for a shared genetic substrate in a study of twins. Psychosom. Med. 2008, 70, 628–636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Siepmann, M.; Weidner, K.; Petrowski, K.; Siepmann, T. Heart Rate Variability: A Measure of Cardiovascular Health and Possible Therapeutic Target in Dysautonomic Mental and Neurological Disorders. Appl. Psychophysiol. Biofeedback 2022, 47, 273–287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olshansky, B. Vagus nerve modulation of inflammation: Cardiovascular implications. Trends Cardiovasc. Med. 2016, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Stuart, M.; Gilmour, A.; Wersching, H.; Heindel, W.; Arolt, V.; Berger, K. The relationship between subtypes of depression and cardiovascular disease: A systematic review of biological models. Transl. Psychiatry 2012, 2, e92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Zhou, J.; Wang, M.; Yang, C.; Sun, G. Cardiovascular disease and depression: A narrative review. Front. Cardiovasc. Med. 2023, 10, 1274595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grenard, J.L.; Munjas, B.A.; Adams, J.L.; Suttorp, M.; Maglione, M.; McGlynn, E.A.; Gellad, W.F. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. J. Gen. Intern. Med. 2011, 26, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahall, M. Prevalence and associations of depression among patients with cardiac diseases in a public health institute in Trinidad and Tobago. BMC Psychiatry 2019, 19, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, X.; Zheng, H.; Liu, M.; Wu, L.; Tian, S.; Wu, W. Association of systemic immune-inflammation index with all-cause and cardiovascular mortality among adults with depression: Evidence from NHANES 2005–2018. BMC Psychiatry 2025, 25, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc. Res. 2004, 64, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Werdermann, M.; Berger, I.; Scriba, L.D.; Santambrogio, A.; Schlinkert, P.; Brendel, H.; Morawietz, H.; Schedl, A.; Peitzsch, M.; King, A.J.F.; et al. Insulin and obesity transform hypothalamic-pituitary-adrenal axis stemness and function in a hyperactive state. Mol. Metab. 2021, 43, 101112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef]
- Jha, M.K.; Qamar, A.; Vaduganathan, M.; Charney, D.S.; Murrough, J.W. Screening and Management of Depression in Patients with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1827–1845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogelzangs, N.; Seldenrijk, A.; Beekman, A.T.; van Hout, H.P.; de Jonge, P.; Penninx, B.W. Cardiovascular disease in persons with depressive and anxiety disorders. J. Affect. Disord. 2010, 125, 241–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link Between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De la Torre, J.C. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc. Psychiatry Neurol. 2012, 2012, 367516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, F.; Yang, J.; Cui, R. Effect of Hypoxic Injury in Mood Disorder. Neural Plast. 2017, 2017, 6986983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lichtman, J.H.; Bigger, J.T., Jr.; Blumenthal, J.A.; Frasure-Smith, N.; Kaufmann, P.G.; Lespérance, F.; Mark, D.B.; Sheps, D.S.; Taylor, C.B.; Froelicher, E.S.; et al. Depression and coronary heart disease: Recommendations for screening, referral, and treatment: A science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Psychiatric Association. Circulation 2008, 118, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337, Erratum in Eur. Heart J. 2022, 43, 4468. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.A.; Dunbar, S.B.; Higgins, M.K.; Musselman, D.L.; Smith, A.L. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J. Psychosom. Res. 2010, 69, 119–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.J.; Wu, J.; Gong, L.J.; Yang, H.S.; Chen, H. Non-invasive vagus nerve stimulation in anti-inflammatory therapy: Mechanistic insights and future perspectives. Front. Neurosci. 