Design and Development of a Wearable Exoskeleton System for Stroke Rehabilitation
Abstract
:1. Introduction
- ▪
- for the healthy (non-paretic) hand to assist the paretic hand to undergo bilateral extension-flexion training simultaneously;
- ▪
- for the exoskeleton rehabilitative device to allow also for the rehabilitation of the fingers;
- ▪
- for the design of the exoskeleton assistive device to accommodate approximately 80–90% users;
- ▪
- for the device to be light-weight, low-cost, and easy to fit onto the forearm.
2. The Human Hand Structure
3. Exoskeleton Structural Design
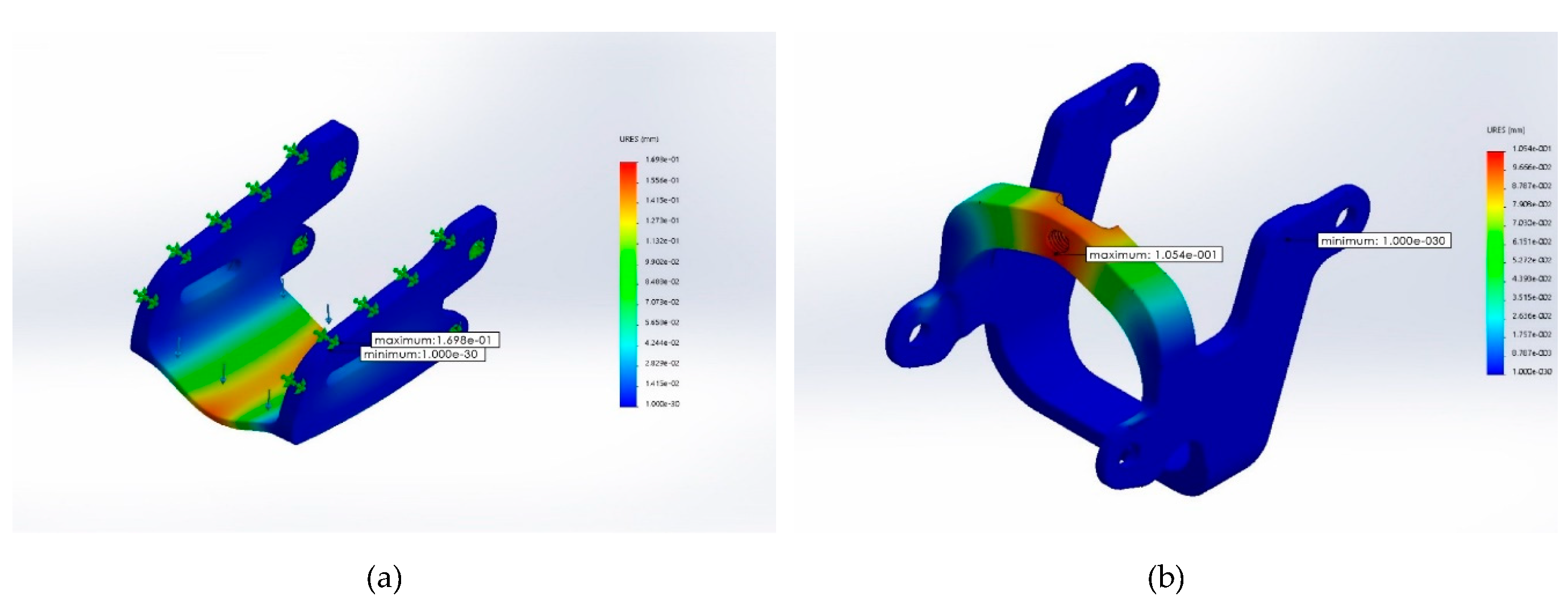
Static Analysis
- Material: Acrylonitrile Butadiene Styrene (ABS plastic)
- Weight: middle open-up exoskeleton at 2.6 g; control movement point at 1.98 g
- Boundary conditions: fixed end in blue (please see Figure 4)
- Force exerted at blue: maximum downward force of 5 kgf, designated force of 2 kgf, and safety index 2.5
- Grid: finite element analysis
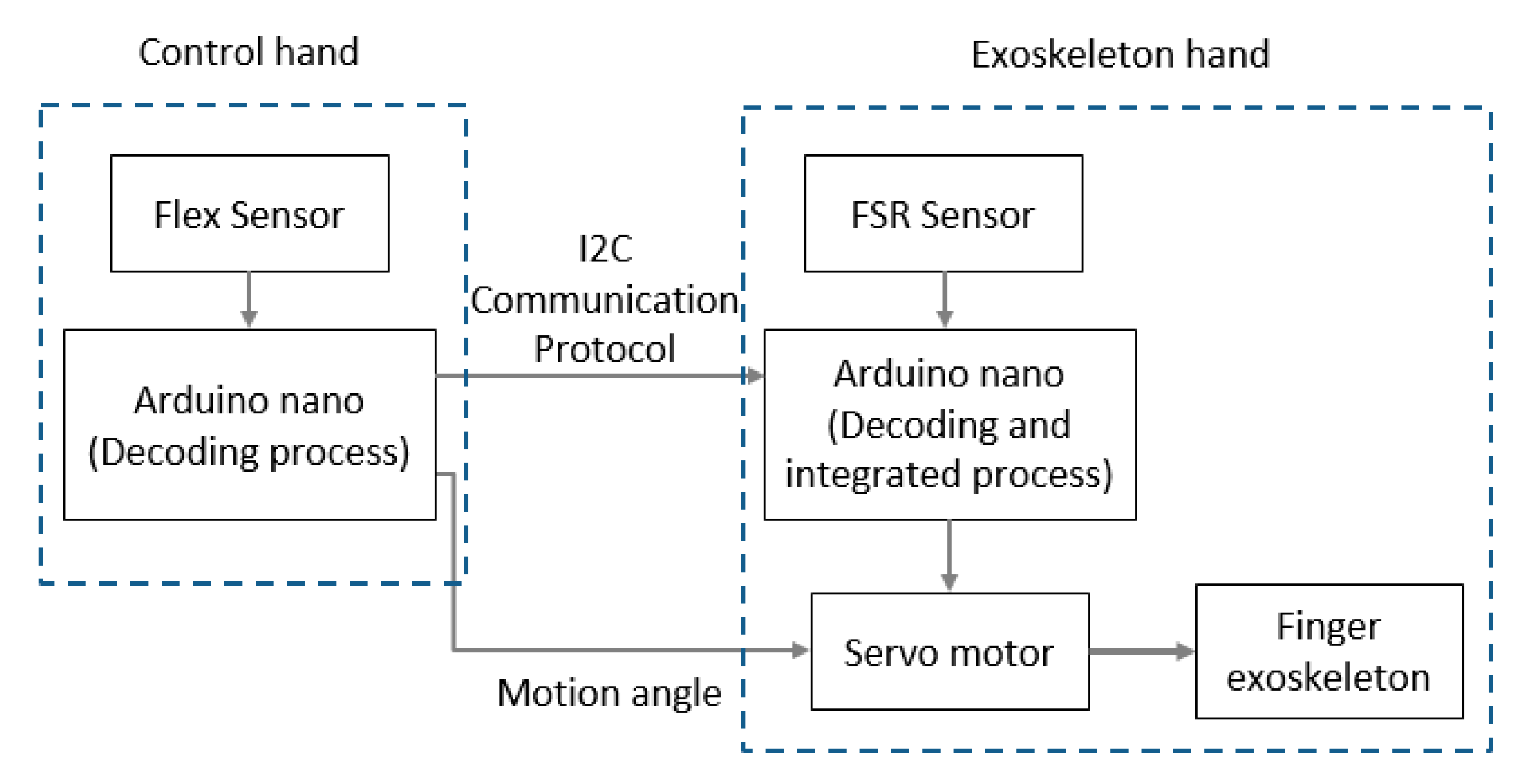
4. Electromechanical Integration Design
4.1. Acceleration and Velocity
4.2. Force Sensitive Resistor Sensor
5. Operating Analysis
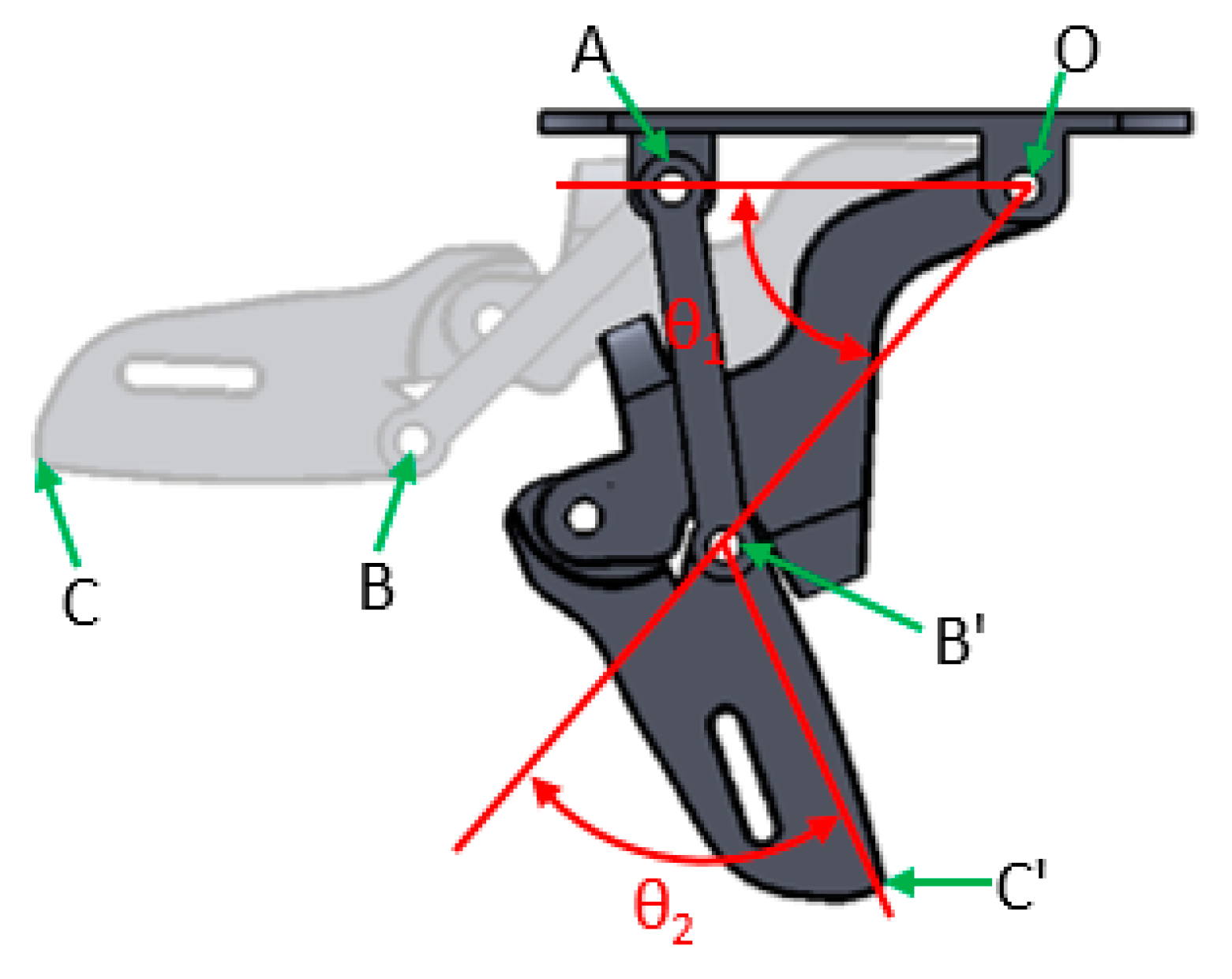
5.1. Parameter Definitions
5.2. Analysis of Movement
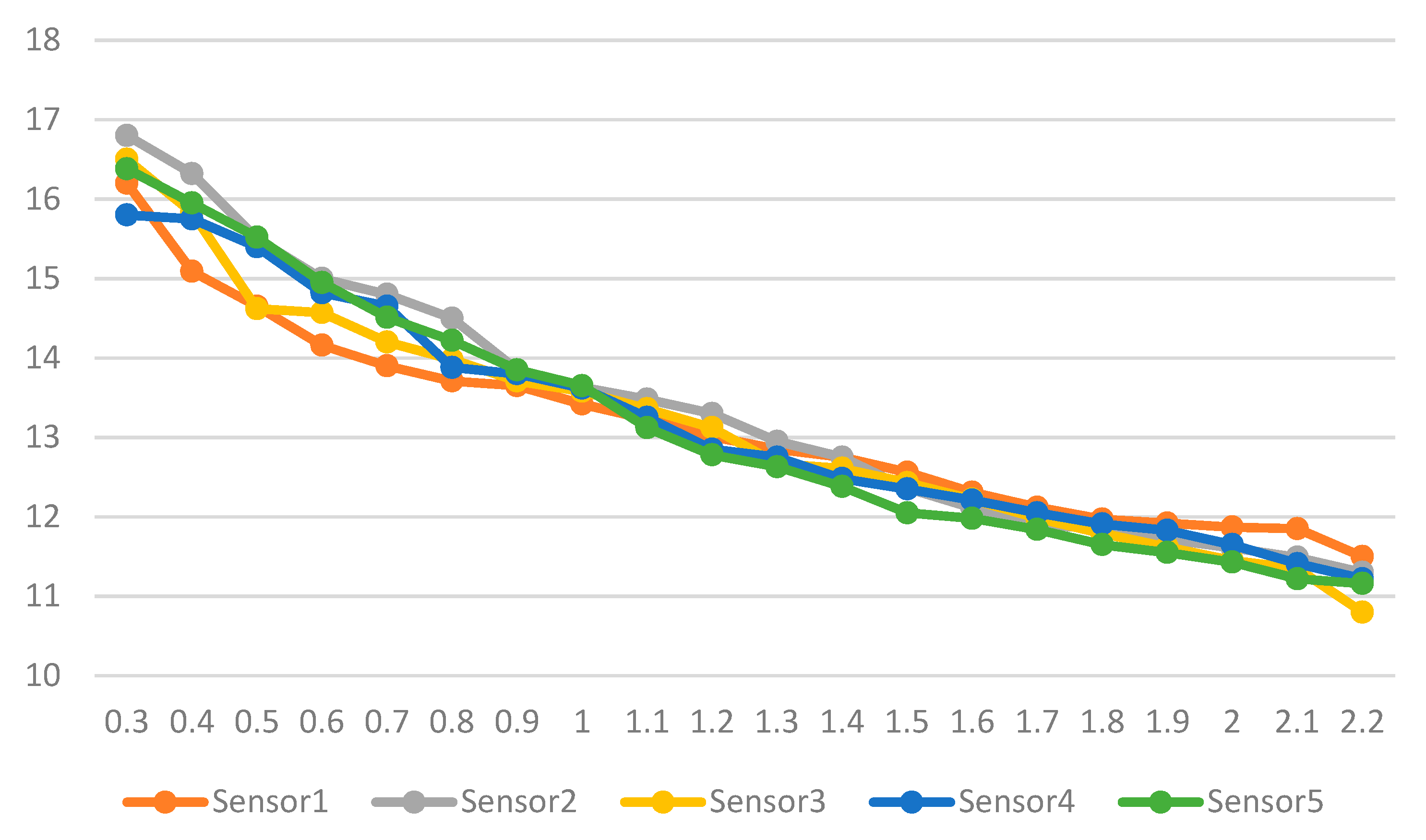
6. Test Results
Simulated Results
7. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DIP | distal interphalangeal joint |
| DOF | degree of freedom |
| IP | interphalangeal joint |
| MCP | metacarpophalangeal joint |
