Integrating Lean Six Sigma into Microbiology Laboratories: Insights from a Literature Review
Abstract
1. Introduction
- RQ-1.—How are KPIs determined, and what data are utilized to select the KPIs and tools for LSS healthcare projects?
- RQ-2.—Do the LSS healthcare projects described in the literature use the control phase as part of a DMAIC process? How important is this stage in healthcare?
- RQ-3.—Are the clinical parameters a part of the LSS optimization process?
- RQ-4.—Is there any standardized system implemented for managing interruption and priorities inside clinical laboratory processes?
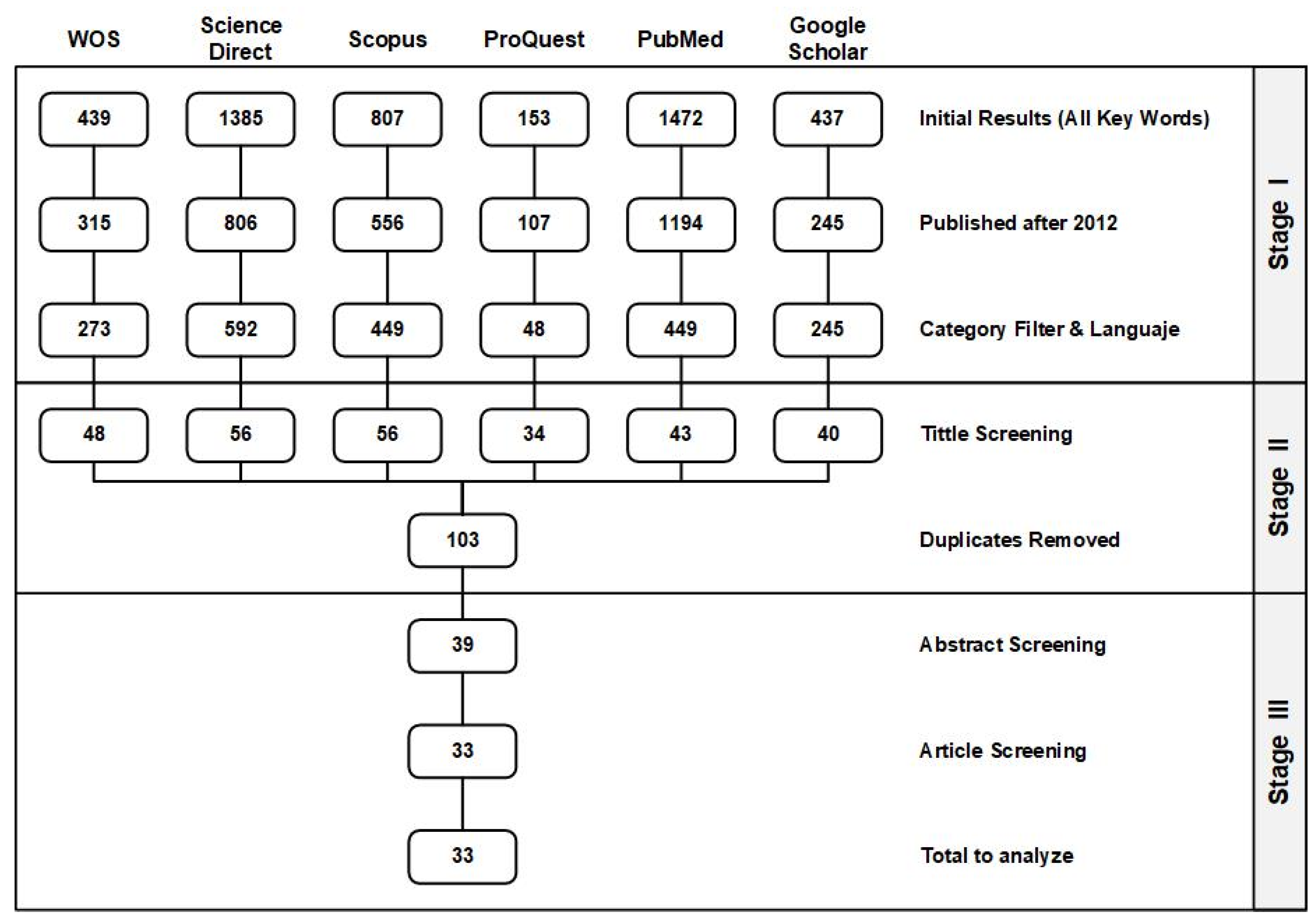
2. Methodology
3. Findings and Results
3.1. Stage I. Initial Results, Publication Year, Language, and Subject Filters
3.2. Stage II: Title Screening
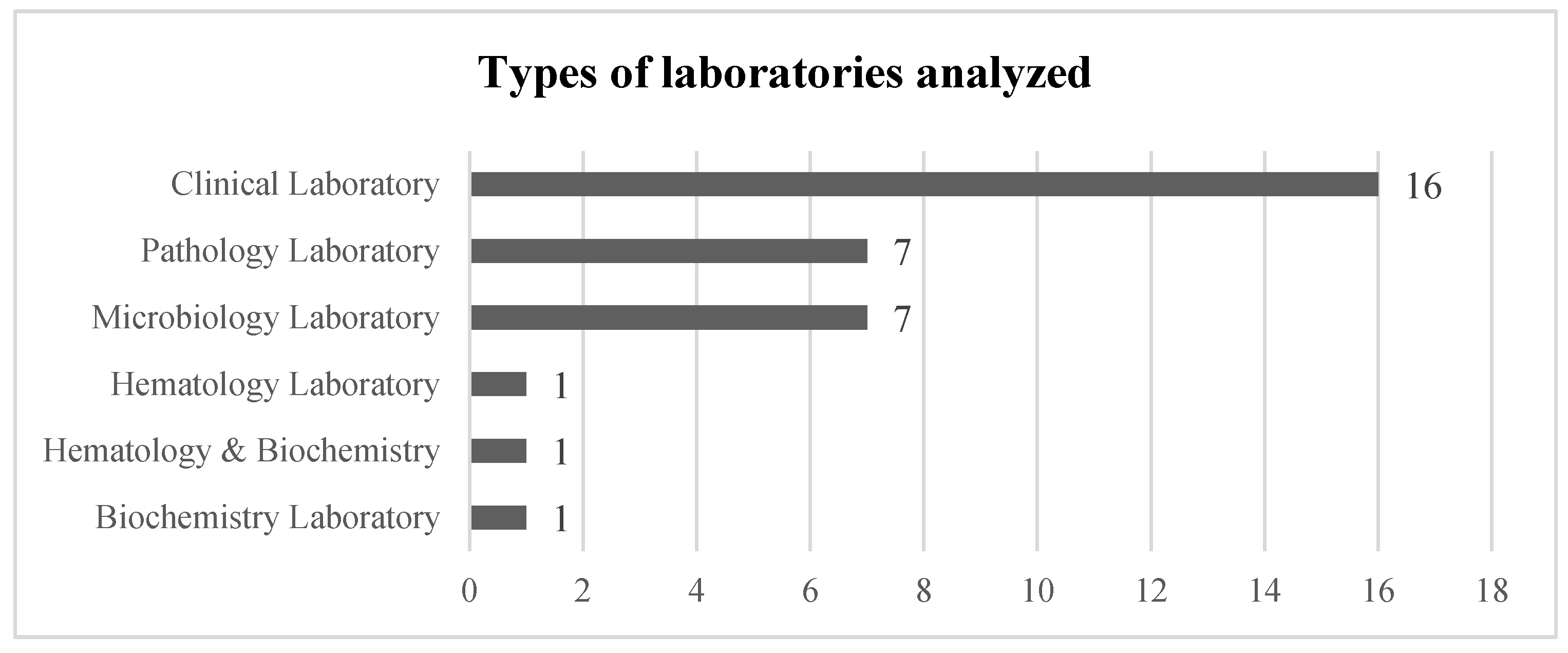
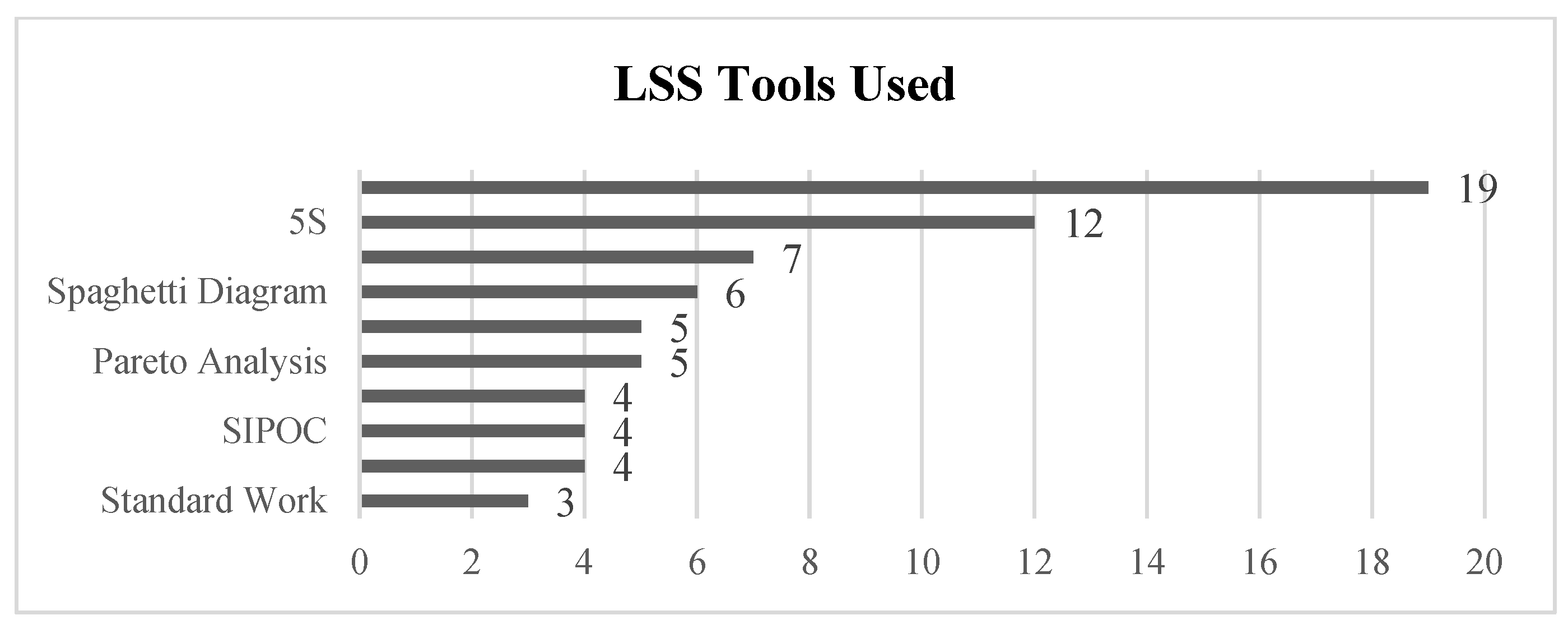
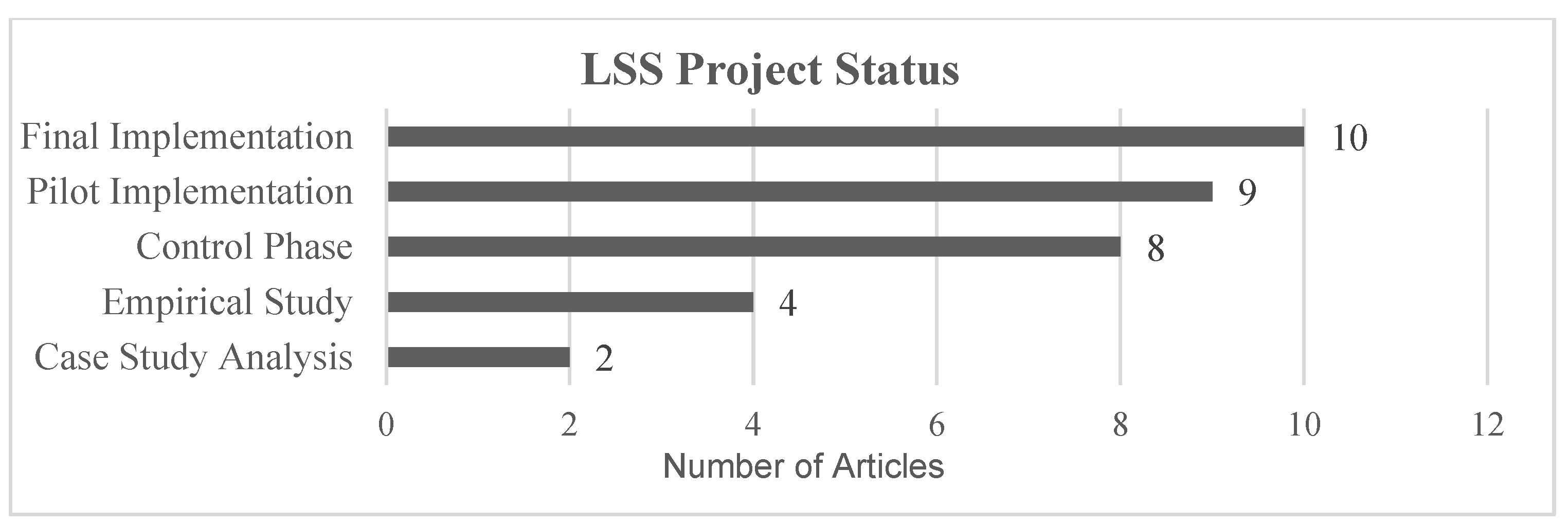
3.3. Stage III: Abstract and Article Analysis
- -
- Empirical Study: the theoretical study of the advantages of LSS, or methodology implementation for a specific field.
