Antioxidant Diets and Lifestyles Could Mitigate the Risk of Sarcopenia with Low Muscle Mass in Women: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. SLM Measurements
2.3. Oxidative Balance Score (Exposure)
2.4. Covariate Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
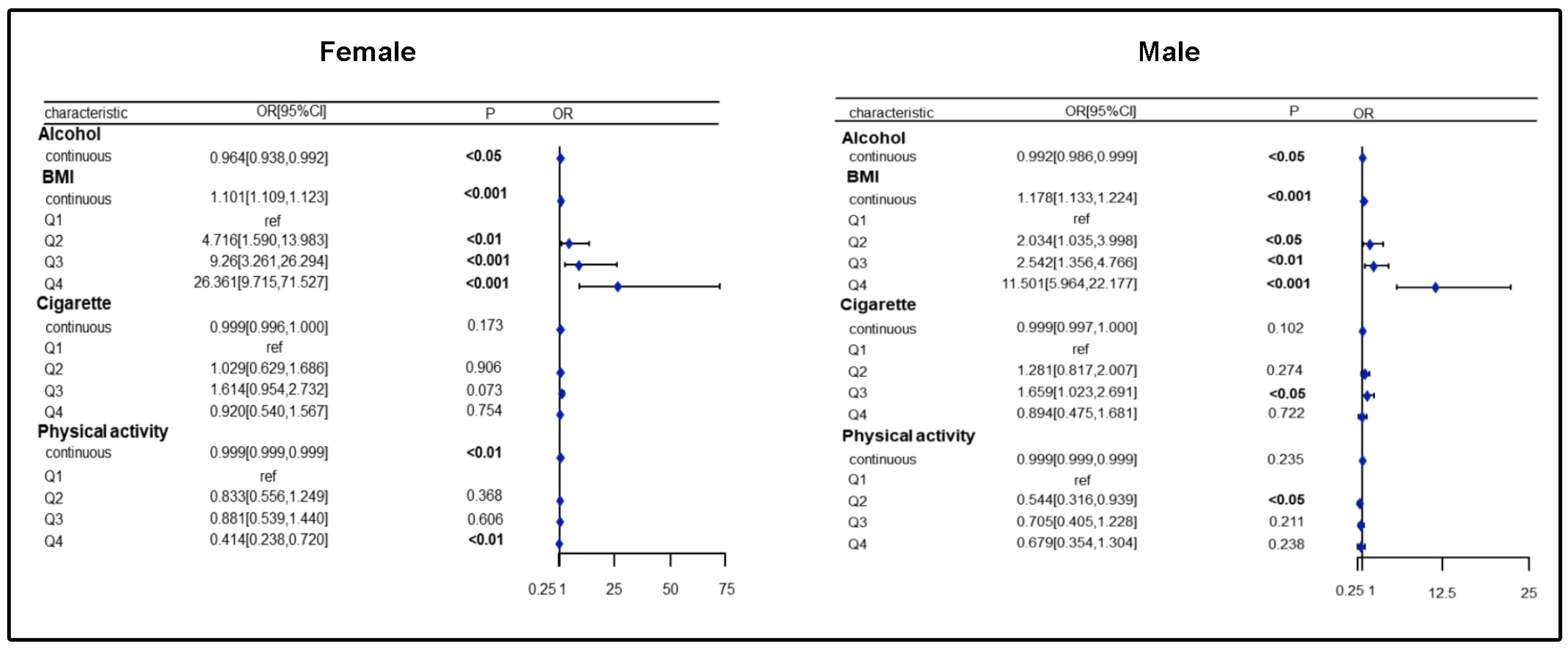
3.2. Association of Nutrients and Lifestyle with SLM Risk in Females/Males
3.3. Association of Different OBSs with SLM Risk Obtained by Gender-Stratified Analysis
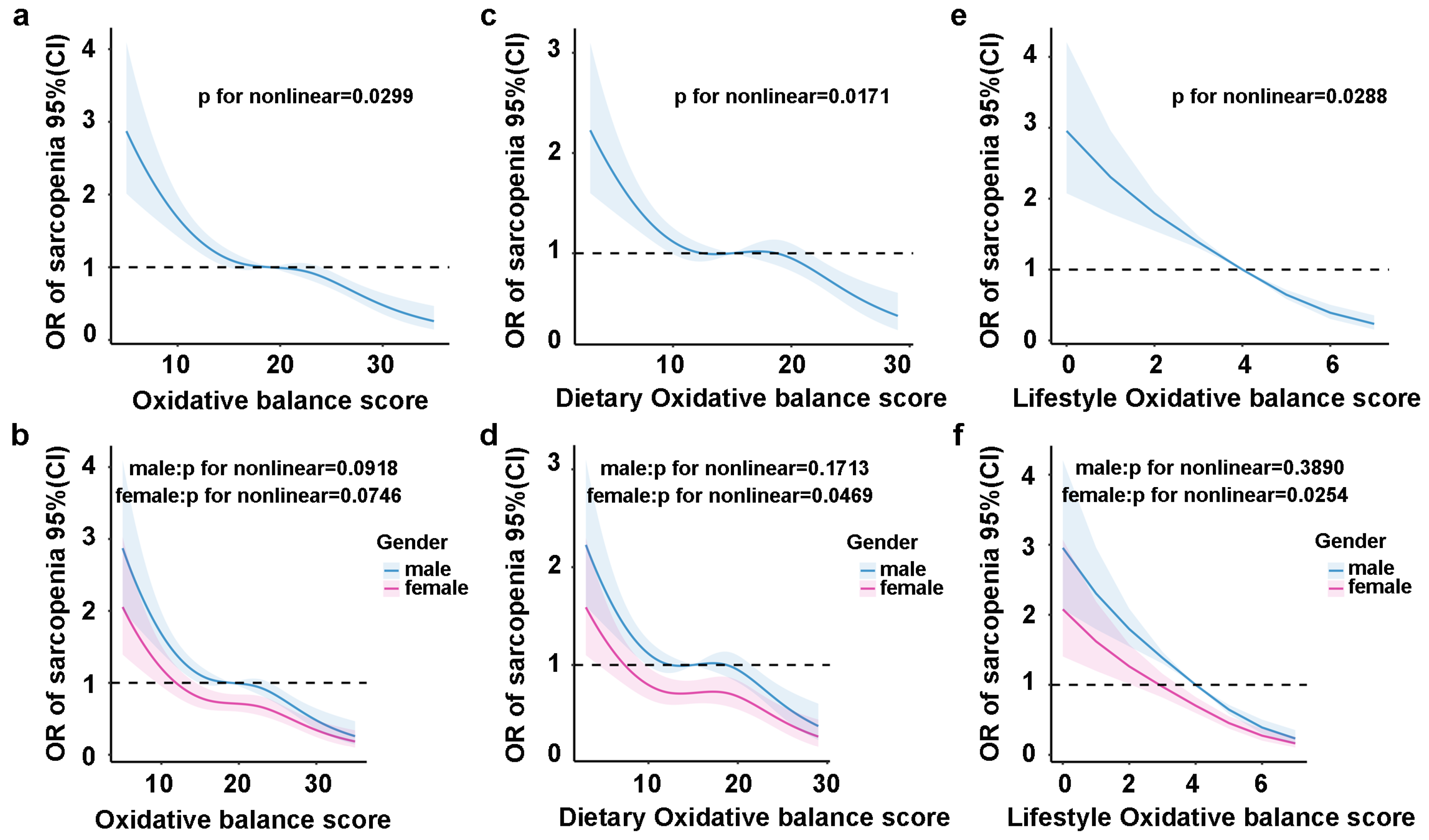
3.4. Restricted Cubic Spline Regression Analysis of the Nonlinear Relationship Between OBS and SLM Risk
3.5. Survival Analysis and Cox Regression Analysis of OBS and All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2021, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Hardee, J.P.; VanderVeen, B.N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin. Cell Dev. Biol. 2016, 54, 53–67. [Google Scholar] [CrossRef]
- Xu, J.; Han, X.; Chen, Q.; Cai, M.; Tian, J.; Yan, Z.; Guo, Q.; Xu, J.; Lu, H. Association between sarcopenia and prediabetes among non-elderly US adults. J. Endocrinol. Investig. 2023, 46, 1815–1824. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Yao, J.; Wang, Y. Prevalence of sarcopenia in patients with COPD through different musculature measurements: An updated meta-analysis and meta-regression. Front. Nutr. 2023, 10, 1137371. [Google Scholar] [CrossRef]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The rate and assessment of muscle wasting during critical illness: A systematic review and meta-analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Arcuri, C.; Giambanco, I.; Bellezza, I.; Minelli, A.; Donato, R. Cellular and molecular mechanisms of sarcopenia: The S100B perspective. J. Cachexia Sarcopenia Muscle 2018, 9, 1255–1268. [Google Scholar] [CrossRef]
