Barriers to Early Utilization of Palliative Care in Heart Failure: A Narrative Review
Abstract
:1. Introduction
2. Stigma of Referring Patients to Palliative Care
3. Terminology as a Barrier to Receive PC
4. Prognosis as Another Barrier
5. Lack of PC Specialists
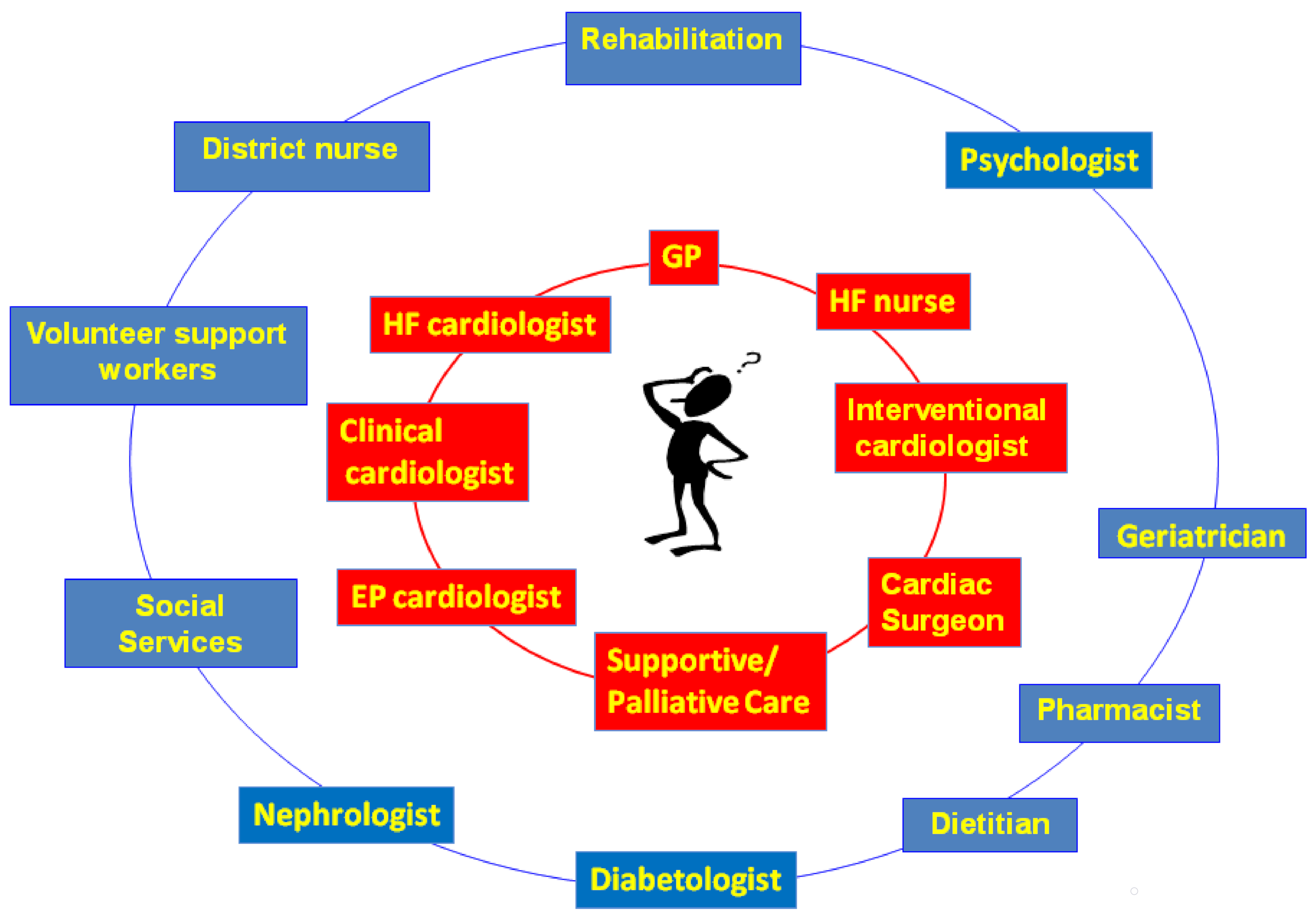
6. A New Model to Early Integrate PC in HF Patients
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.W.; Mc Carthy, E.P.; Lynn, J.; Davis, R.B.; Phillips, R.S. The last six months of life for patients with congestive heart failure. J. Am. Geriatr. Soc. 2000, 48, 101–109. [Google Scholar] [CrossRef]
- World Health Organization. Global Atlas of Palliative Care at the End of Life; Worldwide Palliative Care Alliance: London, UK, 2014. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.P.; Beattie, J.M. Palliative care in heart failure: Facts and numbers. ESC Heart Fail. 2017, 4, 81–87. [Google Scholar] [CrossRef]
- Gadoud, A.; Kane, E.; Macleod, U.; Johnson, M.J.; Macleod, U.; Allgar, V. Palliative care among heart failurepatients in primary care: A comparison to cancer patients using English family practice data. PLoS ONE 2014, 9, e113188. [Google Scholar] [CrossRef]
- Warraich, H.; Wolf, S.P.; Mentz, R.; Rogers, J.G.; Samsa, G.; Kamal, A.H. Characteristics and trends among patients with cardiovascular disease referred to palliative care. JAMA Network Open 2019, 2, e192375. [Google Scholar] [CrossRef]
- Siouta, N.; Van Beek, K.; Payne, S.; Radbruch, L.; Preston, N.; Hasselaar, J.; Centeno, C.; Menten, J. Is the content of guidelines/pathways a barrier for the integration of palliative Care in Chronic Heart Failure (CHF) and chronic pulmonary obstructive disease (COPD)? A comparison with the case of cancer in Europe. BMC Palliat. Care 2017, 16, 62. [Google Scholar] [CrossRef]
- Stocker, R.; Close, H.; Hancock, H.; Hungin, A.P.S. Should heart failure be regarded as a terminal illness requiring palliative care? A study of heart failure patients’, carers’ and clinicians’ understanding of heart failure prognosis and its management. BMJ Supp. Palliat. Care 2017, 7, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Hjelmfors, L.; Sandgren, A.; Strömberg, A.; Mårtensson, J.; Jaarsma, T.; Friedrichsen, M. “I was told that I would not die from heart failure”: Patient perceptions of prognosis communication. Appl. Nurs. Res. 2018, 41, 41–45. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Available online: http://www.who.int/cancer/palliative/definition/en/prologo (accessed on 23 January 2020).