2024, 18, 1490300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gururajan, A.; Naughton, M.E.; Scott, K.A.; O’Connor, R.M.; Moloney, G.; Clarke, G.; Dowling, J.; Walsh, A.; Ismail, F.; Shorten, G.; et al. MicroRNAs as biomarkers for major depression: A role for let-7b and let-7c. Transl. Psychiatry 2016, 6, e862. [Google Scholar] [CrossRef]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Searles, C.D. MicroRNAs and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2024, 26, 51–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez-Moreno, A.C.; Jhund, P.S.; Macdonald, M.R.; Petrie, M.C.; Cleland, J.G.; Böhm, M.; van Veldhuisen, D.J.; Gullestad, L.; Wikstrand, J.; Kjekshus, J.; et al. Fatigue as a predictor of outcome in patients with heart failure: Analysis of CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail. 2014, 2, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Peterson, M.J. Sleep Disturbances in Depression. Sleep Med. Clin. 2015, 10, 17–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Li, G.; Bao, Y.; Liu, M. Role of sleep disorders in patients with cardiovascular disease: A systematic review. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 21, 200257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, A.; Wadhwa, R. Selective Serotonin Reuptake Inhibitors; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Yekehtaz, H.; Farokhnia, M.; Akhondzadeh, S. Cardiovascular considerations in antidepressant therapy: An evidence-based review. J. Tehran Heart Cent. 2013, 8, 169–176. [Google Scholar] [PubMed] [PubMed Central]
- Glassman, A.H. Cardiovascular effects of tricyclic antidepressants. Annu. Rev. Med. 1984, 35, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Low, Y.; Setia, S.; Lima, G. Drug-drug interactions involving antidepressants: Focus on desvenlafaxine. Neuropsychiatr. Dis. Treat. 2018, 14, 567–580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, P.C.; Feist, E.; Pope, J.E.; Nash, P.; Sibilia, J.; Caporali, R.; Balsa, A. What have we learnt from the inhibition of IL-6 in RA and what are the clinical opportunities for patient outcomes? Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720X241283340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tiosano, S.; Yavne, Y.; Watad, A.; Langevitz, P.; Lidar, M.; Feld, J.; Tishler, M.; Aamar, S.; Elkayam, O.; Balbir-Gurman, A.; et al. The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. Eur. J. Clin. Investig. 2020, 50, e13268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kang, H.J.; Jhon, M.; Kim, J.W.; Lee, J.Y.; Walker, A.J.; Agustini, B.; Kim, J.M.; Berk, M. Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front. Psychiatry. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blake, G.J.; Ridker, P.M. Are statins anti-inflammatory? Trials 2000, 1, 161. [Google Scholar] [CrossRef]
- George, M.S.; Aston-Jones, G. Noninvasive techniques for probing neurocircuitry and treating illness: Vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology 2010, 35, 301–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Austelle, C.W.; Cox, S.S.; Wills, K.E.; Badran, B.W. Vagus nerve stimulation (VNS): Recent advances and future directions. Clin. Auton. Res. 2024, 34, 529–547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marino, F.; Failla, C.; Carrozza, C.; Ciminata, M.; Chilà, P.; Minutoli, R.; Genovese, S.; Puglisi, A.; Arnao, A.A.; Tartarisco, G.; et al. Mindfulness-Based Interventions for Physical and Psychological Wellbeing in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 727. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Gómez, A.F. Mindfulness-Based Interventions for Anxiety and Depression. Psychiatr. Clin. North Am. 2017, 40, 739–749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masoumian Hosseini, M.; Masoumian Hosseini, S.T.; Qayumi, K.; Hosseinzadeh, S.; Sajadi Tabar, S.S. Smartwatches in healthcare medicine: Assistance and monitoring; a scoping review. BMC Med. Inf. Decis. Mak. 2023, 23, 248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tubbs, J.D.; Ding, J.; Baum, L.; Sham, P.C. Immune dysregulation in depression: Evidence from genome-wide association. Brain Behav. Immun. Health 2020, 7, 100108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Hakamata, Y.; Hori, H.; Mizukami, S.; Izawa, S.; Yoshida, F.; Moriguchi, Y.; Hanakawa, T.; Inoue, Y.; Tagaya, H. Blunted diurnal interleukin-6 rhythm is associated with amygdala emotional hyporeactivity and depression: A modulating role of gene-stressor interactions. Front. Psychiatry 2023, 14, 1196235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berthold-Losleben, M.; Himmerich, H. The TNF-alpha system: Functional aspects in depression, narcolepsy and psychopharmacology. Curr. Neuropharmacol. 