A Decision Support Method for Prehospital Emergency Care Based on Ranking the Importance of Physiological Variables
Abstract
:1. Introduction
2. Related Work
3. Methods
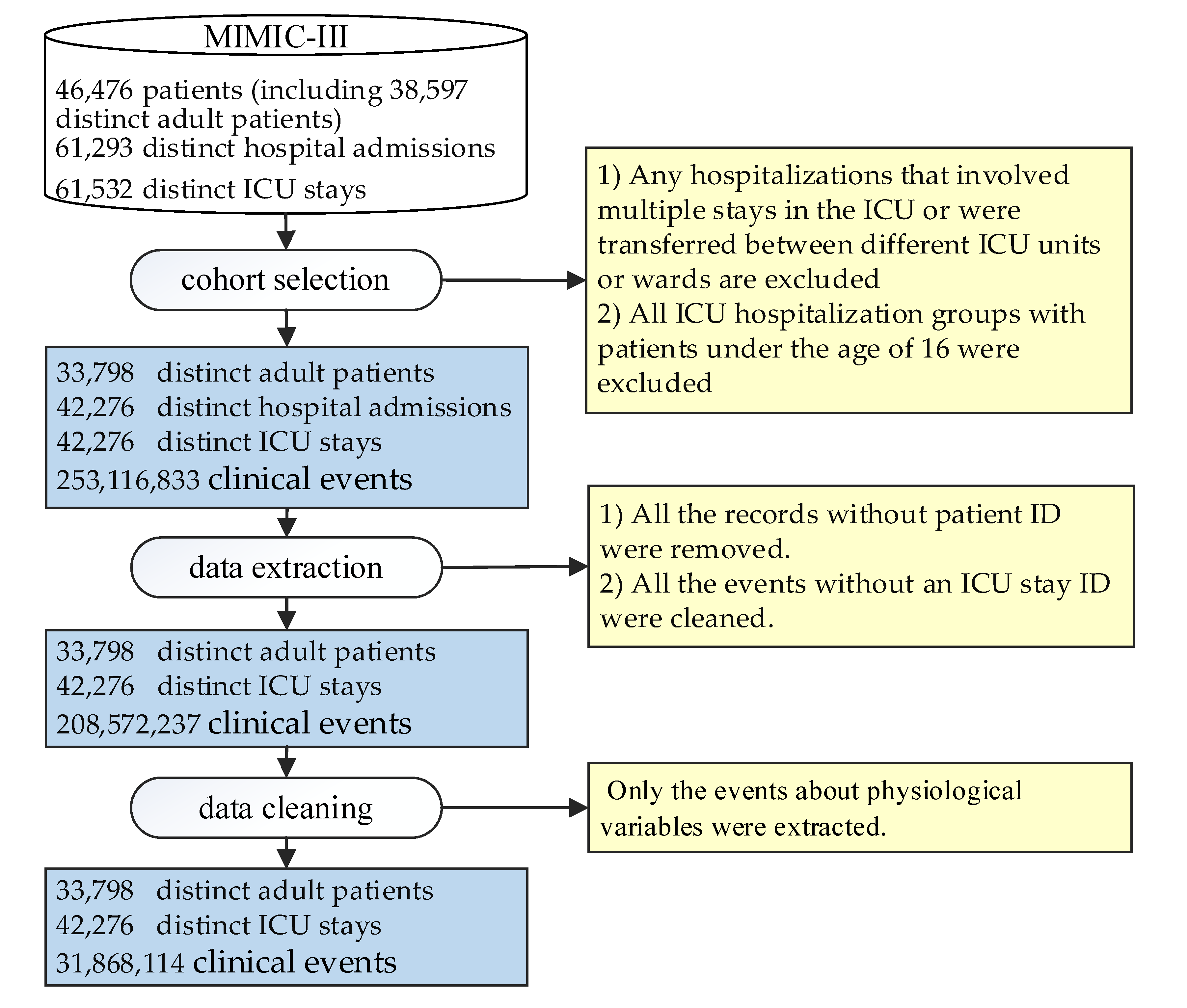
3.1. ICU Patient Database and Data Preprocessing
3.1.1. ICU Database and Data Preprocessing
3.1.2. Cohort Selection
3.1.3. Data Extraction
3.1.4. Data Cleaning
3.2. PV Set Design
3.3. Multinomial Logistic Regression Model
3.3.1. Parameter Estimation
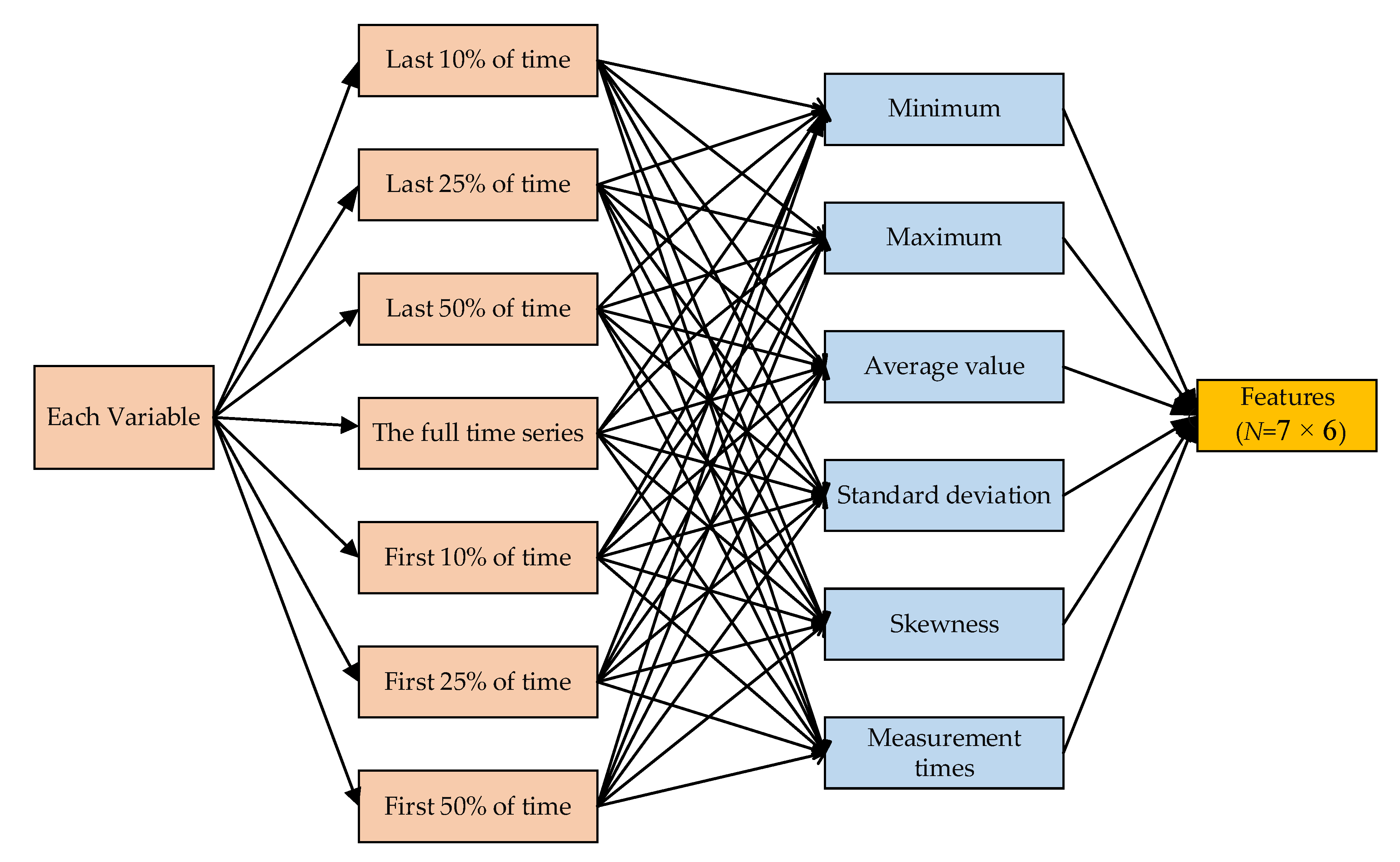
3.3.2. Feature Extraction
4. Experiment and Results
4.1. Experimental Dataset
4.2. Experimental Design
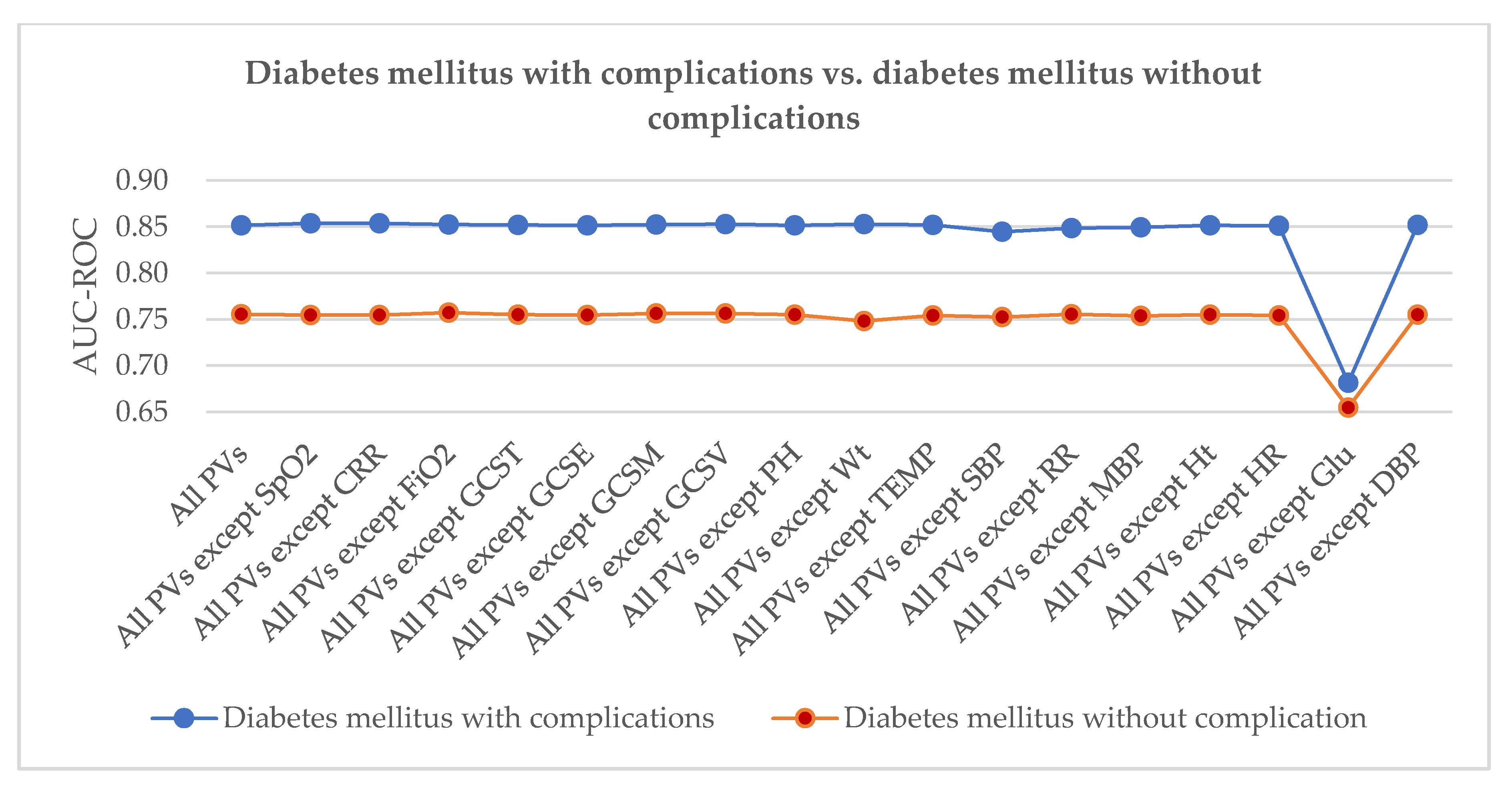
4.3. Results
5. Discussion
5.1. Comparison to Other Methods
5.2. Limitations and Future Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murphy, A.; Wakai, A.; Walsh, C.; Cummins, F.; O’Sullivan, R. Development of key performance indicators for prehospital emergency care. Emerg. Med. J. 2016, 33, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Lee, J.; Sleigh, A.; Banwell, C. Putting Culture into Prehospital Emergency Care: A Systematic Narrative Review of Literature from Lower Middle-Income Countries. Prehosp. Disaster Med. 2019, 34, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Kerner, T.; Schmidbauer, W.; Tietz, M.; Marung, H.; Genzwuerker, H.V. Use of checklists improves the quality and safety of prehospital emergency care. Eur. J. Emerg. Med. 2017, 24, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Glober, N.K.; Sporer, K.A.; Guluma, K.Z.; Serra, J.P.; Barger, J.A.; Brown, J.F.; Gilbert, G.H.; Koenig, K.L.; Rudnick, E.M.; Salvucci, A.A. Acute stroke: Current evidence-based recommendations for prehospital care. West. J. Emerg. Med. 2016, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-Y.; Cho, K.-J.; Kwon, O.; Kwon, J.; Jeon, K.-H.; Park, H.; Lee, Y.; Park, J.; Oh, B.