- Paez, H.G.; Pitzer, C.R.; Alway, S.E. Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia. Cells 2023, 12, 249. [Google Scholar] [CrossRef]
- Li, C.w.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Giresi, P.G.; Stevenson, E.J.; Theilhaber, J.; Koncarevic, A.; Parkington, J.; Fielding, R.A.; Kandarian, S.C. Identification of a molecular signature of sarcopenia. Physiol. Genom. 2005, 21, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yao, J.; Tang, D.; Wang, H.; Zhang, H.; Qiu, J.; Shu, X. Dietary antioxidant capacity and sarcopenia: A study from US population. Nutrition 2025, 130, 112613. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.D.; Anuradha, C.V. Exercise, depletion of antioxidants and antioxidant manipulation. Cell Biochem. Funct. 1998, 16, 269–275. [Google Scholar] [CrossRef]
- Fingeret, M.; Vollenweider, P.; Marques-Vidal, P. No association between vitamin C and E supplementation and grip strength over 5 years: The Colaus study. Eur. J. Nutr. 2018, 58, 609–617. [Google Scholar] [CrossRef]
- Chen, W.; Xiong, B.; Liao, Z.; Xiao, M.; Chen, W. Association between dietary inflammatory index and low muscle mass in diabetes/prediabetes patients. Exp. Gerontol. 2023, 179, 112258. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Degens, H.; El Hajj Fuleihan, G.; Josse, R.; Lips, P.; Morales Torres, J.; Rizzoli, R.; et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos. Int. 2012, 24, 1555–1566. [Google Scholar] [CrossRef]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The Role of Dietary Protein Intake in the Prevention of Sarcopenia of Aging. Nutr. Clin. Pract. 2013, 28, 684–690. [Google Scholar] [CrossRef]
- Xu, W.; Mu, D.; Wang, Y.; Wang, Y.; Wang, C.; Zhang, X. Association between oxidative balance score and sarcopenia in US adults: NHANES 2011–2018. Front. Nutr. 2024, 11, 1342113. [Google Scholar] [CrossRef]
