Morphogenetic Variability as Potential Biomarker of Neurogenic Lesion Degree in Children with Spina Bifida
Abstract
:1. Introduction
2. Methods
2.1. Study Group
2.2. Study Parameters
2.3. Statistical Analysis
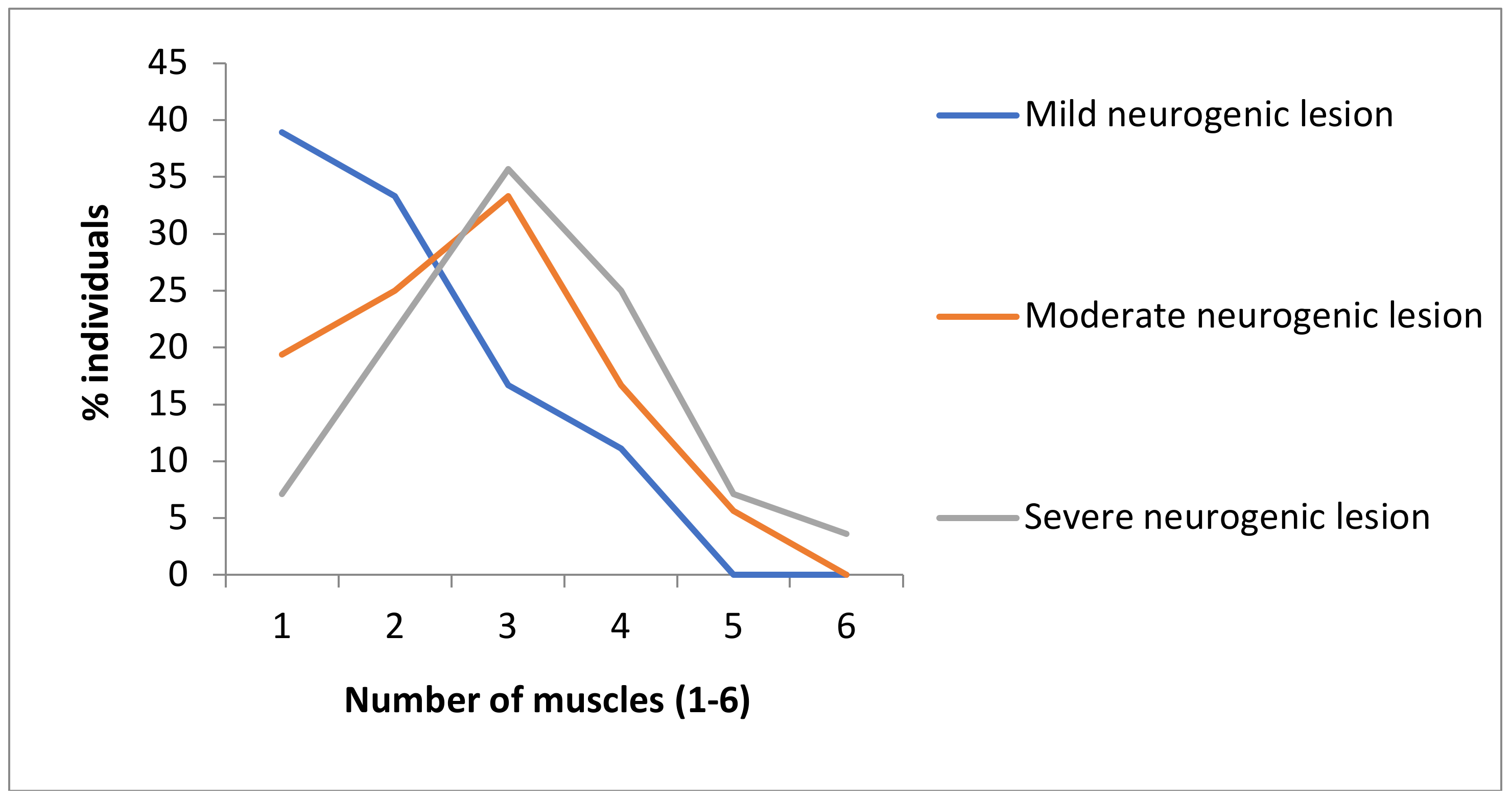
3. Results
4. Discussion
- An increase in individual genetic homozygosity, as well as a decrease in genetic variability in affected groups of individuals, may bring an organism into a specific state of genetic–hysiological homeostasis, which enables easier expression of different degrees of neurogenic lesions in SB patients.