2008, 6, 193–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almutairi, S.; Sivadas, A.; Kwakowsky, A. The Effect of Oral GABA on the Nervous System: Potential for Therapeutic Intervention. Nutraceuticals 2024, 4, 241–259. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed] [PubMed Central]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, P.; Lu, Y.; Pan, B.X.; Zhang, W.H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Ghasemi, M.; Navidhamidi, M.; Rezaei, F.; Azizikia, A.; Mehranfard, N. Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms. Cogn. Affect. Behav. Neurosci. 2022, 22, 431–449. [Google Scholar] [CrossRef]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Golbidi, S.; Frisbee, J.C.; Laher, I. Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1476–H1498. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Jiang, Z.; Chen, X.; Dai, Y.; Zhao, H. Comorbidity of Anxiety and Hypertension: Common Risk Factors and Potential Mechanisms. Int. J. Hypertens. 2023, 2023, 9619388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005, 18, 41–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeLalio, L.J.; Sved, A.F.; Stocker, S.D. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can. J. Cardiol. 2020, 36, 712–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ge, L.; Liu, S.; Li, S.; Yang, J.; Hu, G.; Xu, C.; Song, W. Psychological stress in inflammatory bowel disease: Psychoneuroimmunological insights into bidirectional gut-brain communications. Front. Immunol. 2022, 13, 1016578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, E.A.; Tillisch, K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 2011, 62, 381–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry. 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curtiss, J.E.; Levine, D.S.; Ander, I.; Baker, A.W. Cognitive-Behavioral Treatments for Anxiety and Stress-Related Disorders. Focus 2021, 19, 184–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hofmann, S.G.; Asnaani, A.; Vonk, I.J.; Sawyer, A.T.; Fang, A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cogn. Ther. Res. 2012, 36, 427–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farach, F.J.; Pruitt, L.D.; Jun, J.J.; Jerud, A.B.; Zoellner, L.A.; Roy-Byrne, P.P. Pharmacological treatment of anxiety disorders: Current treatments and future directions. J. Anxiety Disord. 2012, 26, 833–843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, S.A. The effects of benzodiazepines on cognition. J. Clin. Psychiatry 2005, 66 (Suppl. S2), 9–13. [Google Scholar] [PubMed]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus Nerve Stimulation at the Interface of Brain-Gut Interactions. Cold Spring Harb. Perspect. Med. 2019, 9, a034199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The role of interleukin-1β in type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lytrivi, M.; Castell, A.L.; Poitout, V.; Cnop, M. Recent Insights into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Alzamil, H. Elevated Serum TNF-α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Kappelmann, N.; Czamara, D.; Rost, N.; Moser, S.; Schmoll, V.; Trastulla, L.; Stochl, J.; Lucae, S.; CHARGE inflammation working group; Binder, E.B.; et al. Polygenic risk for immuno-metabolic markers and specific depressive symptoms: A multi-sample network analysis study. Brain Behav. Immun. 