- -
- Case Study: an optimization problem is identified in a real environment, presenting the article as a theoretical analysis of LSS tools application to improve the process.
- -
- Pilot Implementation: the process analyzed in the article has been optimized in a real environment and KPIs were measured, but no plan has been established for implementation as a permanent workflow.
- -
- Final Implementation: a permanent optimization workflow was established after process analysis.
- -
- Control Phase: the project was implemented in a real environment, with processes in place to detect deviations in KPIs. In some cases, the author outlined techniques to correct these deviations.
4. Discussion
- RQ-1.—How are KPIs determined, and what data are utilized to select the KPIs and tools for healthcare LSS projects?
- RQ-2.—Do the projects described in the literature use the control phase as part of a DMAIC process? How important is this stage?
- RQ-3.—Are the clinical parameters a part of the LSS optimization?
- RQ-4.—Is there any system for managing interruption and priorities inside the process?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Appendix A
| Title | Year | LSS Tools Used | KPIs (Improved Value) | Laboratory Type | Project Status | |
|---|---|---|---|---|---|---|
| 1 | “Utilization of 5S Visual Management: A Lean Six Sigma Application in Operation of Hospital Clinical Laboratory” [26] | 2013 | - 5S | - Turnaround time (improved >25%) - Cost reduction (revenue +30%) | Clinical Laboratory | Final Implementation |
| 2 | “Adoption of lean principles in a high-volume molecular diagnostic microbiology laboratory” [27] | 2014 | - Value Stream Map - Waste reduction | - Turnaround time (>85% results in <24 h) - Cost reduction (not specified) | Microbiology Laboratory | Control Phase |
| 3 | “Adoption of lean tools in medical laboratory industry: A case study of Namibia” [54] | 2017 | - Standardized work - PDCA - 5S - Value Stream Map | - Turnaround time (N/A—Empirical Study) | Clinical Laboratory | Empirical Study |
| 4 | “Application of lean management systems in pathology laboratory work process and laboratory environment” [28] | 2019 | - 5S - Value Stream Map | - Rejection sigma level of the sample (from 3.61 to 3.9) - Turnaround time (improved by 30%) - Process time (improved by 67%) | Pathology Laboratory | Pilot |
| 5 | “Applying lean flows in pathology laboratory remodeling” [47] | 2014 | - Spaghetti diagram - Value Stream Map | - Turnaround time (not specified) - Cost reduction (not specified) - Time saving (eliminated 16.25 min per day shift) - Test capacity (increased by 20%) | Pathology Laboratory | Final Implementation |
| 6 | “Applying Lean methodologies reduces ED laboratory turnaround times” [90] | 2015 | - Spaghetti diagram - Value Stream Map | - Turnaround time (reduced up to 12) - Cost reduction (reduced $62.400/year) | Clinical Laboratory | Final Implementation |
| 7 | “Conversion of a classical microbiology laboratory to a total automation laboratory enhanced by the application of lean principles” [30] | 2024 | - Spaghetti diagram - Process mapping - Time and Motion - A3 - 5S - Standardized work | - Turnaround time (reduced up to 89%) - Test capacity (increased by 124%) | Microbiology Laboratory | Control Phase |
| 8 | “Experience of lean six sigma quality approach to hospital laboratory services” [48] | 2015 | - Value Stream Map | - Patient waiting time - Check-in time - Number of queue abandonments - Number of front-office check-ins (not specified) | Clinical Laboratory | Pilot |
| 9 | “Externalities of lean implementation in medical laboratories. Process optimization vs. adaptation and flexibility for the future” [67] | 2021 | - Turnaround time - Accuracy and reliability of results (not specified) | Clinical Laboratory | Empirical Study | |
| 10 | “Impact of lean interventions on time buffer reduction in a hospital setting” [55] | 2017 | - 5S - Pareto analysis - Variability reduction | - Turnaround time - Variability (not specified) | Clinical Laboratory | Control Phase |
| 11 | “Improvement of laboratory turnaround time using Lean methodology” [57] | 2018 | - 5S - Value Stream Map - Pareto analysis | - Turnaround time (reduced up to 10 min) | Hematology and Biochemistry | Final Implementation |
| 12 | “Improvement of urgent tests laboratory turnaround time through laboratory lean management” [56] | 2020 | - Waste analysis - Visual control - Standardized work - 5S - Kanban | - Turnaround time (reduced by 4 min) | Clinical Laboratory | Final Implementation |
| 13 | “Improving the efficiency of the center for medical biochemistry, clinical center ni[, by applying lean Six Sigma methodology” [29] | 2014 | - Waste analysis - 5S - Value Stream Map - Pareto analysis | - Turnaround time (reduction >45%) - Patient waiting time (reduction up to 75%) - Reducing congestion in the laboratory | Biochemistry Laboratory | Pilot |
| 14 | “Integrating Lean and Automation for Enhanced Serology Diagnosis Efficiency in Tertiary Healthcare Microbiology Laboratories” [52] | 2024 | - VSM | - Turnaround time (Reduction up to 87.3%) - Cost reduction (reduced >7.1%) - Test per day (66% more test per day) | Microbiology Laboratory | Final Implementation |
| 15 | “Lean healthcare as a tool for improvement: A case study in a clinical laboratory” [49] | 2017 | - Value Stream Map | - Patient waiting time (not specified) | Clinical Laboratory | Pilot |
| 16 | “Lean methodology for pathology laboratories: A case study from a public hospital” [63] | 2019 | - Value Stream Map - Fishbone diagram - Pareto analysis - 5S - Visual management - Standardized eork | - Patient waiting time (improvement of 4.6%) - Turnaround time (reduced by 67%) | Pathology Laboratory | Pilot |