- Cherny, N.I. Stigma associated with “palliative care”: Getting around it or getting over it. Cancer 2009, 115, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Twamley, K.; Craig, F.; Kelly, P.; Hollowell, D.R.; Mendoza, P.; Bluebond-Langner, M. Underlying barriers to referral to paediatric palliative care services: Knowledge and attitudes of health care professionals in a paediatric tertiary care centre in the United Kingdom. J. Child. Health Care 2014, 18, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dalberg, T.; McNinch, N.L.; Friebert, S. Perceptions of barriers and facilitators to early integration of pediatric palliative care: A national survey of pediatric oncology providers. Pediatr. Blood Cancer 2018, 65, e26996. [Google Scholar] [CrossRef]
- Consensus on the Core Ideology of MASCC. Definition of Supportive Care. Available online: https://www.mascc.org/index.php?option=com_content&view=article&id=493:mascc-strategic-plan&catid=30:navigation (accessed on 13 April 2019).
- Fadul, N.; Elsayem, A.; Palmer, J.L.; Del Fabbro, E.; Swint, K.; Li, Z.; Poulter, V.; Bruera, E. Supportive versus palliative care: what’s in a name?: A survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009, 115, 2013–2021. [Google Scholar] [CrossRef]
- Dalal, S.; Palla, S.; Hui, D.; Nguyen, L.; Chacko, R.; Li, Z.; Fadul, N.; Scott, C.; Thornton, V.; Coldman, B.; et al. Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. Oncologist 2011, 16, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K.; Aapro, M.; Kaasa, S.; Ripamonti, C.I.; Scotté, F.; Strasser, F.; Young, A.; Bruera, E.; Herrstedt, J.; Keefe, D.; et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann. Oncol. 2018, 29, 36–43. [Google Scholar] [CrossRef]
- Siouta, N.; Clement, P.; Aertgeerts, B.; Van Beek, K.; Menten, J. Professionals’ perceptions and current practices of integrated palliative care in chronic heart failure and chronic obstructive pulmonary disease: A qualitative study in Belgium. BMC Palliat. Care 2018, 17, 103. [Google Scholar] [CrossRef]
- Katz, N.M. The term “supportive care” is preferable to “palliative care” for consults in the cardiothoracic intensive care unit. J. Thorac. Cardiovasc. Surg. 2018, 155, 2030–2031. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Hua, M.; Takayama, H. Who is not comfortable with the term “palliative care”-patient, family, or surgeon? J. Thorac. Cardiovasc. Surg. 2018, 155, 2032–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.; Mori, M.; Parsons, H.A.; Kim, S.H.; Li, Z.; Damani, S.; Bruera, E. The Lack of Standard Definitions in the Supportive and Palliative Oncology Literature. J. Pain Symptom Manage. 2012, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- MD Dictionary of Cancer Terms. Definitions of palliative care and supportive care. 2019. Available online: http://www.cancer.gov/dictionary (accessed on 8 April 2019).