- An increase in the genetic homozygosity degree may raise genetic loads, thus potentially causing decreased body resistance to developmental disbalances, respectively rising the expression of recessive genes that might be related to spina bifida.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Netto, J.M.; Bastos, A.N.; Figueiredo, A.A.; Perez, L.M. Spinal dysraphism: A neurosurgical review for the urologist. Rev. Urol. 2009, 11, 71–81. [Google Scholar] [PubMed]
- Frey, L.; Hauser, W.A. Epidemiology of neural tube defects. Epilepsia 2003, 44 (Suppl. 3), 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Lu, W.; Zhu, H.; Yang, W.; Shaw, G.M.; Lammer, E.J.; Islam, A.; Finnell, R.H. Genetic polymorphisms in the thioredoxin 2 (TXN2) gene and risk for spina bifida. Am. J. Med. Genet. A 2009, 149A, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvjeticanin, S.; Nikolic, D.; Petronic, I.; Jekic, B.; Damnjanovic, T.; Cirovic, D.; Radlovic, V.; Knezevic, T. Degree of genetic homozygocity among patients with spinal dysraphia. Srp. Arh. Celok. Lek. 2008, 136, 519–523. [Google Scholar] [CrossRef]
- Hol, F.A.; Schepens, M.T.; van Beersum, S.E.; Redolfi, E.; Affer, M.; Vezzoni, P.; Hamel, B.C.; Karnes, P.S.; Mariman, E.C.; Zucchi, I. Identification and characterization of an Xq26-q27 duplication in a family with spina bifida and panhypopituitarism suggests the involvement of two distinct genes. Genomics 2000, 69, 174–181. [Google Scholar] [CrossRef]
- Padmanabhan, R. Etiology, pathogenesis and prevention of neural tube defects. Congenit. Anom. 2006, 46, 55–67. [Google Scholar] [CrossRef]
- Li, Z.; Ren, A.; Zhang, L.; Guo, Z.; Li, Z. A population-based case-control study of risk factors for neural tube defects in four high-prevalence areas of Shanxi province, China. Paediatr. Perinat. Epidemiol. 2006, 20, 43–53. [Google Scholar] [CrossRef]
- Vujkovic, M.; Steegers, E.A.; Looman, C.W.; Ocké, M.C.; van der Spek, P.J.; Steegers-Theunissen, R.P. The maternal Mediterranean dietary pattern is associated with a reduced risk of spina bifida in the offspring. BJOG 2009, 116, 408–415. [Google Scholar] [CrossRef]
- Au, K.S.; Ashley-Koch, A.; Northrup, H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev. Disabil. Res. Rev. 2010, 16, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Online Mendelian Inheritance in Man (OMIM). Available online: http://www.ncbi.nlm.nih.gov (accessed on 24 March 2020).