| PIP | proximal interphalangeal joint |
| ROM | range of motion |
References
- Braithwaite, J.; Mont, D. Disability and Poverty: A Survey of World Bank Poverty Assessment and Implications; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Schaechter, J.D. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog. Neurobiol. 2004, 73, 61–72. [Google Scholar] [CrossRef]
- Hu, X.L.; Tong, K.Y.; Song, R.; Zheng, X.J.; Leung, W.W.F. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabilit. Neural Repair 2009, 23, 837–846. [Google Scholar] [CrossRef] [Green Version]
- Szameitat, A.J.; Shen, S.; Conforto, A.; Sterr, A. Cortical activation during executed, imagined, observed, and passive wrist movements in healthy volun- teers and stroke patients. NeuroImage 2012, 62, 266–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, H.I.; Dipietro, L.; Levy-Tzedek, S.; Fasoli, S.E.; Rykman-Berland, A.; Zipse, J.; Fawcett, J.A.; Stein, J.; Poizner, H.; Lo, A.C.; et al. A paradigm shift for rehabilitation robotics. IEEE Eng. Med. Biol. Mag. 2008, 4, 61–70. [Google Scholar] [CrossRef]
- Hesse, S.; Werner, C. Machines to support motor rehabilitation after stroke. J. Rehabil. Res. Dev. 2006, 43, 671–867. [Google Scholar] [CrossRef] [PubMed]
- Jansen, O.; Grasmuecke, D.; Meindl, R.C.; Tegenthoff, M.; Schwenkreis, P.; Sczesny-Kaiser, M.; Wessling, M.; Schildauer, T.A.; Fisahn, C.; Aach, M. Hybrid Assistive Limb Exoskeleton HAL in the Rehabilitation of Chronic Spinal Cord Injury: Proof of Concept; the Results in 21 Patients. World Neurosurg. 