Haemophilic Pelvic Pseudotumour: A New Surgical Option
Abstract
1. Introduction
2. Case Presentation
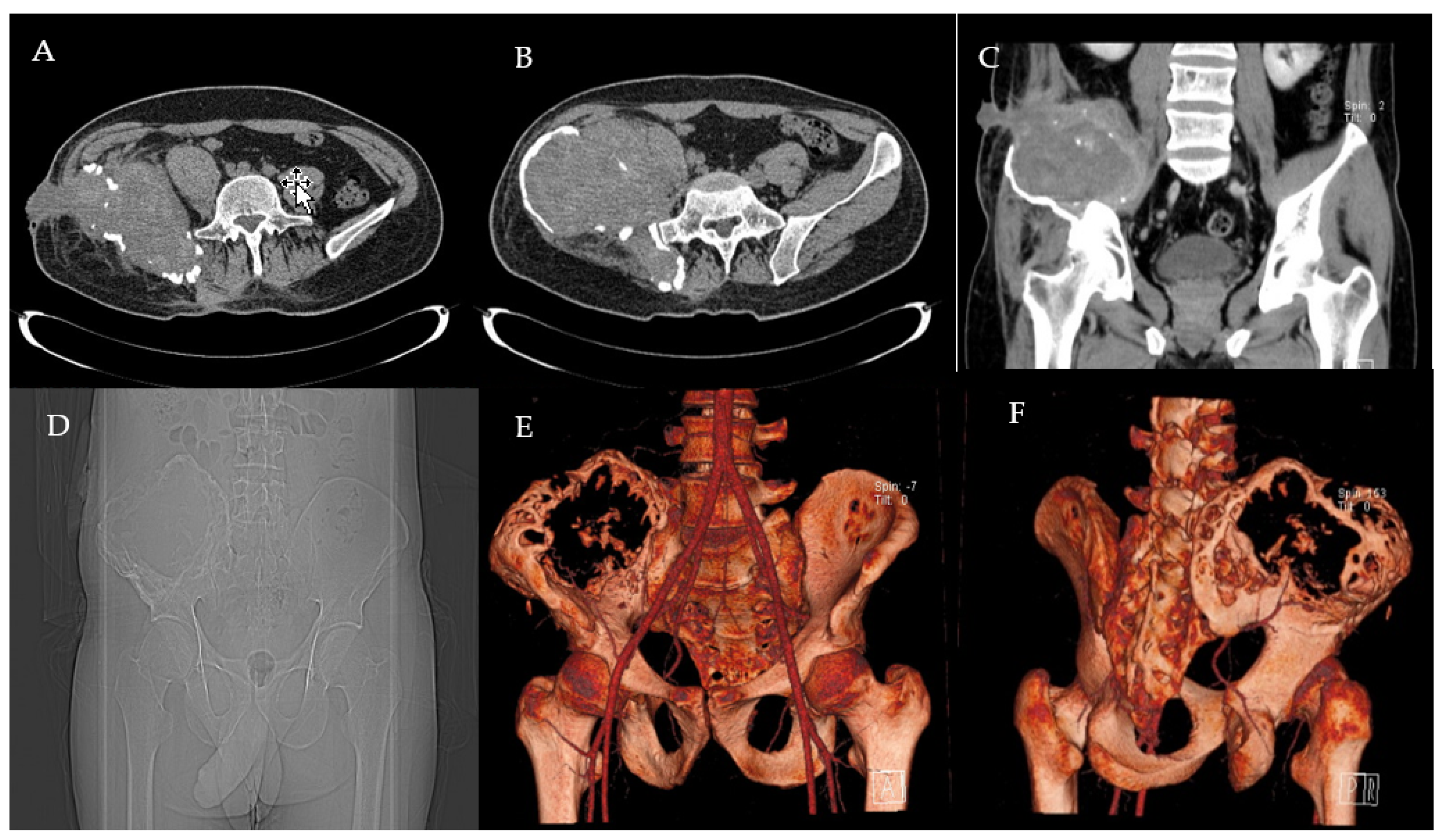
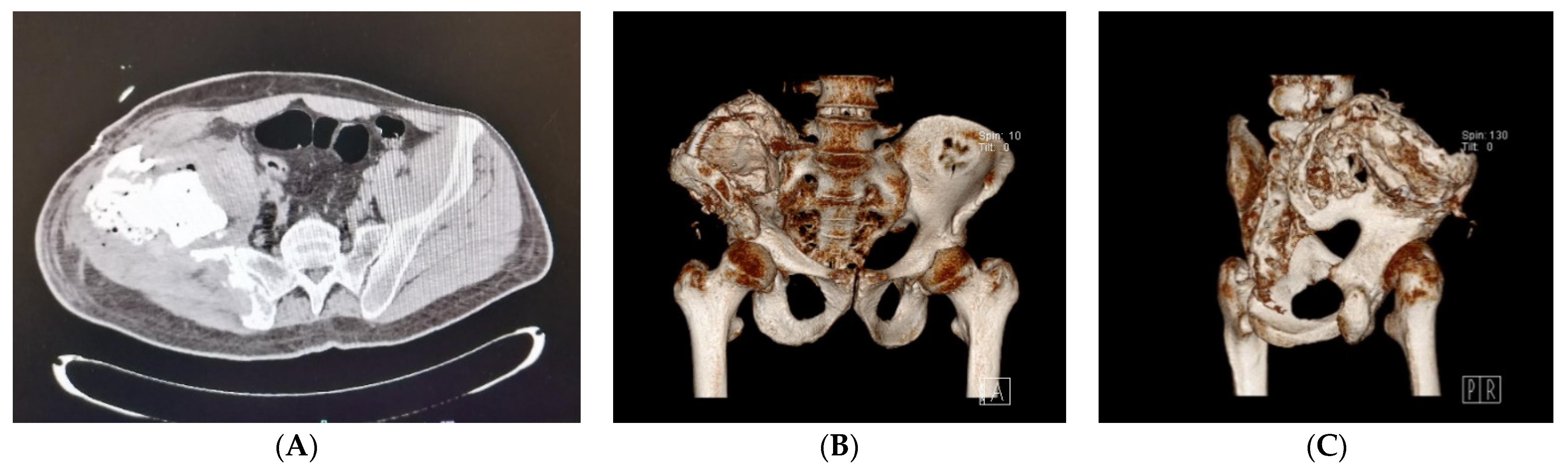
2.1. Clinical History and Instrumental Assessment
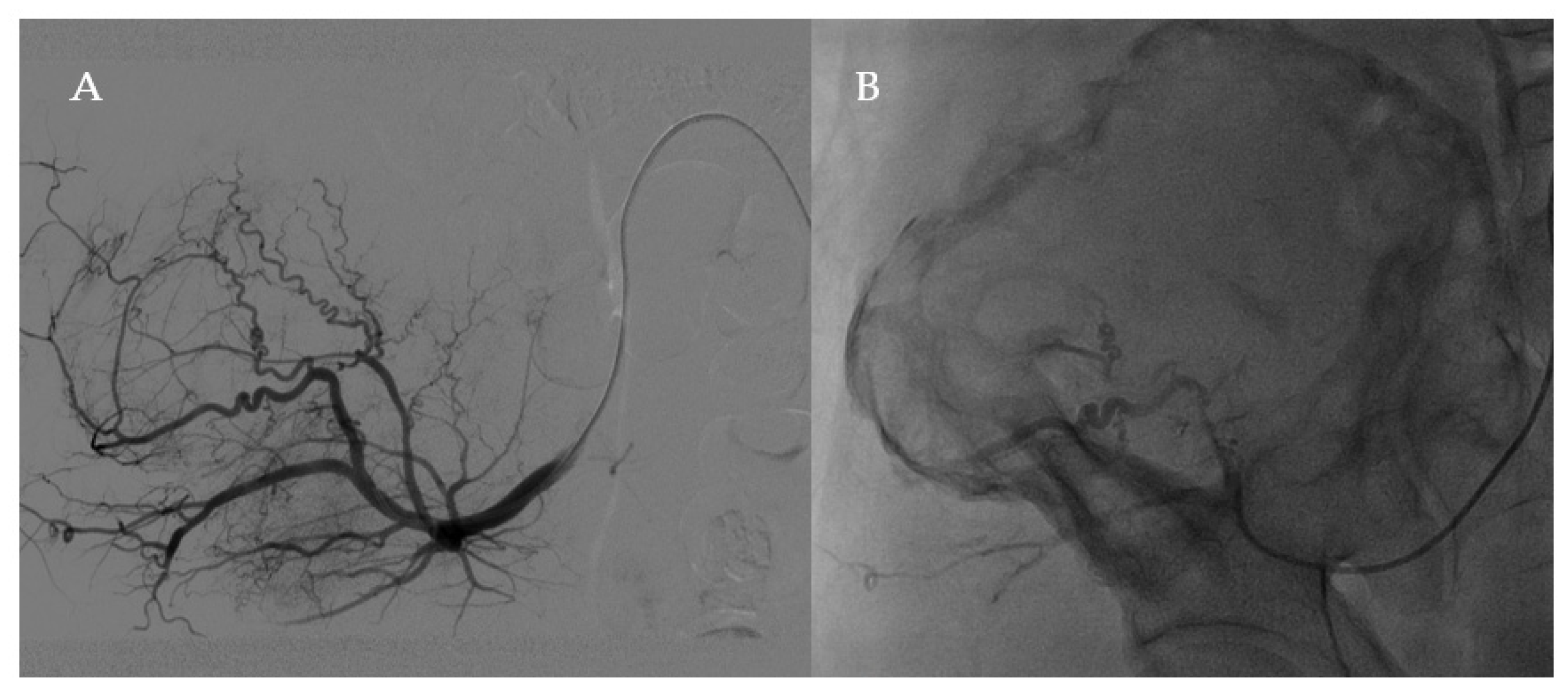
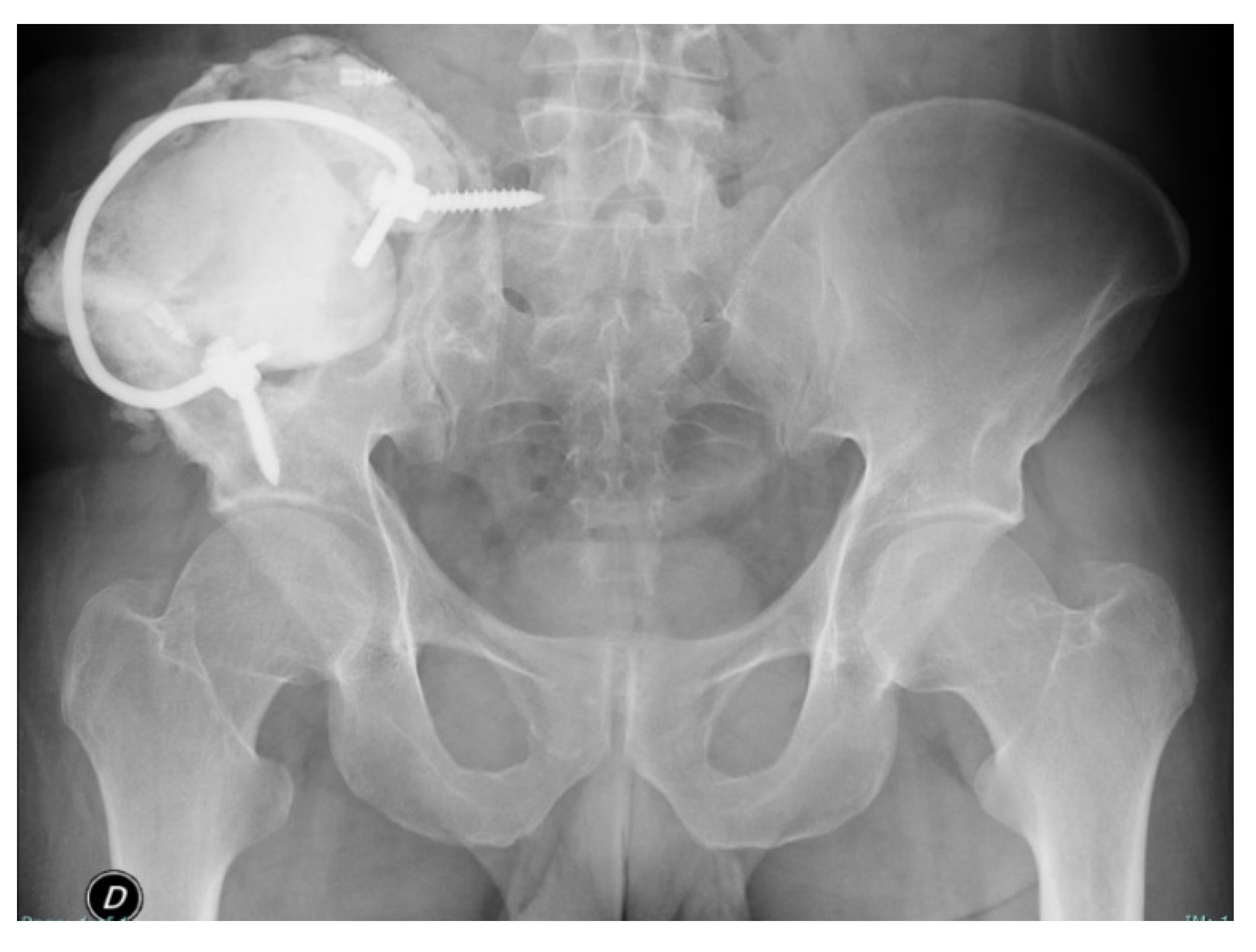
2.2. Surgery
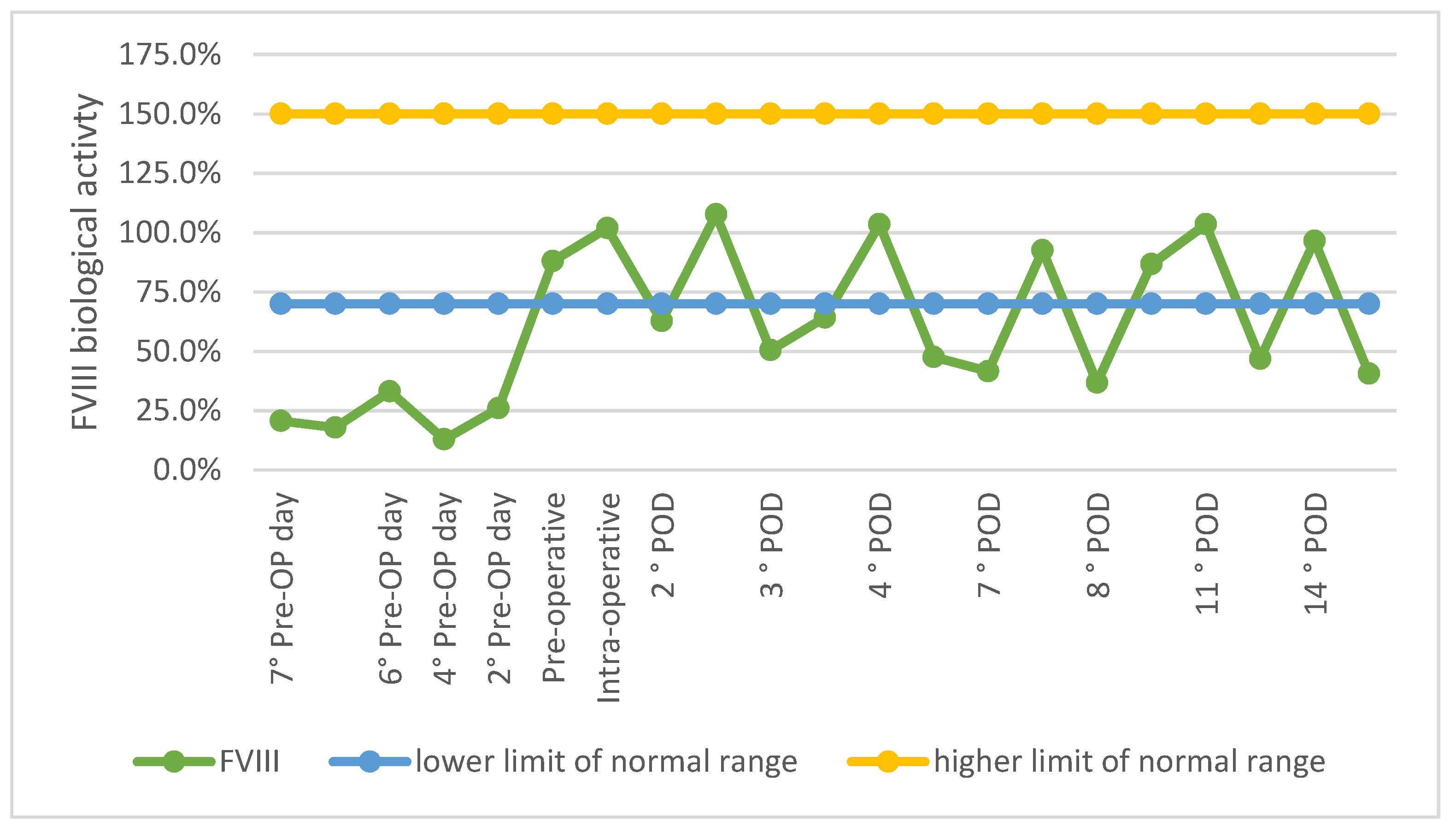
2.3. Postoperative Period
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lobet, S.; Hermans, C.; Lambert, C. Optimal Management of Hemophilic Arthropathy and Hematomas. J. Blood Med. 2014, 5, 207–218. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Hemophilic Pseudotumors: Diagnosis and Management. Arch. Bone Jt. Surg. 2020, 8, 121–130. [Google Scholar] [CrossRef]