- Hui, D.; De La Cruz, M.; Mori, M.; Parsons, H.A.; Kwon, J.H.; Torres-Vigil, I.; Kim, S.H.; Dev, R.; Hutchins, R.; Liem, C.; et al. Concepts and definitions for “supportive care,” “best supportive care,” “palliative care,” and “hospice care” in the published literature, dictionaries, and textbooks. Support. Care Cancer 2013, 21, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Goodlin, S.J.; Hauptman, P.J.; Arnold, R.; Grady, K.; Hershberger, R.E.; Kutner, J.; Masoudi, F.; Spertus, J.; Dracup, K.; Cleary, J.F.; et al. Consensus statement: Palliative and supportive care in advanced heart failure. J. Card. Fail. 2004, 10, 200–209. [Google Scholar] [CrossRef]
- Jaarsma, T.; Beattie, J.M.; Ryder, M.; Rutten, F.H.; McDonagh, T.; Mohacsi, P.; Murray, S.A.; Grodzicki, T.; Bergh, I.; Metra, M.; et al. Palliative care in heart failure: A position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2009, 11, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Lewin, W.H.; Cheung, W.; Horvath, A.N.; Haberman, S.; Patel, A.; Sullivan, D. Supportive Cardiology: Moving Palliative Care Upstream for Patients Living with Advanced Heart Failure. J. Palliat. Med. 2017, 20, 1112–1119. [Google Scholar] [CrossRef]
- Laforest, E.; Hartley, R.; Michel, C.; Cohen, R. Heart Failure Supportive Clinic: Improving palliative care access for patients living with heart failure? J. Pain Symptom Manage. 2008, 56, e112. [Google Scholar] [CrossRef] [Green Version]
- Gandesbery, B.; Dobbie, K.; Gorodeski, E.Z. Outpatient Palliative Cardiology Service Embedded Within a Heart Failure Clinic: Experiences With an Emerging Model of Care. Am. J. Hosp. Palliat. Care 2018, 35, 635–639. [Google Scholar] [CrossRef]
- Caprio, A.J. Palliative care: Renaming as supportive care and integration into comprehensive cancer care. CMAJ 2016, 188, 711–712. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.; Stevenson, L.W.; Grady, K.L.; Goldstein, N.E.; Matlock, D.D.; Arnold, R.M.; Cook, N.R.; Felker, G.M.; Francis, G.S.; Hauptman, P.J.; et al. Decision making in advanced heart failure: A Scientific Statement from the American Heart Association. Circulation 2012, 125, 1928–1952. [Google Scholar] [CrossRef]
- Warraich, H.J.; Allen, L.A.; Mukamal, K.J.; Ship, A.; Kociol, R.D. Accuracy of physician prognosis in heart failure and lung cancer: Comparison between physician estimates and model predicted survival. Palliat. Med. 2016, 30, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Canepa, M.; Fonseca, C.; Chioncel, O.; Laroche, C.; Crespo-Leiro, M.G.; Coats, A.J.S.; Mebazaa, A.; Piepoli, M.F.; Tavazzi, L.; Maggioni, A.P. Performance of Prognostic Risk Scores in Chronic Heart Failure Patients Enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail. 2018, 6, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.H.; Ganjoo, J.; Sharma, S.; Gansor, J.; Senft, S.; Weaner, B.; Dalton, C.; MacKay, K.; Pellegrino, B.; Anantharaman, P.; et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin. J. Am. Soc. Nephrol. 2008, 3, 1379–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, D.J.; Johnson, M.J.; Spruit, M.A. Palliative care needs assessment in chronic heart failure. Curr. Opin. Support. Palliat. Care 2018, 12, 25–31. [Google Scholar] [CrossRef]
- Gómez-Batiste, X.; Martínez-Muñoz, M.; Blay, C.; Amblàs, J.; Vila, L.; Costa, X.; Villanueva, A.; Espaulella, J.; Espinosa, J.; Figuerola, M.; et al. Identifying patients with chronic conditions in need of palliative care in the general population: Development of the NECPAL tool and preliminary prevalence rates in Catalonia. BMJ Support. Palliat. Care 2013, 3, 300–308. [Google Scholar] [CrossRef] [Green Version]
- The National Gold Standards Framework Centre UK. Gold Standards Framework Proactive Identication Guidance. 6th edition. 2016. Available online: http://www.goldstandardsframework.org.uk (accessed on 12 December 2019).