2021, 95, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thaler, J.P.; Schwartz, M.W. Minireview: Inflammation and obesity pathogenesis: The hypothalamus heats up. Endocrinology 2010, 151, 4109–4115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bodnaruc, A.M.; Roberge, M.; Giroux, I.; Aguer, C. The Bidirectional Link between Major Depressive Disorder and Type 2 Diabetes: The Role of Inflammation. Endocrines 2024, 5, 478–500. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Treadway, M.T. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology 2017, 42, 216–241. [Google Scholar] [CrossRef]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes. 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Peyrot, M.; McCarl, L.A.; Collins, E.M.; Serpa, L.; Mimiaga, M.J.; Safren, S.A. Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care 2008, 31, 2398–2403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehdi, S.; Wani, S.U.D.; Krishna, K.L.; Kinattingal, N.; Roohi, T.F. A review on linking stress, depression, and insulin resistance via low-grade chronic inflammation. Biochem. Biophys. Rep. 2023, 36, 101571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, K.; Beekley, J.; Goto, A.; Jeon, C.Y.; Ritz, B.R. Depression and cardiovascular disease events among patients with type 2 diabetes: A systematic review and meta-analysis with bias analysis. J. Diabetes Complicat. 2020, 34, 107710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sgoifo, A.; Carnevali, L.; Alfonso Mde, L.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Z.; Sun, J. Effects of Cognitive Behavioral Therapy-Based Intervention on Improving Glycaemic, Psychological, and Physiological Outcomes in Adult Patients with Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Front. Psychiatry 2020, 11, 711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.; Yang, Y.; Fan, X.W.; Zhang, N.; Wang, S.; Shi, Y.J.; Hu, W.J.; Wang, C.X. Impacts of inflammatory cytokines on depression: A cohort study. BMC Psychiatry 2024, 24, 195. [Google Scholar] [CrossRef]
- Vallejo, S.; Palacios, E.; Romacho, T.; Villalobos, L.; Peiró, C.; Sánchez-Ferrer, C.F. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2014, 13, 158. [Google Scholar] [CrossRef]
- Lim, W.S.; Teoh, S.E.; Tang, A.S.P.; Tan, B.J.M.; Lee, J.Y.; Yau, C.E.; Thumboo, J.; Ng, Q.X. The effects of anti-TNF-α biologics on insulin resistance and insulin sensitivity in patients with rheumatoid arthritis: An update systematic review and meta-analysis. Diabetes Metab. Syndr. 2024, 18, 103001. [Google Scholar] [CrossRef] [PubMed]
- Uzzan, S.; Azab, A.N. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. [Google Scholar] [CrossRef] [PubMed]
- Young-Hyman, D.; de Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.; Peyrot, M. Psychosocial Care for People with Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140, Erratum in Diabetes Care 2017, 40, 287; Erratum in Diabetes Care 2017, 40, 726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbas, Q.; Latif, S.; Ayaz Habib, H.; Shahzad, S.; Sarwar, U.; Shahzadi, M.; Ramzan, Z.; Washdev, W. Cognitive behavior therapy for diabetes distress, depression, health anxiety, quality of life and treatment adherence among patients with type-II diabetes mellitus: A randomized control trial. BMC Psychiatry 2023, 23, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogunmoroti, O.; Osibogun, O.; Spatz, E.S.; Okunrintemi, V.; Mathews, L.; Ndumele, C.E.; Michos, E.D. A systematic review of the bidirectional relationship between depressive symptoms and cardiovascular health. Prev. Med. 2022, 154, 106891. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Sherwood, A.; Smith, P.J.; Watkins, L.; Mabe, S.; Kraus, W.E.; Ingle, K.; Miller, P.; Hinderliter, A. Enhancing Cardiac Rehabilitation with Stress Management Training: A Randomized, Clinical Efficacy Trial. Circulation 2016, 133, 1341–1350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ford, J.; Thomas, F.; Byng, R.; McCabe, R. Use of the Patient Health Questionnaire (PHQ-9) in Practice: Interactions between patients and physicians. Qual. Health Res. 2020, 30, 2146–2159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polcwiartek, C.; O’Gallagher, K.; Friedman, D.J.; Correll, C.U.; Solmi, M.; Jensen, S.E.; Nielsen, R.E. Severe mental illness: Cardiovascular risk assessment and management. Eur. Heart J. 2024, 45, 987–997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reist, C.; Petiwala, I.; Latimer, J.; Raffaelli, S.B.; Chiang, M.; Eisenberg, D.; Campbell, S. Collaborative mental health care: A narrative review. Medicine 2022, 101, e32554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e763–e783. [Google Scholar] [CrossRef] [PubMed]