2018, 110, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lai, W.Y.; Liao, W.H.; Fong, D.T.P.; Chan, K.M. Design and control of a powered knee orthosis for gait assistance. In Proceedings of the 2013 IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Wollongong, Australia, 9–12 July 2013; pp. 816–821. [Google Scholar]
- Kong, K.; Bae, J.; Tomizuka, M. A Compact Rotary Series Elastic Actuator for Human Assistive Systems. IEEE/ASME Trans. Mechatron. 2012, 17, 288–297. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, Z.; Wang, Q. BioKEX: A bionic knee exoskeleton with proxy-based sliding mode control. In Proceedings of the 2015 IEEE International Conference on Industrial Technology, Seville, Spain, 17–19 March 2015; pp. 125–130. [Google Scholar]
- Beyl, P.; Knaepen, K.; Duerinck, S.; Van Damme, M.; Vanderborght, B.; Meeusen, R.; Lefeber, D. Safe and Compliant Guidance by a Powered Knee Exoskeleton for Robot-Assisted Rehabilitation of Gait. Adv. Robot. 2011, 25, 513–535. [Google Scholar] [CrossRef]
- Pratt, J.E.; Krupp, B.T.; Morse, C.J.; Collins, S.H. The RoboKnee: An exoskeleton for enhancing strength and endurance during walking. In Proceedings of the IEEE International Conference on Robotics and Automation. Proceedings. ICRA ’04, New Orleans, LA, USA, 26 April–1 May 2004; pp. 2430–2435. [Google Scholar]