- Chen, K.; Yin, Q.; Guan, J.; Yang, J.; Ma, Y.; Hu, Y.; Chen, C.; Chen, W. Association between the oxidative balance score and low muscle mass in middle-aged US adults. Front. Nutr. 2024, 11, 1358231. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Orav, J.E.; Kanis, J.A.; Rizzoli, R.; Schlögl, M.; Staehelin, H.B.; Willett, W.C.; Dawson-Hughes, B. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos. Int. 2015, 26, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xue, Y.; Wen, H.; Chen, C. Association of oxidative balance score and lung health from the National Health and Nutrition Examination Survey 2007–2012. Front. Nutr. 2023, 9, 961950. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, S.-F.; Chen, L.; Chen, H.-M.; Cheng, X.-E.; Tang, Y.-H.; Mendoza-Núñez, V.M. Association between the Oxidative Balance Score and Telomere Length from the National Health and Nutrition Examination Survey 1999–2002. Oxidative Med. Cell. Longev. 2022, 2022, 1345071. [Google Scholar] [CrossRef]
- Chen, L.; Cai, M.; Li, H.; Wang, X.; Tian, F.; Wu, Y.; Zhang, Z.; Lin, H. Risk/benefit tradeoff of habitual physical activity and air pollution on chronic pulmonary obstructive disease: Findings from a large prospective cohort study. BMC Med. 2022, 20, 70. [Google Scholar] [CrossRef]
- Degens, H.; Gayan-Ramirez, G.; van Hees, H.W.H. Smoking-induced Skeletal Muscle Dysfunction. From Evidence to Mechanisms. Am. J. Respir. Crit. Care Med. 2015, 191, 620–625. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, W.; Fu, L.; Liu, H.; Jin, S.; Ye, X.; Pu, S.; Xue, Y. Muscle quality index and cardiovascular disease among US population-findings from NHANES 2011–2014. BMC Public Health 2023, 23, 2388. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obes. 2023, 16, 1541–1554. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e13. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, A.; Veronese, N.; Solmi, M.; Oh, H.; Shin, J.I.; Jacob, L.; Yang, L.; Haro, J.M.; Smith, L. Fruit and Vegetable Consumption and Sarcopenia among Older Adults in Low- and Middle-Income Countries. Nutrients 2020, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Muscariello, E.; La Rosa, G.; Di Maro, M.; Mondola, P.; Santillo, M. Dual Role of Reactive Oxygen Species in Muscle Function: Can Antioxidant Dietary Supplements Counteract Age-Related Sarcopenia? Int. J. Mol. Sci. 2019, 20, 3815. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Prud’homme, D.; Rabasa-Lhoret, R.; Strychar, I.; Brochu, M.; Lavoie, J.M.; Doucet, É. Effects of the menopausal transition on dietary intake and appetite: A MONET Group Study. Eur. J. Clin. Nutr. 2013, 68, 271–276. [Google Scholar] [CrossRef]
- Legget, K.T.; Cornier, M.-A.; Sarabia, L.; Delao, E.M.; Mikulich-Gilbertson, S.K.; Natvig, C.; Erpelding, C.; Mitchell, T.; Hild, A.; Kronberg, E.; et al. Sex Differences in Effects of Mood, Eating-Related Behaviors, and BMI on Food Appeal and Desire to Eat: A Cross-Sectional Survey Study. Nutrients 2023, 15, 762. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Mikulic, P. Effects of plyometric vs. resistance training on skeletal muscle hypertrophy: A review. J. Sport Health Sci. 2021, 10, 530–535. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, L.; Zhang, Y.; Dang, K.; Cheng, L.; Zhang, J.; Xu, X.; Li, Y. Association of Dietary Inflammatory Index and Dietary Oxidative Balance Score with All-Cause and Disease-Specific Mortality: Findings of 2003–2014 National Health and Nutrition Examination Survey. Nutrients 2023, 15, 3148. [Google Scholar] [CrossRef]
- Mao, Z.; Prizment, A.E.; Lazovich, D.; Bostick, R.M. Associations of dietary and lifestyle oxidative balance scores with mortality risk among older women: The Iowa Women’s Health Study. Eur. J. Nutr. 2021, 60, 3873–3886. [Google Scholar] [CrossRef]