-H. Artificial intelligence algorithm to predict the need for critical care in prehospital emergency medical services. Scand. J. Trauma. Resusc. Emerg. Med. 2020, 28, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.W.; Saria, S.; Ohno-Machado, L.; Shah, A.; Escobar, G. Big Data in Health Care: Using Analytics to Identify and Manage High-risk and High-cost Patients. Health Aff. 2014, 33, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xie, P.; Gao, X.; Cheng, C.; Li, C.; Zhang, H.; Xing, E. Predicting Discharge Medications at Admission Time Based on Deep Learning. arXiv 2017, arXiv:1711.01386. [Google Scholar]
- Lipton, Z.C.; Kale, D.C.; Elkan, C.; Wetzel, R. Learning to Diagnose with LSTM Recurrent Neural Networks. arXiv 2015, arXiv:1511.03677. [Google Scholar]
- Alsholm, L.; Axelsson, C.; Andersson Hagiwara, M.; Niva, M.; Claesson, L.; Herlitz, J.; Magnusson, C.; Rosengren, L.; Jood, K. Interrupted transport by the emergency medical service in stroke/transitory ischemic attack: A consequence of changed treatment routines in prehospital emergency care. Brain Behav. 2019, 9, e01266. [Google Scholar] [CrossRef]
- Huang, W.; Wang, T.B.; He, Y.D.; Zhang, H.; Zhou, X.H.; Liu, H.; Zhang, J.J.; Tian, Z.B.; Jiang, B.G. Trends and characteristics in pre-hospital emergency care in Beijing from 2008 to 2017. Chin. Med. J. (Engl.) 2020, 133, 1268–1275. [Google Scholar] [CrossRef]
- Lindskou, T.A.; Mikkelsen, S.; Christensen, E.F.; Hansen, P.A.; Jørgensen, G.; Hendriksen, O.M.; Kirkegaard, H.; Berlac, P.A.; Søvsø, M.B. The Danish prehospital emergency healthcare system and research possibilities. Scand. J. Trauma. Resusc. Emerg. Med. 2019, 27, 100. [Google Scholar] [CrossRef] [Green Version]
- Turner, S.; Lang, E.S.; Brown, K.; Franke, J.; Workun-Hill, M.; Jackson, C.; Roberts, L.; Leyton, C.; Bulger, E.M.; Censullo, E.M.; et al. Systematic Review of Evidence-Based Guidelines for Prehospital Care. Prehospital Emerg. Care 2020, 1–14. [Google Scholar] [CrossRef]