- Maeda, D.; Tominaga, K.; Oku, T.; Pham, H.T.T.; Saeki, S.; Uemura, M.; Hiroaki, U.; Miyazaki, F. Muscle synergy analysis of human adaptation to a variable-s- tiffness exoskeleton: Human walk with a knee exoskeleton with pneumatic artificial muscles. In Proceedings of the 2012 12th IEEE-RAS International Conference on Humanoid Robots (Humanoids 2012), Osaka, Japan, 29 November–1 December 2012; pp. 638–644. [Google Scholar]
- Karavas, N.; Ajoudani, A.; Tsagarakis, N.; Saglia, J.; Bicchi, A.; Caldwell, D. Tele-Impedance based stiffness and motion augmentation for a knee exoskeleton device. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 2194–2200. [Google Scholar]
- Malcolm, P.; Galle, S.; Derave, W.; De Clercq, D. Bi-articular Knee-Ankle-Foot Exoskeleton Produces Higher Metabolic Cost Reduction than Weight-Matched Mono-articular Exoskeleton. Front. Neurosci. 2018, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cao, Q.; Zhang, C.; Tao, C.; Ji, R. Position solution of a novel four-DOFs self-aligning exoskeleton mechanism for upper limb rehabilitation. Mech. Mach. Theory 2019, 141, 14–39. [Google Scholar] [CrossRef]
- Lo, H.S.; Xie, S.Q. Exoskeleton robots for upper-limb rehabilitation: State of the art and future prospects. Med. Eng. Phys. 2012, 34, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Su, P.; Song, Y.; Dong, M.; Cao, Q. Improvement of human-machine compatibility of upper-limb rehabilitation exoskeleton using passive joints. Robot. Auton. Syst. 2019, 112, 22–31. [Google Scholar] [CrossRef]