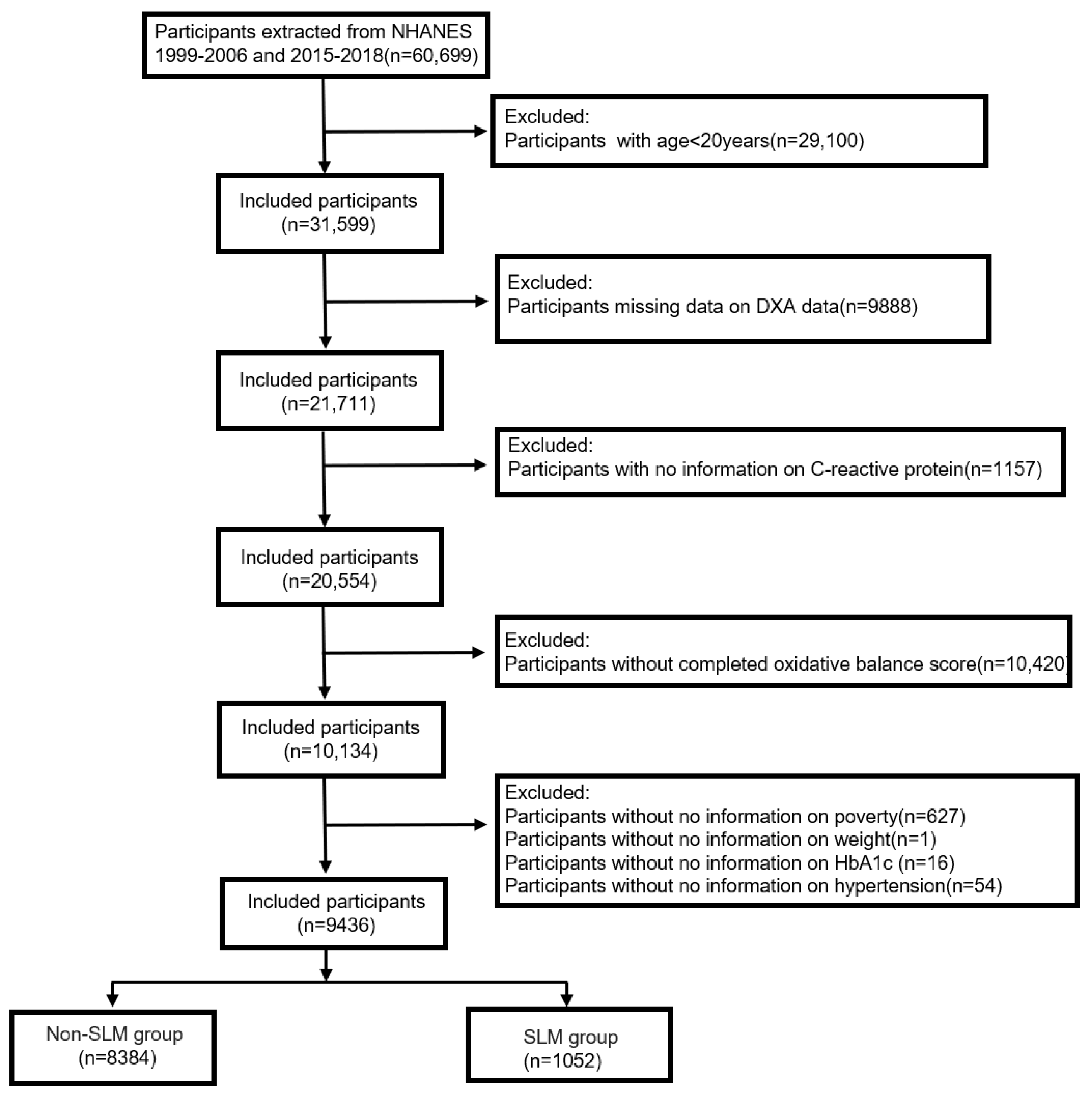


| Characteristic | Overall, N = 9436 | Quartile of OBS | p Value | |||
|---|---|---|---|---|---|---|
| Q1, N = 2770 | Q2, N = 2470 | Q3, N = 2354 | Q4, N = 1842 | |||
| Age (year), Mean (S.E) | 43.94 (0.32) | 44.16 (0.54) | 44.23 (0.51) | 44.22 (0.51) | 43.06 (0.46) | 0.15 |
| Gender, n (%) | >0.9 | |||||
| Female | 4532 (49.45) | 1336 (50.29) | 1182 (49.07) | 1144 (49.20) | 870 (49.21) | |
| Male | 4904 (50.55) | 1434 (49.71) | 1288 (50.93) | 1210 (50.80) | 972 (50.79) | |
| Race, n (%) | <0.001 | |||||
| Non-Hispanic White | 5209 (75.81) | 1322 (69.21) | 1300 (72.14) | 1418 (80.07) | 1169 (82.15) | |
| Non-Hispanic Black | 1769 (9.66) | 744 (15.31) | 471 (10.86) | 327 (6.70) | 227 (5.57) | |
| Mexican American | 1748 (6.12) | 489 (5.87) | 497 (7.07) | 429 (5.65) | 333 (5.93) | |
| Other Hispanic | 368 (3.32) | 112 (3.39) | 108 (4.55) | 93 (2.90) | 55 (2.38) | |
| Other/multiracial | 342 (5.09) | 103 (6.22) | 94 (5.37) | 87 (4.70) | 58 (3.98) | |
| Education, n (%) | <0.001 | |||||
| Less Than 9th Grade | 902 (3.80) | 381 (6.72) | 255 (4.16) | 180 (2.79) | 86 (1.37) | |
| 9–11th Grade | 1223 (8.39) | 459 (11.05) | 321 (9.25) | 277 (7.44) | 166 (5.60) | |
| High School Grad/GED | 2220 (23.71) | 707 (27.33) | 619 (25.46) | 533 (24.13) | 361 (17.34) | |
| Some College or AA degree | 2772 (32.34) | 810 (34.84) | 723 (32.44) | 717 (32.06) | 522 (29.77) | |
| College Graduate or above | 2311 (31.76) | 412 (20.05) | 548 (28.68) | 646 (33.58) | 705 (45.92) | |
| PIR, n (%) | <0.001 | |||||
| <1.3 | 2110 (15.36) | 792 (21.80) | 571 (15.82) | 442 (12.51) | 305 (11.06) | |
| >3.5 | 3767 (50.52) | 851 (40.01) | 947 (49.64) | 1,039 (53.57) | 930 (59.56) | |
| 1.3–3.5 | 3559 (34.12) | 1127 (38.19) | 952 (34.54) | 873 (33.93) | 607 (29.38) | |