- Ee, C.; Lake, J.; Firth, J.; Hargraves, F.; de Manincor, M.; Meade, T.; Marx, W.; Sarris, J. An integrative collaborative care model for people with mental illness and physical comorbidities. Int. J. Ment. Health Syst. 2020, 14, 83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Buys, N.; Ferguson, S.; Li, Z.; Shi, Y.C.; Li, L.; Sun, J. The evaluation of cognitive-behavioral therapy-based intervention on type 2 diabetes patients with comorbid metabolic syndrome: A randomized controlled trial. Diabetol. Metab. Syndr. 2023, 15, 158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richardson, B.; MacPherson, A.; Bambico, F. Neuroinflammation and neuroprogression in depression: Effects of alternative drug treatments. Brain Behav. Immun. Health 2022, 26, 100554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Zonneveld, S.M.; van den Oever, E.J.; Haarman, B.C.M.; Grandjean, E.L.; Nuninga, J.O.; van de Rest, O.; Sommer, I.E.C. An Anti-Inflammatory Diet and Its Potential Benefit for Individuals with Mental Disorders and Neurodegenerative Diseases-A Narrative Review. Nutrients 2024, 16, 2646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamasaki, H. The Effects of Mindfulness on Glycemic Control in People with Diabetes: An Overview of Systematic Reviews and Meta-Analyses. Medicines 2023, 10, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tovote, K.A.; Fleer, J.; Snippe, E.; Bas, I.V.; Links, T.P.; Emmelkamp, P.M.; Sanderman, R.; Schroevers, M.J. Cognitive behavioral therapy and mindfulness-based cognitive therapy for depressive symptoms in patients with diabetes: Design of a randomized controlled trial. BMC Psychol. 2013, 1, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezeamii, V.C.; Okobi, O.E.; Wambai-Sani, H.; Perera, G.S.; Zaynieva, S.; Okonkwo, C.C.; Ohaiba, M.M.; William-Enemali, P.C.; Obodo, O.R.; Obiefuna, N.G. Revolutionizing Healthcare: How Telemedicine Is Improving Patient Outcomes and Expanding Access to Care. Cureus 2024, 16, e63881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Darwish, T.; Korouri, S.; Pasini, M.; Cortez, M.V.; IsHak, W.W. Integration of Advanced Health Technology Within the Healthcare System to Fight the Global Pandemic: Current Challenges and Future Opportunities. Innov. Clin. Neurosci. 2021, 18, 31–34. [Google Scholar] [PubMed] [PubMed Central]
- Subramanian, M.; Wojtusciszyn, A.; Favre, L.; Boughorbel, S.; Shan, J.; Letaief, K.B.; Pitteloud, N.; Chouchane, L. Precision medicine in the era of artificial intelligence: Implications in chronic disease management. J. Transl. Med. 2020, 18, 472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manfro, P.H.; Anselmi, L.; Barros, F.; Gonçalves, H.; Murray, J.; Oliveira, I.O.; Tovo-Rodrigues, L.; Wehrmeister, F.C.; Menezes, A.M.B.; Mondelli, V.; et al. Youth depression and inflammation: Cross-sectional network analyses of C-Reactive protein, interleukin-6 and symptoms in a population-based sample. J. Psychiatr. Res. 2022, 150, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.M.; GholamHosseini, H.; Connolly, M.J. Mobile healthcare applications: System design review, critical issues and challenges. Australas. Phys. Eng. Sci. Med. 2015, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, S.; Szot-Karpińska, K.; Niedziółka-Jönsson, J.; de Marco, A.; Jönsson-Niedziółka, M. NFC Smartphone-Based Electrochemical Microfluidic Device Integrated with Nanobody Recognition for C-Reactive Protein. ACS Sens. 2024, 9, 3066–3074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahub, S.; Kumar, R.M.; Lin, K.C.; Banga, I.; Choi, N.K.; Garcia, N.M.; Muthukumar, S.; Rubin, D.T.; Prasad, S. Continuous Monitoring of CRP, IL-6, and Calprotectin in Inflammatory Bowel Disease Using a Perspiration-Based Wearable Device. Inflamm. Bowel Dis. 2024, 23, izae054. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Wu, J.; Yu, Z.; Bair, M.J.; Kean, J.; Stump, T.; Monahan, P.O. Patient Health Questionnaire Anxiety and Depression Scale: Initial Validation in Three Clinical Trials. Psychosom. Med. 2016, 78, 716–727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, V.V.; Villaflores, C.W.; Chuong, L.H.; Leuchter, R.K.; Kilaru, A.S.; Vangala, S.; Sarkisian, C.A. Association Between In-Person vs Telehealth Follow-up and Rates of Repeated Hospital Visits Among Patients Seen in the Emergency Department. JAMA Netw. Open. 2022, 5, e2237783. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.S.; Krowski, N.; Rodriguez, B.; Tran, L.; Vela, J.; Brooks, M. Telehealth and patient satisfaction: A systematic review and narrative analysis. BMJ Open 2017, 7, e016242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomes, N.; Pato, M.; Lourenço, A.R.; Datia, N. A Survey on Wearable Sensors for Mental Health Monitoring. Sensors 2023, 23, 1330. [Google Scholar] [CrossRef]
- Li, K.; Cardoso, C.; Moctezuma-Ramirez, A.; Elgalad, A.; Perin, E. Heart Rate Variability Measurement through a Smart Wearable Device: Another Breakthrough for Personal Health Monitoring? Int. J. Environ. Res. Public Health. 2023, 20, 7146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, S.-Y.; Cho, M.-K. Effects of Continuous Glucose Monitoring on Glycemic Control in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Lin, K.C.; Whang, J.; Shahub, S.; Churcher, N.K.M.; Helmus, D.; Muthukumar, S.; Sands, B.; Prasad, S. Longitudinal monitoring of IL-6 and CRP in inflammatory bowel disease using IBD-AWARE. Biosens. Bioelectron. X 2024, 16, 100435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernando, D.; Roca, S.; Sancho, J.; Alesanco, Á.; Bailón, R. Validation of the Apple Watch for Heart Rate Variability Measurements during Relax and Mental Stress in Healthy Subjects. Sensors 2018, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.C.; Lin, K.C.; Shahub, S.; Ramasubramanya, A.; Fagan, A.; Muthukumar, S.; Prasad, S.; Bajaj, J.S. A novel sweat sensor detects inflammatory differential rhythmicity patterns in inpatients and outpatients with cirrhosis. NPJ Digit. Med. 2024, 7, 382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koopman, F.A.; Chavan, S.S.; Miljko, S.; Grazio, S.; Sokolovic, S.; Schuurman, P.R.; Mehta, A.D.; Levine, Y.A.; Faltys, M.; Zitnik, R.; et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2016, 113, 8284–8289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindsay, E.K.; Marsland, A.L.; Cole, S.W.; Dutcher, J.M.; Greco, C.M.; Wright, A.G.C.; Brown, K.W.; Creswell, J.D. Mindfulness-Based Stress Reduction Reduces Proinflammatory Gene Regulation but Not Systemic Inflammation Among Older Adults: A Randomized Controlled Trial. Psychosom. Med. 2024, 86, 463–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Christopher, M.; Bowen, S.; Witkiewitz, K.; Grupe, D.; Goerling, R.; Hunsinger, M.; Oken, B.; Korecki, T.; Rosenbaum, N. A multisite feasibility randomized clinical trial of mindfulness-based resilience training for aggression, stress, and health in law enforcement officers. BMC Complement. Med. Ther. 2024, 24, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villalba, D.K.; Lindsay, E.K.; Marsland, A.L.; Greco, C.M.; Young, S.; Brown, K.W.; Smyth, J.M.; Walsh, C.P.; Gray, K.; Chin, B.; et al. Mindfulness training and systemic low-grade inflammation in stressed community adults: Evidence from two randomized controlled trials. PLoS ONE 2019, 14, e0219120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grammoustianou, A.; Saeidi, A.; Longo, J.; Risch, F.; Ionescu, A.M. Real-time detection of C-reactive protein in interstitial fluid using electrochemical impedance spectroscopy, towards wearable health monitoring. arXiv 2024, arXiv:2407.16734. Available online: https://arxiv.org/abs/2407.16734 (accessed on 20 January 2025).