| 17 | “Lean Six Sigma in Laboratory Process” [59] | 2023 | - DMAIC - SIPOC - CTQ - Pareto analysis - Fishbone diagram - Whys analysis - Value Stream Map | - Cost reduction (reduction of 40%) - Turnaround time (reduction of 40%) | Microbiology Laboratory | Control Phase |
| 18 | “Lean six sigma methodologies improve clinical laboratory efficiency and reduce turnaround times” [81] | 2018 | - DMAIC | - Turnaround time (Reduced by 9 min) - Reducing errors (Reduced from 30% to 3%) | Clinical Laboratory | Final Implementation |
| 19 | “Lean thinking in hospital: Case study at the pathology laboratory” [91] | 2015 | - Waste analysis - Spaghetti diagram - Value Stream Map | - Takt time - Error minimization (not Specified) | Pathology Laboratory | Pilot |
| 20 | “Lean-Agile adaptations in clinical laboratory accredited ISO 15189” [53] | 2015 | - 5S | - Cost Reduction (2 technicians fewer) - Turnaround time (no variation after cost reduction) | Clinical Laboratory | Control Phase |
| 21 | “Optimizing the Supply Chain of Intensive Care Unit Blood Culture Samples to Clinical Microbiology Laboratory Using Lean Six Sigma” [83] | 2023 | - Waste analysis - Value Stream Map - Process activity analysis | - Turnaround time (reduced >55%) | Microbiology Laboratory | Final Implementation |
| 22 | “Preliminary results of Lean method implementation in a pathology lab from Northeastern Brazil” [50] | 2015 | - Value Stream Map - Spaghetti diagram - A3 | - Patient waiting time (reduced 55%) - % of correct and completed task. (improved 122.2%) | Pathology Laboratory | Pilot |
| 23 | “Providing critical laboratory results on time, every time to help reduce emergency department length of stay: How our laboratory achieved a six sigma level of performance” [65] | 2013 | - Automation with LSS philosophy | - Turnaround time - Patient length of stay - Return of investment (not Specified) - Productivity (improvement of 35%) | Clinical Laboratory | Final Implementation |
| 24 | “Reducing patient waiting time in a pathology department using the Six Sigma methodology” [64] | 2013 | - DMAIC - CTQ - SIPOC - C&E diagram - PCA - Gemba Walk | - Patient waiting time (reduced by 50%) | Pathology Laboratory | Control Phase |
| 25 | “Reducing the turnaround time of laboratory samples by using Lean Six Sigma methodology in a tertiary-care hospital in India” [69] | 2020 | - DMAIC - CTQ - SIPOC - Value Stream Map - I-MR - Fishbone diagram | - Turnaround time (reduced by 49%) | Clinical Laboratory | Case Study |
| 26 | “Reducing turnaround time for routine outpatient biochemical tests through Lean Six Sigma: A case study in China” [92] | 2024 | - DMAIC - VSM - VOC - Pareto analysis | - Turnaround time (reduced by 59%) | Clinical Laboratory | Control Phase |
| 27 | “Reducing turnaround time in a pathology laboratory using the lean methodology.” [31] | 2023 | - Process flowcharts - Fishbone diagram - Kaizen - A3 - Spaghetti diagram | - Turnaround time (reduced from 9.7 to 9 h) | Pathology Laboratory | Pilot |
| 28 | “The Impact of Lean in Medical Laboratory Industry: Empirical Studies in Namibia” [54] | 2017 | - SOP - Root cause analysis - Visual management - 5S - PDCA - Kanban - Value Stream Map | - Turnaround time - Quality improvement - Market share - Cost reduction - Reduced waste (not specified) | Clinical Laboratory | Empirical Study |
| 29 | “The Optimization of Total Laboratory Automation by Simulation of a Pull-Strategy” [51] | 2015 | - Value Stream Map | - Turnaround time (54.5% time saving) | Clinical Laboratory | Case Study |
| 30 | “The role of the clinical laboratory in the future of health care: Lean microbiology” [68] | 2014 | - PDCA - 5S | - Number of corrected reports. - Turnaround time (not specified) | Microbiology Laboratory | Empirical Study |
| 31 | “Using Lean Six Sigma to improve timeliness of clinical laboratory test results in a university hospital in Egypt” [93] | 2022 | - DMAIC - Spaghetti diagram - Why-why | - Turnaround time (not specified) | Hematology Laboratory | Control Phase |
| 32 | “Vision of a Microbiology Laboratory of Excellence using Lean” [58] | 2018 | - Seven wastes - DMADV - SIPOC - Process map - Fishbone diagram - CTQ - Value Stream Map - Pareto diagram | - Delay of results - Waiting time - Physical space needs (not specified) | Microbiology Laboratory | Final Implementation |
| 33 | “Workflow optimization in a clinical laboratory using Lean management principles in the pre-analytical phase” [32] | 2021 | - Lean philosophy | - Turnaround time (reduction of 13%) | Clinical Laboratory | Pilot |
References
- Patel, A.S.; Patel, K.M. Critical Review of Literature on Lean Six Sigma Methodology. Int. J. Lean Six Sigma 2020, 12, 627–674. [Google Scholar] [CrossRef]
- Narusawa, T.; Shook, J. Kaizen Express—Fundamentals for Your Lean Journey; Borton, The Lean Enterprise Institute: Boston, MA, USA, 2009. [Google Scholar]
- Muraliraj, J.; Zailani, S.; Kuppusamy, S.; Santha, C. Annotated Methodological Review of Lean Six Sigma. Int. J. Lean Six Sigma 2018, 9, 2–49. [Google Scholar] [CrossRef]
- Siregar, K.; Ariani, F.; Ginting, E.; Dinda, M.P.T. Lean Six Sigma for Manufacturing Industry: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 505, 012056. [Google Scholar] [CrossRef]
- Costa, L.B.M.; Godinho Filho, M.; Fredendall, L.D.; Paredes Gomez, F.J. Lean, Six Sigma and Lean Six Sigma in the Food Industry: A Systematic Literature Review. Trends Food Sci. Technol. 2018, 82, 122–133. [Google Scholar] [CrossRef]
- Thomas, A.J.; Francis, M.; Fisher, R.; Byard, P. Implementing Lean Six Sigma to Overcome the Production Challenges in an Aerospace Company. Prod. Plan. Control. 2016, 27, 591–603. [Google Scholar] [CrossRef]