- Anderson, C.L.; Alvarez, O.; Saigal, G.; Davis, J.A. Hemophilic Pseudotumor: An Important Differential Diagnosis of an Intracranial Mass. J. Pediatr. Hematol. Oncol. 2015, 37, 219–222. [Google Scholar] [CrossRef]
- Caviglia, H.; Candela, M.; Landro, M.E.; Price, A.L.D.; Neme, D.; Galatro, G.A. Haemophilia Pseudotumours in Patients with Inhibitors. Haemophilia 2015, 21, 681–685. [Google Scholar] [CrossRef]
- Caviglia, H.A.; Daniela, N.; Miguel, C.; Guillermo, C.; Eulalia, M.; Gustavo, G. Pseudotumor Surgery in Haemophilia a Patient: Comparative Results between Inhibitor and Non-Inhibitor Patients. J. Blood. Disord. 2015, 2, 1020. [Google Scholar]
- Said, B.; Forsyth, A.; Solimeno, L.P.; Kouides, P.A.; Poudyal, B.S. Surgical Excision of a Pseudotumour under the Coverage of the Plasma-Derived Factor X Concentrate in a Patient with Severe Factor X Deficiency: A Case Report. Haemoph. Off. J. World Fed. Hemoph. 2021, 27, e543–e546. [Google Scholar] [CrossRef]
- Valentino, L.A.; Martinowitz, U.; Doolas, A.; Murali, P. Surgical Excision of a Giant Pelvic Pseudotumour in a Patient with Haemophilia A. Haemoph. Off. J. World Fed. Hemoph. 2006, 12, 541–544. [Google Scholar] [CrossRef]
- Frioui, S.; Jemni, S. Une Complication Musculaire Rarissime de l’hémophilie: La Myosite Ossifiante. Pan. Afr. Med. J. 2015, 22, 149. [Google Scholar] [CrossRef]
- Klein, A.A.; Arnold, P.; Bingham, R.M.; Brohi, K.; Clark, R.; Collis, R.; Gill, R.; McSporran, W.; Moor, P.; Rao Baikady, R.; et al. AAGBI Guidelines: The Use of Blood Components and Their Alternatives 2016. Anaesthesia 2016, 71, 829–842. [Google Scholar] [CrossRef]