- Ohbe, H.; Isogai, S.; Nakajima, M.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Physician-manned prehospital emergency care in tertiary emergency centers in Japan. Acute Med. Surg. 2019, 6, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, D.; McKinlay, J.; Jefferys, S.; Wright, C. Military Prehospital Emergency Care: Defining and professionalising the levels of care provided along the Operational Patient Care Pathway. BMJ Mil. Health 2019, 165, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.A.; Magnusson, C.; Herlitz, J.; Seffel, E.; Axelsson, C.; Munters, M.; Strömsöe, A.; Nilsson, L. Adverse events in prehospital emergency care: A trigger tool study. BMC Emerg. Med. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; O’Hara, R.; Hirst, E.; Weyman, A.; Turner, J.; Mason, S.; Quinn, T.; Shewan, J.; Siriwardena, A.N. Multiple triangulation and collaborative research using qualitative methods to explore decision making in pre-hospital emergency care. BMC Med. Res. Methodol. 2017, 17, 11. [Google Scholar] [CrossRef]
- Bashiri, A.; Savareh, B.A.; Ghazisaeedi, M. Promotion of prehospital emergency care through clinical decision support systems: Opportunities and challenges. Clin. Exp. Emerg. Med. 2019, 6, 288–296. [Google Scholar] [CrossRef]
- Howard, I.; Cameron, P.; Wallis, L.; Castrén, M.; Lindström, V. Identifying quality indicators for prehospital emergency care services in the low to middle income setting: The South African perspective. Afr. J. Emerg. Med. 2019, 9, 185–192. [Google Scholar] [CrossRef]
- Wang, D. Analysis of the Complexity Patterns in Respiratory Data. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; pp. 2556–2560. [Google Scholar]
- Ritsinger, V.; Tanoglidi, E.; Malmberg, K.; Näsman, P.; Rydén, L.; Tenerz, Å.; Norhammar, A. Sustained Prognostic Implications of Newly Detected Glucose Abnormalities in Patients with Acute Myocardial Infarction: Long-Term Follow-Up of The Glucose Tolerance in Patients with Acute Myocardial Infarction Cohort. Diabetes Vasc. Dis. Res. 2015, 12, 23–32. [Google Scholar] [CrossRef]