| CRP, Mean (S.E) | 0.40 (0.01) | 0.50 (0.03) | 0.38 (0.02) | 0.39 (0.02) | 0.33 (0.03) | <0.001 |
| HbA1c (%) | 5.40 (0.01) | 5.51 (0.04) | 5.42 (0.03) | 5.37 (0.02) | 5.30 (0.03) | <0.001 |
| SLM, n (%) | <0.001 | |||||
| Yes | 1052 (7.25) | 417 (11.32) | 307 (8.36) | 225 (5.81) | 103 (3.21) | |
| No | 8483 (92.75) | 2353 (88.68) | 2163 (91.64) | 2129 (94.19) | 1739 (96.79) | |
| Smoke, n (%) | <0.001 | |||||
| Yes | 4502 (47.69) | 1499 (55.03) | 1201 (47.69) | 1082 (45.54) | 720 (42.06) | |
| No | 4934 (52.31) | 1271 (44.97) | 1269 (52.31) | 1272 (54.46) | 1122 (57.94) | |
| Hypertension, n (%) | <0.001 | |||||
| Yes | 2736 (26.29) | 954 (31.62) | 706 (25.14) | 656 (26.02) | 420 (21.96) | |
| No | 6700 (73.71) | 1816 (68.38) | 1764 (74.86) | 1698 (73.98) | 1422 (78.04) | |
| Diabetes mellitus, n (%) | 0.002 | |||||
| Yes | 914 (6.93) | 332 (9.29) | 259 (7.14) | 198 (6.31) | 125 (4.82) | |
| No | 8522 (93.07) | 2438 (90.71) | 2211 (92.86) | 2156 (93.69) | 1717 (95.18) | |
| Q1 | Q2 (OR 95%CI) | p | Q3 (OR 95%CI) | p | Q4 (OR 95%CI) | p | p for Trend | p for Interaction | |
|---|---|---|---|---|---|---|---|---|---|
| Total OBS | |||||||||
| Unadjusted | |||||||||
| Female | Ref | 0.97 (0.64, 1.46) | 0.882 | 0.67 (0.43, 1.05) | 0.082 | 0.31 (0.17, 0.55) | <0.001 | <0.001 | 0.015 |
| Male | Ref | 0.55 (0.38, 0.82) | 0.003 | 0.36 (0.23, 0.57) | <0.001 | 0.23 (0.13, 0.39) | <0.001 | <0.001 | |
| Adjusted | |||||||||
| Female | Ref | 0.94 (0.54, 1.62) | 0.808 | 0.71 (0.42, 1.21) | 0.202 | 0.34 (0.19, 0.61) | 0.003 | 0.001 | 0.219 |
| Male | Ref | 0.59 (0.40, 0.86) | 0.007 | 0.43 (0.26, 0.72) | 0.002 | 0.35 (0.19, 0.65) | 0.001 | <0.001 | |
| Dietary OBS | |||||||||
| Unadjusted | |||||||||
| Female | Ref | 1.05 (0.68, 1.63) | 0.827 | 0.75 (0.49, 1.15) | 0.189 | 0.50 (0.31, 0.81) | 0.006 | 0.006 | 0.008 |
| Male | Ref | 0.53 (0.36, 0.77) | 0.001 | 0.47 (0.30, 0.74) | 0.002 | 0.25 (0.15, 0.42) | <0.001 | <0.001 | |