- da Silva, I.B.; Cabeça, M.G.; Barbosa, G.F.; Shiki, S.B. Lean Six Sigma for the Automotive Industry through the Tools and Aspects within Metrics: A Literature Review. Int. J. Adv. Manuf. Technol. 2022, 119, 1357–1383. [Google Scholar] [CrossRef]
- Teich, S.; Faddoul, F. Lean Management—The Journey from Toyota to Healthcare. Rambam Maimonides Med. J. 2013, 4, e0007. [Google Scholar] [CrossRef]
- Sony, M.; Antony, J.; McDermott, O. The Impact of Healthcare 4.0 on the Healthcare Service Quality: A Systematic Literature Review. Hosp. Top. 2023, 101, 288–304. [Google Scholar] [CrossRef]
- Lee, J.Y.; McFadden, K.L.; Lee, M.K.; Gowen, C.R. U.S. Hospital Culture Profiles for Better Performance in Patient Safety, Patient Satisfaction, Six Sigma, and Lean Implementation. Int. J. Prod. Econ. 2021, 234, 108047. [Google Scholar] [CrossRef]
- Cosio-Gil, Y.; Omara, M.; Watson, C.; Casey, J.; Chakhunashvili, A.; Miguel, M.G.-S.; Kahlem, P.; Keuchkerian, S.; Kirchberger, V.; Luce-Garnier, V.; et al. The Roadmap for Implementing Value-Based Healthcare in European University Hospitals—Consensus Report and Recommendations. Value Health 2022, 25, 1148–1156. [Google Scholar] [CrossRef]
- Bianchini de Barros, L.; de Camargo Bassi, L.; Caldas, L.P.; Sarantopoulos, A.; Zeferino, E.B.B.; Minatogawa, V.; Gasparino, R.C. Lean Healthcare Tools for Processes Evaluation: An Integrative Review. Int. J. Environ. Res. Public Health 2021, 18, 7389. [Google Scholar] [CrossRef] [PubMed]
- Narayanamurthy, G.; Gurumurthy, A.; Subramanian, N.; Moser, R. Assessing the Readiness to Implement Lean in Healthcare Institutions—A Case Study. Int. J. Prod. Econ. 2018, 197, 123–142. [Google Scholar] [CrossRef]
- Shortell, S.M.; Blodgett, J.C.; Rundall, T.G.; Henke, R.M.; Reponen, E. Lean Management and Hospital Performance: Adoption vs. Implementation. Jt. Comm. J. Qual. Patient Saf. 2021, 47, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Lugo, C.; Tlapa, D.; Baez-Lopez, Y.; Limon-Romero, J.; Ontiveros, S.; Perez-Sanchez, A.; Tortorella, G. Assessing the Impact of Lean Healthcare on Inpatient Care: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5609. [Google Scholar] [CrossRef]
- Schonberger, R.J. Reconstituting Lean in Healthcare: From Waste Elimination toward ‘Queue-Less’ Patient-Focused Care. Bus. Horiz. 2018, 61, 13–22. [Google Scholar] [CrossRef]
- Tlapa, D.; Zepeda-Lugo, C.A.; Tortorella, G.L.; Baez-Lopez, Y.A.; Limon-Romero, J.; Alvarado-Iniesta, A.; Rodriguez-Borbon, M.I. Effects of Lean Healthcare on Patient Flow: A Systematic Review. Value Health 2020, 23, 260–273. [Google Scholar] [CrossRef]
- Oliveira, R.T.K.; Gohr, C.F.; Santos, L.C. Implementação do lean healthcare: Experiências e lições aprendidas em hospitais brasileiros. Rev. Adm. Empresas 2018, 58, 30–43. [Google Scholar] [CrossRef]
- Sales Coll, M.; De Castro, R.; de Echagüen, A.O.; Ibáñez, V.M. Economic Impact of Lean Healthcare Implementation on the Surgical Process. Healthcare 2024, 12, 512. [Google Scholar] [CrossRef]
- Kam, A.W.; Collins, S.; Park, T.; Mihail, M.; Stanaway, F.F.; Lewis, N.L.; Polya, D.; Fraser-Bell, S.; Roberts, T.V.; Smith, J.E.H. Using Lean Six Sigma Techniques to Improve Efficiency in Outpatient Ophthalmology Clinics. BMC Health Serv. Res. 2021, 21, 38. [Google Scholar] [CrossRef]
- Pierce, A.; Teeling, S.P.; McNamara, M.; O’Daly, B.; Daly, A. Using Lean Six Sigma in a Private Hospital Setting to Reduce Trauma Orthopedic Patient Waiting Times and Associated Administrative and Consultant Caseload. Healthcare 2023, 11, 2626. [Google Scholar] [CrossRef]
- Boronat, F.; Budia, A.; Broseta, E.; Ruiz-Cerdá, J.L.; Vivas-Consuelo, D. Aplicación de La Metodología Lean Healthcare En Un Servicio de Urología de Un Hospital Terciario Como Herramienta de Mejora de La Eficiencia. Actas Urol. Esp. 2018, 42, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Carreras, P.I.; Garcia-Sabater, J.J.; Marin-Garcia, J.A. Applying Value Stream Mapping to Improve the Delivery of Patient Care in the Oncology Day Hospital. Int. J. Environ. Res. Public Health 2022, 19, 4265. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, N. Lean Principles for Healthcare. In Lean Thinking for Healthcare; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–11. [Google Scholar] [CrossRef]
- Rathi, R.; Vakharia, A.; Shadab, M. Lean Six Sigma in the Healthcare Sector: A Systematic Literature Review. Mater. Today Proc. 2022, 50, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Satyadi, C. 236 Utilization of 5S Visual Management: A Lean Six Sigma Application in Operation of Hospital Clinical Laboratory. Am. J. Clin. Pathol. 2013, 140, A236. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Mandrekar, J.N.; Yao, J.D. Adoption of Lean Principles in a High-Volume Molecular Diagnostic Microbiology Laboratory. J. Clin. Microbiol. 2014, 52, 2689–2693. [Google Scholar] [CrossRef]
- Akdag, H.C.; Bolat, H.B.; Arslan, A.H.; Karacakaya, E. Application of Lean Management Systems in Pathology Laboratory Work Process and Laboratory Environment. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Toronto, ON, Canada, 23–25 October 2019; pp. 468–479. [Google Scholar]
- Stoiljković, P.; Milosavljević, P.; Mladenović, S.; Pavlović, D.; Todorović, M. Improving the Efficiency of the Center for Medical Biochemistry, Clinical Center NiŠ, by Applying Lean Six Sigma Methodology. J. Med. Biochem. 2014, 33, 299–307. [Google Scholar] [CrossRef]
- Trigueiro, G.; Oliveira, C.; Rodrigues, A.; Seabra, S.; Pinto, R.; Bala, Y.; Granado, M.G.; Vallejo, S.; Gonzalez, V.; Cardoso, C. Conversion of a Classical Microbiology Laboratory to a Total Automation Laboratory Enhanced by the Application of Lean Principles. Microbiol. Spectr. 2024, 12, e02153-23. [Google Scholar] [CrossRef]