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
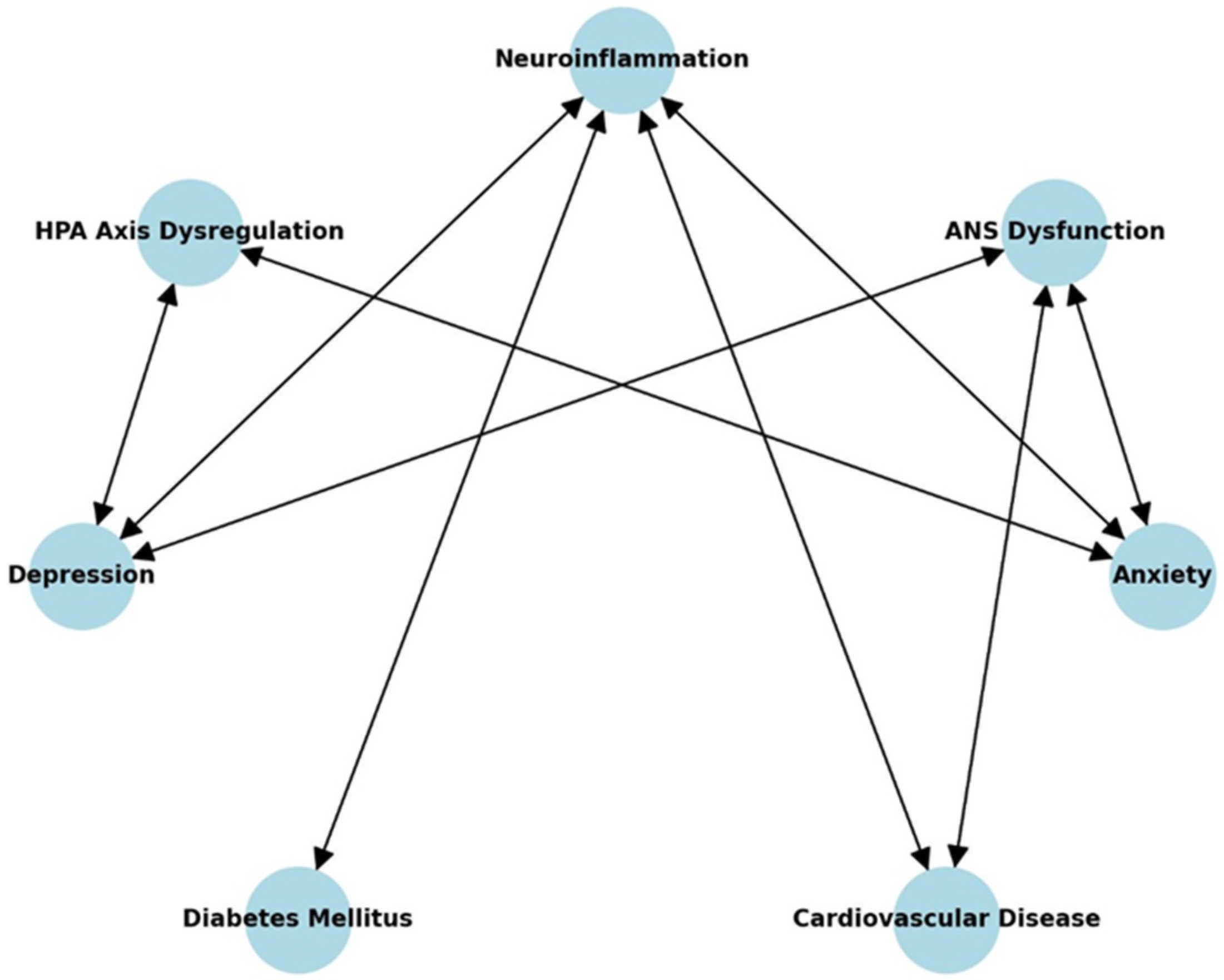

| Pathway | Mechanism | References |
|---|---|---|
| Neuroinflammation → HPA Axis Dysregulation | Chronic inflammation disrupts cortisol regulation | Mikulska et al., 2021 [7] |
| Neuroinflammation → ANS Dysfunction | Inflammation increases sympathetic activation | Kenwood et al., 2022 [8] |
| HPA Axis Dysregulation → Depression | Sustained cortisol elevation damages neural circuits | Herman et al., 2016 [9] |
| ANS Dysfunction → Anxiety | Autonomic imbalance amplifies stress responses | Kenwood et al., 2022 [8] |
| Depression → Cardiovascular Disease | Inflammation and autonomic dysfunction increase risk | Vaccarino et al., 2020 [10] |
| Anxiety → Cardiovascular Disease | Chronic stress and inflammation impair vascular function | Tuomisto et al., 2006 [11] |
| Depression → Diabetes Mellitus | Inflammatory cytokines worsen insulin resistance | Alzoubi et al., 2018 [12] |
| Pathway | Mechanism | References |
|---|---|---|
| IL-6 → Systemic Inflammation | IL-6 promotes chronic systemic inflammation and disrupts immune homeostasis | [30] |
| CRP → Systemic Inflammation | Elevated CRP levels are associated with increased risk of psychiatric and cardiovascular diseases | [31] |
| TNF-α → Systemic Inflammation | TNF-α induces pro-inflammatory cascades, exacerbating metabolic and psychiatric conditions | [32] |
| Systemic Inflammation → Anxiety | Systemic inflammation contributes to hyperactive stress responses and emotional dysregulation | [33] |
| Systemic Inflammation → Depression | Inflammation alters neurotransmitter systems and promotes depressive symptoms | [34] |