- Vanegas, M.I.; Ghilardi, M.F.; Kelly, S.P.; Blangero, A. Machine learning for EEG-based biomarkers in Parkinson’s disease. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; pp. 2661–2665. [Google Scholar]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of The Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef] [Green Version]
- Mårtensson, J.; Egi, M.; Bellomo, R. Blood Glucose Control in Critical Care. Crit. Care Nephrol. Third Ed. 2017, 6, 464–469. [Google Scholar]
- DeQuattro, K.; Urisman, A.; Margaretten, M. A 36-Year-Old Man With Renal Failure, Fever, and Hypocomplementemia. Arthritis Care Res. (Hoboken) 2019, 71, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tumer, N.B.; Kunt, A.T.; Gunaydin, S. Preoperative urinary pH is associated with acute kidney injury after cardiac surgery in non-diabetic patients. Heart Surg. Forum 2019, 22, E456–E461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Lei, X.; Guo, L.; Zhang, A. Predicting the associations between microbes and diseases by integrating multiple data sources and path-based HeteSim scores. Neurocomputing 2019, 323, 76–85. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Cao, Z.; Zhao, N.; Chen, C.; Wang, D.; Lei, K.C.; Leng, D.; Zhang, X.D. Prediction Indicators for Acute Exacerbations of Chronic Obstructive Pulmonary Disease by Combining Non-linear analyses and Machine. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine, (BIBM), Madrid, Spain, 3–6 December 2018; pp. 2515–2521. [Google Scholar]
- Böhning, D. Multinomial logistic regression algorithm. Ann. Inst. Stat. Math. 1992, 44, 197–200. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Pollard, T.J.; Shen, L.; Lehman, L.-w.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R.G. MIMIC-III, a Freely Accessible Critical Care Database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef] [Green Version]