- Yasemin, A.; Usubütün, A.; Durur, F.; Kutlu, G. Reducing Turnaround Time in a Pathology Laboratory Using the Lean Methodology. Int. J. Lean Six Sigma 2023, 14, 520–533. [Google Scholar] [CrossRef]
- Letelier, P.; Guzmán, N.; Medina, G.; Calcumil, L.; Huencho, P.; Mora, J.; Quiñones, F.; Jara, J.; Reyno, C.; Farías, J.; et al. Workflow Optimization in a Clinical Laboratory Using Lean Management Principles in the Pre-Analytical Phase. J. Med. Biochem. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Antony, J.; Lizarelli, F.L.; Fernandes, M.M. A Global Study into the Reasons for Lean Six Sigma Project Failures: Key Findings and Directions for Further Research. IEEE Trans. Eng. Manag. 2022, 69, 2399–2414. [Google Scholar] [CrossRef]
- Tsai, E.R.; Tintu, A.N.; Demirtas, D.; Boucherie, R.J.; de Jonge, R.; de Rijke, Y.B. A Critical Review of Laboratory Performance Indicators. Crit. Rev. Clin. Lab. Sci. 2019, 56, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.J.; Carwardine, Z.L.; Hallworth, M.J.; Kilpatrick, E.S. Using Clinical Guidelines to Assess the Potential Value of Laboratory Medicine in Clinical Decision-Making. Biochem. Med. 2021, 31, 010703. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Khangulov, V.S.; O’hara, M.; Arnaout, R. Reduction in Laboratory Turnaround Time Decreases Emergency Room Length of Stay. Open Access Emerg. Med. 2018, 10, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Umut, B.; Sarvari, P. Applying Lean Tools in The Clinical Laboratory to Reduce Turnaround Time for Blood Test Results. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 4, 164–169. [Google Scholar]
- López-Hernández, I.; López-Cerero, L.; Fernández-Cuenca, F.; Pascual, Á. El Papel Del Laboratorio de Microbiología En El Diagnóstico de Infecciones Por Bacilos Gramnegativos Multirresistentes. Importancia de La Determinación de Mecanismos de Resistencias. Med. Intensiv. 2022, 46, 455–464. [Google Scholar] [CrossRef]
- Bouza, E.; Muñoz, P.; Burillo, A. Role of the Clinical Microbiology Laboratory in Antimicrobial Stewardship. Med. Clin. North Am. 2018, 102, 883–898. [Google Scholar] [CrossRef]
- MacArthur, R.D.; Miller, M.; Albertson, T.; Panacek, E.; Johnson, D.; Teoh, L.; Barchuk, W. Adequacy of Early Empiric Antibiotic Treatment and Survival in Severe Sepsis: Experience from the MONARCS Trial. Clin. Infect. Dis. 2004, 38, 284–288. [Google Scholar] [CrossRef]
- Yuan, S.; Astion, M.L.; Schapiro, J.; Limaye, A.P. Clinical Impact Associated with Corrected Results in Clinical Microbiology Testing. J. Clin. Microbiol. 2005, 43, 2188–2193. [Google Scholar] [CrossRef]
- De Plato, F.; Fontana, C.; Gherardi, G.; Privitera, G.P.; Puro, V.; Rigoli, R.; Viaggi, B.; Viale, P. Collection, Transport and Storage Procedures for Blood Culture Specimens in Adult Patients: Recommendations from a Board of Italian Experts. Clin. Chem. Lab. Med. 2019, 57, 1680–1689. [Google Scholar] [CrossRef]
- Torraco, R.J. Writing integrative literature reviews: Using the past and present to explore the future. Hum. Resour. Dev. Rev. 2016, 15, 404–428. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hayes, K.J.; Reed, N.; Fitzgerald, A.; Watt, V. Applying Lean Flows in Pathology Laboratory Remodelling. J. Health Organ. Manag. 2014, 28, 229–246. [Google Scholar] [CrossRef]
- Miniati, R.; Frosini, F.; Cecconi, G.; Dori, F.; Iadanza, E.; Vezzosi, S.; Curiardi, M.; Belardinelli, A. Experience of Lean Six Sigma Quality Approach to Hospital Laboratory Services. IFMBE Proc. 2015, 45, 609–612. [Google Scholar] [CrossRef]
- de Oliveira, K.B.; dos Santos, E.F.; Junior, L.V.G. Lean Healthcare as a Tool for Improvement: A Case Study in a Clinical Laboratory. Adv. Intell. Syst. Comput. 2017, 482, 129–140. [Google Scholar] [CrossRef]
- Quetz, J.S.; Dantas, I.F.; Hirth, C.G.; Brasil, C.G.; Juaçaba, S.F. Preliminary Results of Lean Method Implementation in a Pathology Lab from Northeastern Brazil. J. Bras. Patol. Med. Lab. 2015, 51, 33–38. [Google Scholar] [CrossRef]
- Yang, T.; Wang, T.K.; Li, V.C.; Su, C.L. The Optimization of Total Laboratory Automation by Simulation of a Pull-Strategy. J. Med. Syst. 2015, 39, 162. [Google Scholar] [CrossRef]
- Acero, R.; Torralba, M.; Valverde, E.D.; Roc, L.; Rezusta, A. Integrating Lean and Automation for Enhanced Serology Diagnosis Efficiency in Tertiary Healthcare Microbiology Laboratories. Appl. Sci. 2024, 14, 241. [Google Scholar] [CrossRef]
- Pérez, C.V.; Guerrero, G.S.; Garzón, F.G.; Garcia, A.S. Lean-Agile Adaptations in Clinical Laboratory Accredited ISO 15189. Appl. Sci. 2015, 5, 1616–1638. [Google Scholar] [CrossRef]
- Michael, M.; Hilma, D.I.; Harmony, M.; Mbohwa, C. The Impact of Lean in Medical Laboratory Industry: Empirical Studies in Namibia. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 25 October 2017; Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000418109600083 (accessed on 23 February 2025).
- Roemeling, O.P.; Land, M.J.; Ahaus, K.; Slomp, J.; van den Bijllaardt, W. Impact of Lean Interventions on Time Buffer Reduction in a Hospital Setting. Int. J. Prod. Res. 2017, 55, 4802–4815. [Google Scholar] [CrossRef]
- Salbiah, I.; Hami, R.; Hashim, H.; Nizam, S.M.; Harani, M.S.; Sairi, S.; Salleh, M.J.M. Improvement of Urgent Tests Laboratory Turnaround Time through Laboratory Lean Management. Malays. J. Med. Health Sci. 2020, 16, 15–21. [Google Scholar]
- Shradha, G.; Kapil, S.; Sharma, M. Improvement of Laboratory Turnaround Time Using Lean Methodology. Int. J. Health Care Qual. Assur. 2018, 31, 295–308. [Google Scholar] [CrossRef]
- Cruz, S.A.; Morales, J.A. Vision of a Microbiology Laboratory of Excellence Using Lean Methodology. 2018. Available online: https://prcr.cobimet.org/server/api/core/bitstreams/c3e2e9c4-a325-40a4-8bb7-65c8619af7a7/content (accessed on 23 February 2025).