| Systemic Inflammation → Diabetes Mellitus | Inflammatory cytokines worsen insulin resistance, leading to metabolic dysfunction | [35] |
| Systemic Inflammation → ANS Dysfunction | Chronic inflammation disrupts autonomic nervous system balance, increasing cardiovascular risk | [36] |
| Systemic Inflammation → Cardiovascular Disease | Inflammation promotes endothelial dysfunction, increasing CVD risk | [37] |
| HPA Axis Dysregulation → Depression | Sustained cortisol elevation damages neural circuits, contributing to depression | [38] |
| ANS Dysfunction → Cardiovascular Disease | Autonomic dysfunction exacerbates vascular dysfunction and increases cardiovascular events | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cătălina, G.R.; Gheorman, V.; Gheorman, V.; Forțofoiu, M.-C. The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases. Healthcare 2025, 13, 837. https://doi.org/10.3390/healthcare13070837
Cătălina GR, Gheorman V, Gheorman V, Forțofoiu M-C. The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases. Healthcare. 2025; 13(7):837. https://doi.org/10.3390/healthcare13070837
Chicago/Turabian StyleCătălina, Grecu Ramona, Victor Gheorman, Veronica Gheorman, and Mircea-Cătălin Forțofoiu. 2025. "The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases" Healthcare 13, no. 7: 837. https://doi.org/10.3390/healthcare13070837
APA StyleCătălina, G. R., Gheorman, V., Gheorman, V., & Forțofoiu, M.-C. (2025). The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases. Healthcare, 13(7), 837. https://doi.org/10.3390/healthcare13070837