- Marinkovic, D.; Cvjeticanin, S. Studies of human population-genetic variations. The frequencies of ABO blood types and homozygously recessive traits among top sportsmen and young intellectuals. Arch. Biol. Sci. 1991, 43, 5–6. [Google Scholar]
- Cvjeticanin, S.; Marinkovic, D. Genetic variability in the group of patients with congenital hip dislocation. Genetika 2005, 41, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Cvjeticanin, S.; Marinkovic, D. Genetic variability and frequencies of ABO blood types among different samples of patients from Serbia. Korean J. Genetics 2005, 27, 35–40. [Google Scholar]
- Pesut, D.P.; Marinkovic, D. Lung cancer and pulmonary tuberculosis- a comparative population-genetic study. Balk. J. Med. Genet. 2009, 12, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Milasinovic, S.; Cvjeticanin, S.; Brdar, R.; Nikolic, D. Morphogenetic variability and genetic loads among patients with different expression of developmental hip dysplasia. Genetika 2017, 49, 1035–1045. [Google Scholar] [CrossRef]
- Marinkovic, D.; Cvjeticanin, S. Anthropogenetic Homozygosity and Adaptive Variability: HRC-Test in Studies of Human Populations; Monographs DCLXXII, Book 8; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2013. [Google Scholar]
- Chang, C.K.; Wong, T.T.; Huang, B.S.; Chan, R.C.; Yang, T.F. Spinal dysraphism: A cross-sectional and retrospective multidisciplinary clinic-based study. J. Chin. Med. Assoc. 2008, 71, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Sonoo, M. Electrodiagnosis of neuromuscular disorders. Brain Nerve 2007, 59, 241–250. [Google Scholar]
- Petronic, I.; Nikolic, D.; Cirovic, D.; Cvjeticanin, S.; Knezevic, T.; Raicevic, M.; Brdar, R.; Dzamic, D.; Janic, N.; Golubovic, Z. Distribution of affected muscles and degree of neurogenic lesion in patients with spina bifida. Arch. Med. Sci. 2011, 7, 1049–1054. [Google Scholar] [CrossRef]
- Preston, D.C.; Shapiro, B.E. Needle electromyography. Fundamentals, normal and abnormal patterns. Neurol. Clin. 2002, 20, 361–396. [Google Scholar]
- Ciesla, N.; Dinglas, V.; Fan, E.; Kho, M.; Kuramoto, J.; Needham, D. Manual muscle testing: A method of measuring extremity muscle strength applied to critically Ill patients. J. Vis. Exp. 2011, 50, 2632. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Beaudin, A.E.; Stover, P.J. Insights into metabolic mechanisms underlying folate-responsive neural tube defects: a minireview. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.; Loughna, S.C.; Stanier, P.M.; Jensson, O.; Moore, G.E. X-linked Spina Bifida: A Linkage Analysis; (Series) Miami Short Reports; Advances in Gene Technology: The Molecular Biology of Human Genetic Disease; IRL Press: New York, NY, USA, 1991; p. 33. [Google Scholar]
- Chih-Ping, C. Chromosomal abnormalities associated with neural tube defects (II): Partial aneuploidy. Taiwan J. Obstet. Gynecol. 2007, 46, 336–351. [Google Scholar]