- Rivera, M.Y.; De Jesus, A.O.; Vázquez, Y.A.; Cantillo, Y.C. Lean Six Sigma in Laboratory Process. In Proceedings of the IISE Annual Conference and Expo, New Orleans, LA, USA, 21–23 May 2023; Available online: https://www.proquest.com/openview/555c7643e53ad2d2c36ada8653f2cdef/1.pdf?cbl=51908&pq-origsite=gscholar (accessed on 23 February 2025).
- Yuceel-Timur, I.; Thierry, E.; Chainier, D.; Ndao, I.; Labrousse, M.; Grélaud, C.; Bala, Y.; Barraud, O. Retrospective Evaluation of Rapid Genotypic ID and Phenotypic AST Systems on Positive Blood Culture Turnaround Time and Simulated Potential Impacts on Bloodstream Infection Management. J. Antimicrob. Chemother. 2024, 79 (Suppl. S1), i26–i31. [Google Scholar] [CrossRef]
- Barenfanger, J.; Drake, C.; Kacich, G. Clinical and Financial Benefits of Rapid Bacterial Identification and Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 1999, 37, 1415–1418. [Google Scholar] [CrossRef]
- Genwei, A.; Zhang, Y.; Guo, K.; Zhao, L.; Li, Z.; Hai, H.; Jia, E.; Liu, J. The Impact of Optimizing Microbial Diagnosis Processes on Clinical and Healthcare Economic Outcomes in Hospitalized Patients with Bloodstream Infections. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 2147–2157. [Google Scholar] [CrossRef]
- Fatih, D.; Akbulut, Y. Lean Methodology for Pathology Laboratories: A Case Study from a Public Hospital. Turk Patoloji Derg. 2019, 35, 228–236. [Google Scholar] [CrossRef]
- Gijo, E.V.; Antony, J.; Hernandez, J.; Scaria, J. Reducing Patient Waiting Time in a Pathology Department Using the Six Sigma Methodology. Leadersh. Health Serv. 2013, 26, 253–267. [Google Scholar] [CrossRef]
- Blick, K.E. Providing Critical Laboratory Results on Time, Every Time to Help Reduce Emergency Department Length of Stay: How Our Laboratory Achieved a Six Sigma Level of Performance. Am. J. Clin. Pathol. 2013, 140, 193–202. [Google Scholar] [CrossRef]
- Mutingi, M.; Isack, H.D.; Kandjeke, H.; Mbohwa, C. Adoption of Lean Tools in Medical Laboratory Industry: A Case Study of Namibia. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Rabat, Morocco, 11–13 April 2017; pp. 895–901. [Google Scholar]
- Andreea, A.S.; Vasile, V.; Veres, C. Externalities of Lean Implementation in Medical Laboratories. Process Optimization vs. Adaptation and Flexibility for the Future. Int. J. Environ. Res. Public Health 2021, 18, 12309. [Google Scholar] [CrossRef]
- Linoj, S.; Novak-Weekley, S. The Role of the Clinical Laboratory in the Future of Health Care: Lean Microbiology. J. Clin. Microbiol. 2014, 52, 1812–1817. [Google Scholar] [CrossRef]
- Lokesh, K.; Samanta, A.K.; Varaprasad, G. Reducing the Turnaround Time of Laboratory Samples by Using Lean Six Sigma Methodology in a Tertiary-Care Hospital in India. In Proceedings of the 2020 International Conference on System, Computation, Automation and Networking, ICSCAN, Pondicherry, India, 3–4 July 2020. [Google Scholar] [CrossRef]
- Cruz, V.C.; Pinilla, L.S.; Olaso, J.R.O.; Gandarias, N.T.; de Lacalle, N.L. Identification of Key Performance Indicators in Project-Based Organisations through the Lean Approach. Sustainability 2020, 12, 5977. [Google Scholar] [CrossRef]
- Ativa, A.-Z.; Vlachos, I.; Amer, Y. Application of Lean Six Sigma to Reduce Patient Waiting Time: Literature Review. Int. J. Qual. Res. 2021, 15, 241–258. [Google Scholar] [CrossRef]
- Mohamed, K.; Khalid, P. Improving Laboratory Results Turnaround Time by Reducing Pre Analytical Phase. Stud. Health Technol. Inform. 2014, 202, 71–74. [Google Scholar] [CrossRef]
- Eubank, T.A.; Long, S.W.; Perez, K.K. Role of Rapid Diagnostics in Diagnosis and Management of Patients with Sepsis. J. Infect. Dis. 2021, 222, S103–S109. [Google Scholar] [CrossRef]
- Binita, G.; Singh, B.; Chawla, R.; Gupta, V.K.; Mallika, V. Turn Around Time (TAT) as a Benchmark of Laboratory Performance. Indian J. Clin. Biochem. 2010, 25, 376–379. [Google Scholar] [CrossRef]
- Starolis, M.W. The Contamination Monitoring Toolbox: Best Practices for Molecular Microbiology Testing. Clin. Microbiol. Newsl. 2024, 47, 21–27. [Google Scholar] [CrossRef]
- Justine, E.; Samarasekara, H. Blood Culture Contamination Rate as a Pre-Analytical Quality Indicator: 12-Month Data Analysis from a Metropolitan Microbiology Laboratory. Pathology 2024, 56, S120–S121. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Almutairi, A.Z.; Alharbi, N.L.; Alenezi, R.F.; Alturkostani, M.A.; Ozbak, H.A.; Islam, F.A. Blood Culture Contamination in a Tertiary Care Hospital of Saudi Arabia. Saudi Med. J. 2020, 41, 508–515. [Google Scholar] [CrossRef]
- Xu, K.; Gilani, S.; Wang, H.; Fallon, J. Blood Culture Contamination in the Clinical Microbiology Laboratory of a Teaching Hospital. Am. J. Clin. Pathol. 2019, 152 (Suppl. S1), S133. [Google Scholar] [CrossRef]
- Nuray, A.; Şölen, E.Y.; Yılmaz, N. Urine Culture Contamination Rates at a University Hospital. Klimik Derg. 2021, 34, 182–185. [Google Scholar] [CrossRef]
- Mason, S.E.; Nicolay, C.R.; Darzi, A. The Use of Lean and Six Sigma Methodologies in Surgery: A Systematic Review. Surgeon 2015, 13, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Inal, T.C.; Ozturk, O.G.; Kibar, F.; Cetiner, S.; Matyar, S.; Daglioglu, G.; Yaman, A. Lean Six Sigma Methodologies Improve Clinical Laboratory Efficiency and Reduce Turnaround Times. J. Clin. Lab. Anal. 2018, 32, e22180. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.J.; Khanduja, D. Perspectives of Control Phase to Manage Six Sigma Implements: An Empirical Study. Int. J. Bus. Excell. 2014, 7, 88–111. [Google Scholar] [CrossRef]