- Nef, T.; Guidali, M.; Riener, R. ARMin III—Arm therapy exoskeleton with an ergonomic shoulder actuation. Appl. Bionics Biomech. 2009, 6, 127–142. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, S.; Wei, R.; Perez, M.; Shepard, B.; Koeneman, E.; Koeneman, J.; He, J. RUPERT: An exoskeleton robot for assisting rehabilitation of arm functions. In Proceedings of the 2008 Virtual Rehabilitation, Reno, NV, USA, 8–12 March 2008. [Google Scholar]
- Perry, J.C.; Rosen, J.; Burns, S. Upper-Limb Powered Exoskeleton Design. IEEE/ASME Trans. Mechatron. 2007, 12, 408–417. [Google Scholar] [CrossRef]
- Bataller, A.; Cabrera, J.A.; Clavijo, M.; Castillo, J.J. Evolutionary synthesis of mechanisms applied to the design of an exoskeleton for finger rehabilitation. Mech. Mach. Theory 2016, 105, 31–43. [Google Scholar] [CrossRef]
- Iqbal, J.; Khan, H.; Tsagarakis, N.G.; Caldwell, D.G. A novel exoskeleton robotic system for hand rehabilitation—Conceptualization to prototyping. Biocybern. Biomed. Eng. 2014, 34, 79–89. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: a global response is needed. Bull. World Health Organ. 2016, 94, 634–634A. [Google Scholar] [CrossRef]
- Geurts, A.C.; Hendricks, H.T.; Limbeek, V.J.; Zwarts, M.J. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002, 83, 1629–1637. [Google Scholar]
- Winstein, C.J.; Rose, D.K.; Tan, S.M.; Lewthwaite, R.; Chui, H.C.; Azen, S.P. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Arch. Phys. Med. Rehabil. 2004, 85, 620–628. [Google Scholar] [CrossRef]