- Jensson, O.; Arnason, A.; Gunnarsdottir, H.; Petursdottir, I.; Fossdal, R.; Hreidarsson, S. A family showing apparent X linked inheritance of both anencephaly and spina bifida. J. Med. Genet. 1988, 25, 227–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Yang, W.; Lu, W.; Zhang, J.; Shaw, G.M.; Lammer, E.J.; Finnell, R.H. A known functional polymorphism (Ile120Val) of the human PCMT1 gene and risk of spina bifida. Mol. Genet. Metab. 2006, 87, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, D.; Cvjeticanin, S.; Petronic, I.; Jekic, B.; Brdar, R.; Damnjanovic, T.; Bunjevacki, V.; Maksimovic, N. Degree of genetic homozygosity and distribution of ABO blood types among patients with spina bifida occulta and spina bifida aperta. Arch. Med. Sci. 2010, 6, 854–859. [Google Scholar] [CrossRef] [Green Version]
- Mominoki, K.; Kinutani, M.; Wakisaka, H.; Saito, S.; Kobayashi, N.; Fujiwara, T.; Matsuda, S. Leg dysfunctions in a hatched chick model of spina bifida aperta. Exp. Neurol. 2006, 197, 133–142. [Google Scholar] [CrossRef]
- Marinkovic, D.; Cvjeticanin, S. Population-genetic study of Balkan endemic nephrophaty in Serbia. Genetika 2007, 43, 1134–1138. [Google Scholar]

| Homozygously Recessive Characteristics | Kappa Value (Control Sample N = 100) | ||
|---|---|---|---|
| Test | Retest - 1 | Retest - 2 | |
| Blond Hair | 0.94 | 0.96 | 0.98 |
| Straight Hair | 0.98 | 1.00 | 1.00 |
| Double Hair Whorl | 1.00 | 1.00 | 1.00 |
| Opposite Hair Whorl Orientation | 0.96 | 1.00 | 1.00 |
| Soft Hair | 0.96 | 0.98 | 1.00 |
| Continuous Hair Line | 0.96 | 1.00 | 1.00 |
| Attached Ear Lobe | 0.96 | 1.00 | 1.00 |
| Ear Without Darwinian notch | 1.00 | 1.00 | 1.00 |
| Blue Eyes | 0.96 | 1.00 | 1.00 |
| Color Blindness | 1.00 | 1.00 | 1.00 |
| Right Thumb over Left Thumb | 1.00 | 1.00 | 1.00 |
| Hand Clasping Pattern | 1.00 | 1.00 | 1.00 |
| Proximal thumb extensibility | 0.98 | 1.00 | 1.00 |
| Left-handedness | 1.00 | 1.00 | 1.00 |
| Index finger longer than the ring finger | 0.98 | 1.00 | 1.00 |
| Spina Bifida | Total | Paralysis | Paresis | ||
|---|---|---|---|---|---|
| N | N (%) | p* | N (%) | p* | |
| SBO | 76 | 0 (0) | <0.0001 | 36 (47.4) | 0.6069 |
| SBA | 46 | 22 (47.8) | 24 (52.2) | ||
| Total | 122 | 22 (18) | - | 60 (49.2) | - |
| HRC | Affected Muscles | |||
|---|---|---|---|---|
| p * | p ** | p * | p ** | |
| All groups | - | <0.0001 | - | <0.0054 |
| Mild and Moderate | 0.0093 | - | 0.0615 | - |
| Moderate and Severe | 0.9045 | - | 0.1211 | - |
| Mild and Severe | <0.0005 | - | 0.0029 | - |
| Mild and control | 0.0160 | - | - | - |
| Moderate and control | <0.0001 | - | - | - |
| Severe and control | <0.0001 | - | - | - |
| Degree of neurogenic lesion/Degree of genetic homozygosity | Pearson correlation | |||
| 0.320 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronic, I.; Marinkovic, D.; Nikolic, D.; Cirovic, D.; Golubovic, Z.; Milanovic, F.; Cvjeticanin, S. Morphogenetic Variability as Potential Biomarker of Neurogenic Lesion Degree in Children with Spina Bifida. Healthcare 2020, 8, 68. https://doi.org/10.3390/healthcare8010068
Petronic I, Marinkovic D, Nikolic D, Cirovic D, Golubovic Z, Milanovic F, Cvjeticanin S. Morphogenetic Variability as Potential Biomarker of Neurogenic Lesion Degree in Children with Spina Bifida. Healthcare. 2020; 8(1):68. https://doi.org/10.3390/healthcare8010068
Chicago/Turabian StylePetronic, Ivana, Dragoslav Marinkovic, Dejan Nikolic, Dragana Cirovic, Zoran Golubovic, Filip Milanovic, and Suzana Cvjeticanin. 2020. "Morphogenetic Variability as Potential Biomarker of Neurogenic Lesion Degree in Children with Spina Bifida" Healthcare 8, no. 1: 68. https://doi.org/10.3390/healthcare8010068