| Adjusted | |||||||||
| Female | Ref | 0.95 (0.56, 1.59) | 0.833 | 0.78 (0.47, 1.29) | 0.319 | 0.54 (0.34,0.86) | 0.010 | 0.018 | 0.159 |
| Male | Ref | 0.58 (0.40, 0.86) | 0.007 | 0.56 (0.34, 0.91) | 0.022 | 0.41 (0.24, 0.70) | 0.002 | 0.001 | |
| Lifestyle OBS | |||||||||
| Unadjusted | |||||||||
| Female | Ref | 0.66 (0.47, 0.93) | 0.018 | 0.53 (0.37, 0.76) | <0.001 | 0.13 (0.07, 0.26) | <0.001 | <0.001 | 0.941 |
| Male | Ref | 0.57 (0.36, 0.90) | 0.017 | 0.51 (0.32, 0.81) | 0.005 | 0.29 (0.15, 0.56) | <0.001 | <0.001 | |
| Adjusted | |||||||||
| Female | Ref | 0.60 (0.42, 0.86) | 0.007 | 0.45 (0.32, 0.63) | <0.001 | 0.14 (0.07, 0.29) | <0.001 | <0.001 | 0.795 |
| Male | Ref | 0.56 (0.34, 0.92) | 0.023 | 0.45 (0.28, 0.72) | 0.001 | 0.25 (0.12, 0.51) | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Y.; Li, Q.; Liu, W.; Wu, Y. Antioxidant Diets and Lifestyles Could Mitigate the Risk of Sarcopenia with Low Muscle Mass in Women: A Retrospective Study. Healthcare 2025, 13, 910. https://doi.org/10.3390/healthcare13080910
Li S, Zhang Y, Li Q, Liu W, Wu Y. Antioxidant Diets and Lifestyles Could Mitigate the Risk of Sarcopenia with Low Muscle Mass in Women: A Retrospective Study. Healthcare. 2025; 13(8):910. https://doi.org/10.3390/healthcare13080910
Chicago/Turabian StyleLi, Shanshan, Yiqiong Zhang, Qian Li, Wenjun Liu, and Yue Wu. 2025. "Antioxidant Diets and Lifestyles Could Mitigate the Risk of Sarcopenia with Low Muscle Mass in Women: A Retrospective Study" Healthcare 13, no. 8: 910. https://doi.org/10.3390/healthcare13080910
APA StyleLi, S., Zhang, Y., Li, Q., Liu, W., & Wu, Y. (2025). Antioxidant Diets and Lifestyles Could Mitigate the Risk of Sarcopenia with Low Muscle Mass in Women: A Retrospective Study. Healthcare, 13(8), 910. https://doi.org/10.3390/healthcare13080910