- Moss, A.H.; Lunney, J.R.; Culp, S.; Auber, M.; Kurian, S.; RogersJ Dower, J.; Abraham, J. Prognostic significance of the “surprise” question in cancer patients. J. Palliat. Med. 2010, 13, 834–837. [Google Scholar]
- Haga, K.; Murray, S.; Reid, J.; Ness, A.; O’Donnell, M.; Yellowlees, D.; Denvir, M.A. Identifying community based chronic heart failure patients in the last year of life: A comparison of the Gold Standards Framework Prognostic Indicator Guide and the Seattle Heart Failure Model. Heart 2012, 98, 579–583. [Google Scholar] [CrossRef]
- Straw, S.; Byrom, R.; Gierula, J.; Paton, M.F.; Koshy, A.; Cubbon, R.; Drozd, M.; Kearney, M.; Witte, K.K. Predicting one-year mortality in heart failure using the ‘Surprise Question’: A prospective pilot study. Eur. J. Heart Fail. 2018. (Epub ahead of print). [Google Scholar] [CrossRef] [Green Version]
- Noppe, D.; Veen, H.I.; Mooren, K. COPD patients in need of palliative care: Identification after hospitalization through the surprise question. Chron. Respir. Dis. 2019, 16. [Google Scholar] [CrossRef]
- Patel, R.B.; Warraich, H.J.; Butler, J.; Vaduganathan, M. Surprise, surprise: Improving the referral pathway to palliative care interventions in advanced heart failure. Eur. J. Heart Fail. 2018. [Google Scholar] [CrossRef] [Green Version]
- Morton, G.; Masters, J.; Cowburn, P.J. Multidisciplinary team approach to heart failure management. Heart 2018, 104, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.H.; Wolf, S.P.; Troy, J.; Leff, V.; Dahlin, C.; Rotella, J.D.; Handzo, G.; Rodgers, P.E.; Myers, E.R. Policy Changes Key To Promoting Sustainability And Growth Of The Specialty Palliative Care Workforce. Health Aff (Millwood). 2019, 38, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupu, D.; Quigley, L.; Mehfoud, N.; Salsberg, E.S. The Growing Demand for Hospice and Palliative Medicine Physicians: Will the Supply Keep Up? J. Pain Symptom Manage. 2018, 55, 1216–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewin, W.; Schaefer, K. Integrating palliative care into routine care of patients with heart failure: Models for clinical collaboration. Heart Fail. Rev. 2017, 22, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Nooruddin, Z.; Didwaniya, N.; Dev, R.; De La Cruz, M.; Kim, S.H.; Kwon, J.H.; Hutchins, R.; Liem, C.; Bruera, E. Concepts and Definitions for “Actively Dying,” “End of Life,” “Terminally Ill,” “Terminal Care,” and “Transition of Care”: A Systematic Review. J. Pain Symptom Manage. 2014, 47, 77–89. [Google Scholar] [CrossRef]
- Rogers, J.G.; Patel, C.B.; Mentz, R.J.; Granger, B.B.; Steinhauser, K.E.; Fiuzat, M.; Adams, P.A.; Speck, A.; Johnson, K.S.; Krishnamoorthy, A. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J. Am. Coll. Cardiol. 2017, 70, 331–341. [Google Scholar] [CrossRef]
- Sobanski, P.; Alt-Epping, B.; Currow, D.; Goodlin, S.J.; Grodzicki, T.; Hogg, K.; Janssen, D.J.A.; Johnson, M.J.; Krajnik, M.; Leget, C.; et al. Palliative care for people living with heart failure-European Association for Palliative Care Task Force expert position statement. Cardiovasc. Res. 2019. (Epub ahead of print). [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.T.; Petrie, M.C.; Jackson, C.E.; Jhund, P.S.; Wright, A.; Gardner, R.S.; Sonecki, P.; Pozzi, A.; McSkimming, P.; McConnachie, A.; et al. Which patients with heart failure should receive specialist palliative care? Eur. J. Heart Fail. 2018, 20, 1338–1347. [Google Scholar] [CrossRef]
- Masterson Creber, R.; Russell, D.; Doole, F.; Baik, D.; Goyal, P.; Hummel, S.; Hummel, E.K.; Bowles, K.H. Use of the Palliative Performance Scale to estimate survival among home hospice patients with heart failure. ESC Heart Fail. 2019, 6, 371–378. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanò, M. Barriers to Early Utilization of Palliative Care in Heart Failure: A Narrative Review. Healthcare 2020, 8, 36. https://doi.org/10.3390/healthcare8010036
Romanò M. Barriers to Early Utilization of Palliative Care in Heart Failure: A Narrative Review. Healthcare. 2020; 8(1):36. https://doi.org/10.3390/healthcare8010036
Chicago/Turabian StyleRomanò, Massimo. 2020. "Barriers to Early Utilization of Palliative Care in Heart Failure: A Narrative Review" Healthcare 8, no. 1: 36. https://doi.org/10.3390/healthcare8010036