- Legg, L.; Drummond, A.; Langhorne, P. Occupational Therapy for Patients with Problems in Activities of Daily Living after Stroke; Cochrane Library: London, UK, 2006. [Google Scholar]
- Taub, E.; Uswatte, G.; Elbert, T. New Treatments in neurorehabilitation founded on basic research. Nat. Rev. Neurosci. 2002, 3, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.N.; Carnethon, M.; Dai, S.; Desimone, G. Heart Disease and Stroke Statistics-2010 Update: A Report From the American Heart Association. Circulation 2010, 121, 46–215. [Google Scholar]
- Liao, W.W.; Wu, C.Y.; Hsieh, Y.W.; Lin, K.C.; Chang, W.Y. Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: A randomized controlled trial. Clin. Rehabil. 2012, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, E.L.; Wisdom, S.B.; Stone, L.; Foster, C.; Galasko, D.; Llewellyn, D.M. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999, 353, 2035–2036. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Smits, M.; Ribbers, G.M.; Stam, H.J.; Geest, J.N.; Bussmann, J.B. The neuronal correlates of mirror therapy: An fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatry 2011, 82, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohle, C.; Pullen, J.; Nakaten, A.; Kust, J.; Rietz, C.; Karbe, H. Mirror therapy promotes recovery from severs hemiparesis: A randomized controlled trial. Neurorehabilit. Neural Repair 2009, 23, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cauraugh, J.H.; Summers, J.J. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog. Neurobiol. 