- Harutyunyan, H.; Khachatrian, H.; Kale, D.C.; Ver Steeg, G.; Galstyan, A. Multitask Learning and Benchmarking with Clinical Time Series Data. Sci. Data 2017, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.; Moody, G.; Scott, D.J.; Celi, L.A.; Mark, R.G. Predicting In-Hospital Mortality of ICU Patients: The Physionet/Computing in Cardiology Challenge 2012. In Proceedings of the 2012 Computing in Cardiology, Krakow, Poland, 9–12 September 2012; pp. 245–248. [Google Scholar]
- Saltelli, A.; Tarantola, S.; Campolongo, F.; Ratto, M. Sensitivity Analysis in Practice: A Guide to Assessing Scientific Models; Wiley Online Library: Hoboken, NJ, USA, 2004; Volume 1. [Google Scholar]
- Carmeliet, E. History of the Disorders of Cardiac Rhythm. J. Cardiovasc. Pharmacol. 1999, 33, 173. [Google Scholar] [CrossRef]




| No. | Disease Name | No. | Disease Name |
|---|---|---|---|
| D1 | Acute and unspecified renal failure | D13 | Disorders of lipid metabolism |
| D2 | Acute cerebrovascular disease | D14 | Essential hypertension |
| D3 | Acute myocardial infarction | D15 | Hypertension with complications and secondary hypertension |
| D4 | Cardiac dysrhythmias | D16 | Fluid and electrolyte disorders |
| D5 | Chronic kidney disease | D17 | Gastrointestinal hemorrhage |
| D6 | Congestive heart failure; non-hypertensive | D18 | Other liver diseases |
| D7 | Chronic obstructive pulmonary disease and bronchiectasis | D19 | Other lower respiratory diseases |
| D8 | Complications of surgical procedures or medical care | D20 | Other upper respiratory diseases |
| D9 | Conduction disorder | D21 | Pleurisy; pneumothorax; pulmonary collapse |
| D10 | Coronary atherosclerosis and other heart diseases | D22 | Pneumonia (except that caused by tuberculosis or sexually transmitted diseases) |
| D11 | Diabetes mellitus with complications | D23 | Respiratory failure; insufficiency; arrest (adult) |
| D12 | Diabetes mellitus without complications | D24 | Septicemia (except in labor) |
| No. | PV Name (Abbreviation) | No. | PV Name (Abbreviation) |
|---|---|---|---|
| PV1 | Oxygen saturation (SpO2) | PV10 | Temperature (TEMP) |