- Sancho, D.; Cacho, R.A.; Lavilla, M.J.; Corral, M.; Carrillo-López, A.; Rezusta, A. Optimizing the Supply Chain of Intensive Care Unit Blood Culture Samples to Clinical Microbiology Laboratory Using Lean Six Sigma. In Advances in Science and Technology; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2023; Volume 132. [Google Scholar] [CrossRef]
- Mlinarić, A.; Vogrinc, Ž.; Drenšek, Z. Effect of Sample Processing and Time Delay on Cell Count and Chemistry Tests in Cerebrospinal Fluid Collected from Drainage Systems. Biochem. Medica 2018, 28, 030705. [Google Scholar] [CrossRef]
- Eisinger, S.W.; Schwartz, M.; Dam, L.; Riedel, S. Evaluation of the BD Vacutainer Plus Urine C&S Preservative Tubes Compared with Nonpreservative Urine Samples Stored at 4 °C and Room Temperature. Am. J. Clin. Pathol. 2013, 140, 306–313. [Google Scholar] [CrossRef]
- Cuthberson, L.; Rogers, G.B.; Walker, A.W.; Oliver, A.; Hafiz, T.; Hoffman, L.R.; Carroll, M.P.; Parkhill, J.; Bruce, K.D.; Van Der Gast, C.J. Time between Collection and Storage Significantly Influences Bacterial Sequence Composition in Sputum Samples from Cystic Fibrosis Respiratory Infections. J. Clin. Microbiol. 2014, 52, 3011–3016. [Google Scholar] [CrossRef]
- Jenkins, S.V.; Vang, K.B.; Gies, A.; Griffin, R.J.; Se-Ran, J.; Nookawe, I.; Dings, R.P.M. Sample storage conditions induce post-collection biases in microbiome profiles. BMC Microbiol. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Antillon, M.; Saad, N.J.; Baker, S.; Pollard, A.J.; Pitzer, V.A. The Relationship Between Blood Sample Volume and Diagnostic Sensitivity of Blood Culture for Typhoid and Paratyphoid Fever: A Systematic Review and Meta-Analysis. J Infect Dis. 2018, 218 (Suppl. S4), S255–S267. [Google Scholar] [CrossRef]
- Connell, T.G.; Rele, M.; Cowley, D.; Buttery, J.P.; Curtis, N. How Reliable Is a Negative Blood Culture Result? Volume of Blood Submitted for Culture in Routine Practice in a Children’s Hospital. Pediatrics 2007, 119, 891–896. [Google Scholar] [CrossRef]
- White, B.A.; Baron, J.M.; Dighe, A.S.; Camargo, C.A.; Brown, D.F.M. Applying Lean Methodologies Reduces ED Laboratory Turnaround Times. Am. J. Emerg. Med. 2015, 33, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Frosini, F.; Miniati, R.; Cecconi, G.; Dori, F.; Iadanza, E.; Vezzosi, S.; Mechi, M.T.; Belardinelli, A. Lean Thinking in Hospital: Case Study at the Pathology Laboratory. IFMBE Proc. 2015, 45, 613–616. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, X.; Miao, Y. Reducing Turnaround Time for Routine Outpatient Biochemical Tests through Lean Six Sigma: A Case Study in China. J. Eval. Clin. Pract. 2024, 31, e14116. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.; Sultan, M.; Yassine, O.G.; Zaki, A.; Elamir, H.; Guirguis, W. Using Lean Six Sigma to Improve Timeliness of Clinical Laboratory Test Results in a University Hospital in Egypt. Int. J. Lean Six Sigma 2022, 13, 1159–1183. [Google Scholar] [CrossRef]





| Core Search Terms (Group 1): |
| Laboratory Lean Laboratory DMAIC |
| Generic Related Topic (Group 2): |
| Healthcare DMAIC Clinical Laboratory Lean |
| Specific Topic (Group 3): |
| Microbiology Laboratory Lean Microbiology Laboratory Management Microbiology Laboratory Six Sigma |
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Stage I | (+) Articles containing the search terms; (+) Articles in Spanish or English; (+) Relevant to healthcare: laboratory, healthcare, microbiology, pathology, etc. | (−) Preprint and editorials; (−) Published before 2012; (−) Not relevant to healthcare. |
| Stage II | (+) Article titles containing references to Lean, Six Sigma, or Lean Six Sigma about clinical laboratories. | (−) Article titles do not contain references to Lean, Six Sigma, or Lean Six Sigma applied to clinical laboratories; (−) Articles duplicated. |
| Stage III | (+) Articles abstract with scope on Lean, Six Sigma, or both in a clinical laboratory; (+) Articles including LSS tools or methodology. | (−) Articles without a clear LSS methodology, KPIs, or tools. |
| Turnaround Time | Cost Reduction | Patient Waiting | Accuracy and Reliability | Test Capacity | |
|---|---|---|---|---|---|
| Biochemistry Laboratory | 1 | 1 | 1 | ||
| Clinical Laboratory | 14 | 4 | 2 | 3 | 1 |
| Hematology and Biochemistry | 1 | ||||
| Hematology Laboratory | 1 | ||||
| Microbiology Laboratory | 6 | 3 | 1 | 1 | |
| Pathology Laboratory | 3 | 1 | 3 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho, D.; Rezusta, A.; Acero, R. Integrating Lean Six Sigma into Microbiology Laboratories: Insights from a Literature Review. Healthcare 2025, 13, 917. https://doi.org/10.3390/healthcare13080917
Sancho D, Rezusta A, Acero R. Integrating Lean Six Sigma into Microbiology Laboratories: Insights from a Literature Review. Healthcare. 2025; 13(8):917. https://doi.org/10.3390/healthcare13080917
Chicago/Turabian StyleSancho, David, Antonio Rezusta, and Raquel Acero. 2025. "Integrating Lean Six Sigma into Microbiology Laboratories: Insights from a Literature Review" Healthcare 13, no. 8: 917. https://doi.org/10.3390/healthcare13080917
APA StyleSancho, D., Rezusta, A., & Acero, R. (2025). Integrating Lean Six Sigma into Microbiology Laboratories: Insights from a Literature Review. Healthcare, 13(8), 917. https://doi.org/10.3390/healthcare13080917