2005, 75, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Cauraugh, J.H.; Garry, M.I.; Hiraga, C.Y.; Loftus, A.; Kagerer, F.A.; Summers, J.J. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A TMS study. J. Neurol. Sci. 2007, 252, 76–82. [Google Scholar]
- Grebenstein, M.; Chalon, M.; Friedl, W.; Haddadin, S.; Wimbock, T.; Hirzinger, G.; Siegwart, R. The hand of the DLR hand arm system: Designed for inter-action. Int. J. Robot. Res. 2012, 31, 1531–1555. [Google Scholar] [CrossRef]
- Durand, S.; Marin, F.; Oberlin, C.; Ho Ba Tho, M.C. Morphogenesis of the human palma arch using three-dimensional geometric modeling. Clin. Anat. 2011, 24, 874–879. [Google Scholar] [CrossRef]
- Diffrient, N.; Tilley, A.R. Humanscale Manual; IA Collaborative Ventures, LLC: Chicago, IL, USA, 2017; (Original work published 1974). [Google Scholar]
- Susanto, E.A.; Tong, R.K.; Ockenfeld, C.; Ho, N.S. Efficacy of robot-assisted fingers training in chronic stroke survivors: A pilot randomized-controlled trial. J. Neuroeng. Rehabil. 2015, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.W.; Tsai, S.Y.; Chang, J.Y. Design and development of the wearable hand exoskeleton system for rehabilitation of hand impaired patients. In Proceedings of the 2014 IEEE International Conference on Automation Science and Engineering (CASE), Taipei, Taiwan, 18–22 August 2014. [Google Scholar]