| PV2 | Capillary refill rate (CRR) | PV11 | Systolic blood pressure (SBP) |
| PV3 | Fraction inspired oxygen (FiO2) | PV12 | Respiratory rate (RR) |
| PV4 | Glasgow coma scale total (GCST) | PV13 | Mean blood pressure (MBP) |
| PV5 | Glasgow coma scale eye opening (GCSE) | PV14 | Height (Ht) |
| PV6 | Glasgow coma scale motor response (GCSM) | PV15 | Heart rate (HR) |
| PV7 | Glasgow coma scale verbal response (GCSV) | PV16 | Glucose (Glu) |
| PV8 | pH (PH) | PV17 | Diastolic blood pressure (DBP) |
| PV9 | Weight (Wt) |
| Rank | Conduction Disorder | Coronary Atherosclerosis and Other Heart Disease | Diabetes Mellitus with Complications | Diabetes Mellitus without Complications |
|---|---|---|---|---|
| 1 | HR 1 | HR | Glu | Glu |
| 2 | CRR | CRR | SBP | Weight |
| 3 | SpO2 | SpO2 | RR | SBP |
| 4 | RR | GCSM | MBP | MBP |
| 5 | GCST | Glu | HR | TEMP |
| 6 | TEMP | RR | PH | HR |
| 7 | GCSV | TEMP | Ht | GCSE |
| 8 | DBP | SBP | GCSE | SpO2 |
| 9 | GCSE | MBP | GCST | CRR |
| 10 | Glu | DBP | TEMP | Ht |
| 11 | GCSM | GCST | DBP | DBP |
| 12 | PH | GCSE | GCSM | PH |
| 13 | Ht | PH | FiO2 | GCST |
| 14 | SBP | GCSV | GCSV | RR |
| 15 | MBP | FiO2 | Weight | GCSM |
| 16 | FiO2 | Ht | SpO2 | GCSV |
| 17 | Weight | Weight | CRR | FiO2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, S.; Li, F.; Rao, G. A Decision Support Method for Prehospital Emergency Care Based on Ranking the Importance of Physiological Variables. Healthcare 2020, 8, 295. https://doi.org/10.3390/healthcare8030295
Zhang L, Zhao S, Li F, Rao G. A Decision Support Method for Prehospital Emergency Care Based on Ranking the Importance of Physiological Variables. Healthcare. 2020; 8(3):295. https://doi.org/10.3390/healthcare8030295
Chicago/Turabian StyleZhang, Li, Shuying Zhao, Fang Li, and Guozheng Rao. 2020. "A Decision Support Method for Prehospital Emergency Care Based on Ranking the Importance of Physiological Variables" Healthcare 8, no. 3: 295. https://doi.org/10.3390/healthcare8030295