- Bryanton, C.; Bosse, J.; Brien, M.; McLean, J.; McCormick, A.; Sveistrup, H. Feasibility, Motivation, and selective motor control: Virtual reality compared to conventional home exercise in children with cerebral palsy. Cyberpsychol. Behav. 2006, 9, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.H.; You, S.H.; Hallett, M.; Cho, Y.W.; Park, C.M.; Cho, S.H. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: An experimenter-blind preliminary study. Arch. Phys. Med. Rehabil. 2005, 86, 2218–2223. [Google Scholar] [CrossRef] [PubMed]






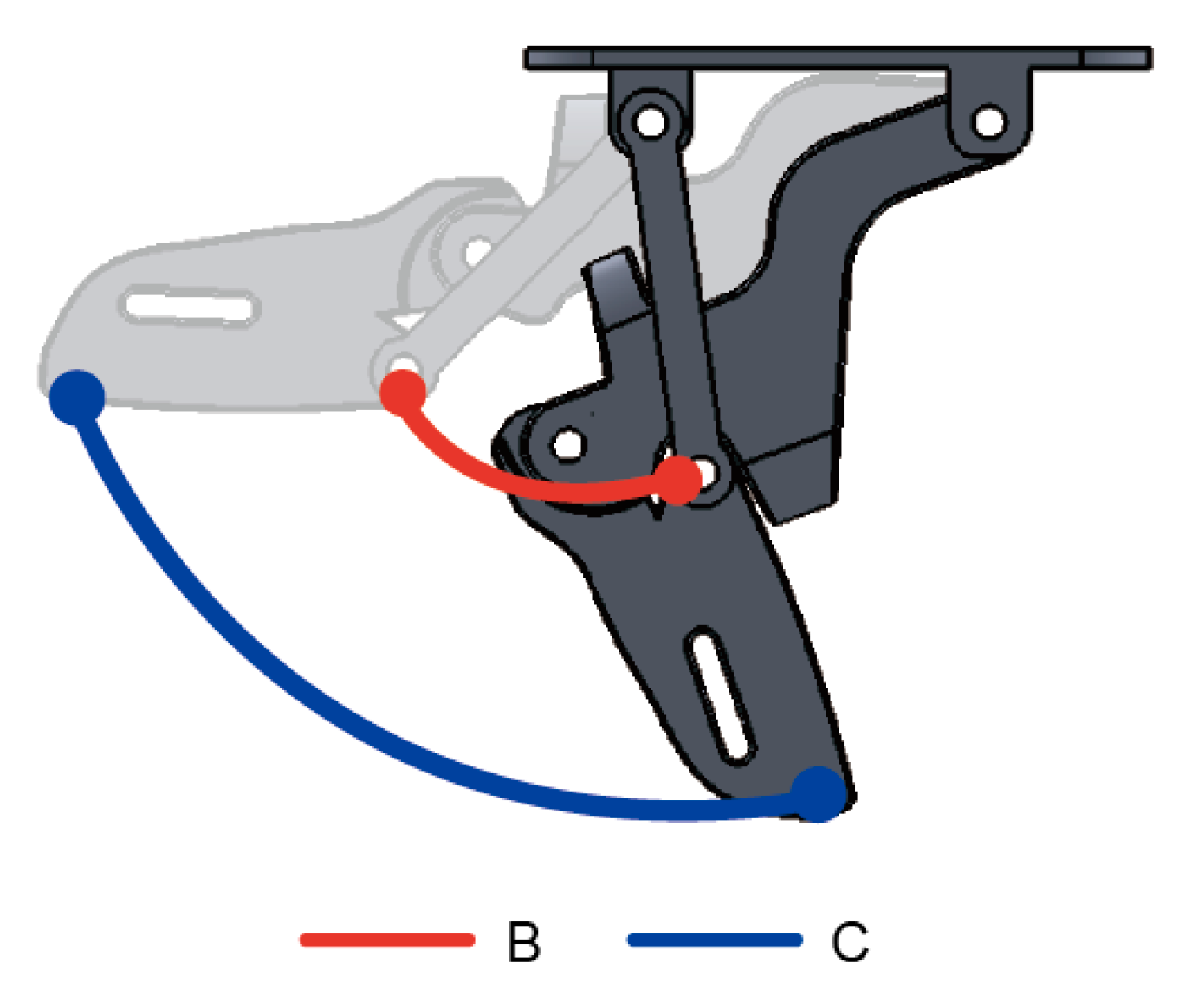

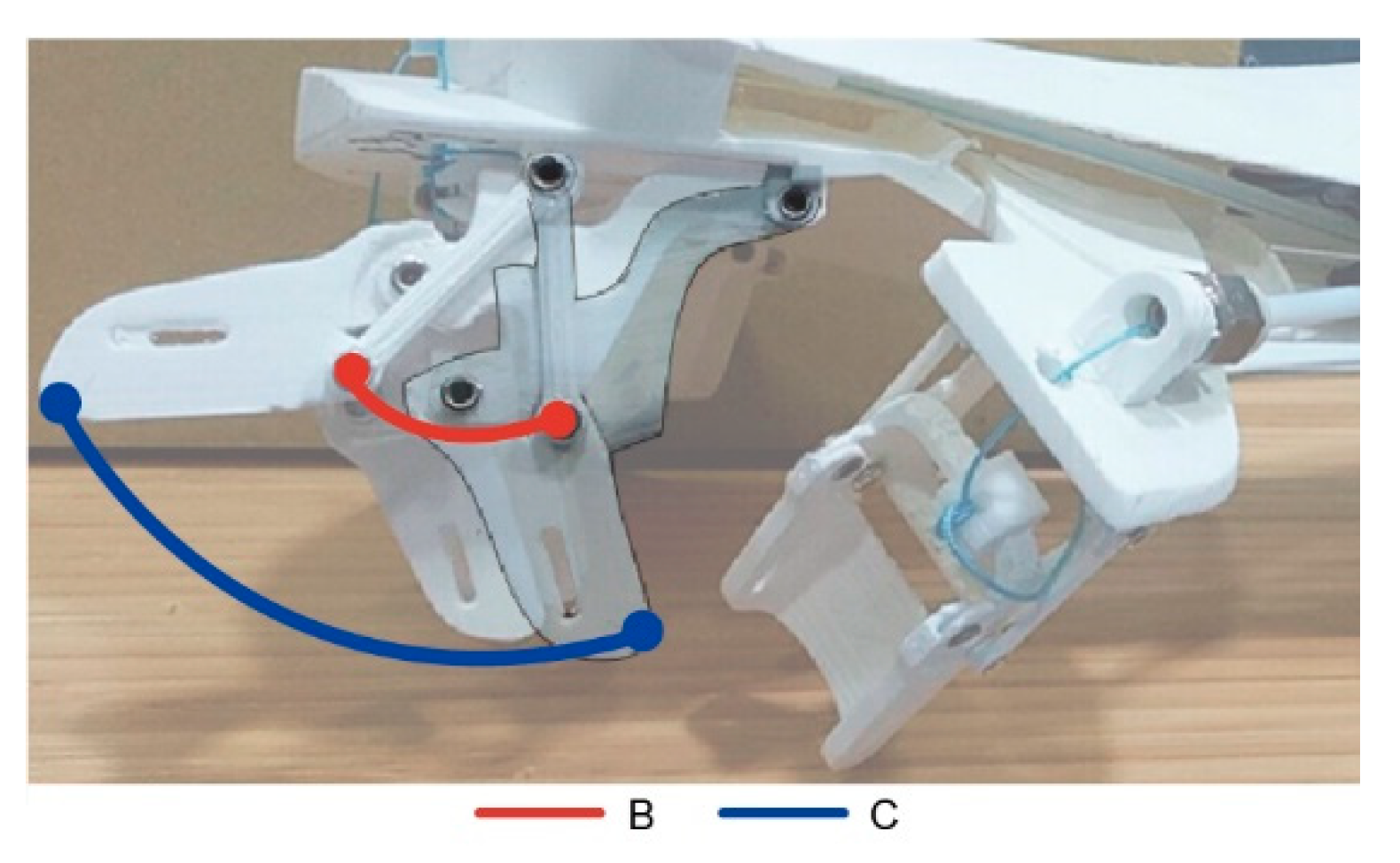
| Labels | MCP | PIP | DIP | Transverse DOF—Thumb | Weight | Clinical Testing |
|---|---|---|---|---|---|---|
| The design in this study | 70° | 90° | N.A. | 35° | 800g | No |
| Susanto et al., 2015 [39] | 55° | 65° | N.A. | N.A. | >1kg | Yes |
| Pu et al., 2014 [40] | 90° | 80° | 100° | N.A. | 700g | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.-K.; Wang, Y.-L.; Chang, H.-C.; Chen, C.-C. Design and Development of a Wearable Exoskeleton System for Stroke Rehabilitation. Healthcare 2020, 8, 18. https://doi.org/10.3390/healthcare8010018
Ou Y-K, Wang Y-L, Chang H-C, Chen C-C. Design and Development of a Wearable Exoskeleton System for Stroke Rehabilitation. Healthcare. 2020; 8(1):18. https://doi.org/10.3390/healthcare8010018
Chicago/Turabian StyleOu, Yang-Kun, Yu-Lin Wang, Hua-Cheng Chang, and Chun-Chih Chen. 2020. "Design and Development of a Wearable Exoskeleton System for Stroke Rehabilitation" Healthcare 8, no. 1: 18. https://doi.org/10.3390/healthcare8010018