- Kamal, A.F.; Waryudi, A.; Kurniawan, A.; Lubis, A.M.; Gatot, D. Various Surgical Treatment of Hemophilic Pseudotumor: A Case Series. Arch. Bone Jt. Surg. 2019, 7, 514–522. [Google Scholar]
- Liu, S.; Zhou, X.; Song, A.; Huo, Z.; Wang, Y.; Liu, Y. Successful Resection of Giant Abdominal Hemophilic Pseudotumor. Med. Baltim. 2019, 98, e17998. [Google Scholar] [CrossRef]
- Lin, S.; Tong, K.; Wang, G.; Zhong, Z.; Cao, S.; Feng, Z. Clinical Characteristics and Surgical Treatment of Haemophilic Pseudotumor: A Retrospective Analysis of Thirty-Four Patients. Haemoph. Off. J. World Fed. Hemoph. 2020, 26, 873–881. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, G.; Xu, Y.; Yang, Y.; Mao, Z.; Lv, J.; Liu, F.; Chen, B. Surgical Treatment for Patients with Hemophilic Pseudotumor-Related Femoral Fracture: A Retrospective Study. J. Orthop. Surg. 2021, 16, 275. [Google Scholar] [CrossRef]
- Panotopoulos, J.; Ay, C.; Trieb, K.; Funovics, P.T.; Stockhammer, V.; Lang, S.; Holinka, J.; Windhager, R.; Pabinger, I.; Wanivenhaus, H.A. Surgical Treatment of the Haemophilic Pseudotumour: A Single Centre Experience. Int. Orthop. 2012, 36, 2157–2162. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Surgical Approaches to Hemophilic Arthropathy. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2019, 30, S11–S13. [Google Scholar] [CrossRef]
- Stafford, J.M.; James, T.T.; Allen, A.M.; Dixon, L.R. Hemophilic Pseudotumor: Radiologic-Pathologic Correlation. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2003, 23, 852–856. [Google Scholar] [CrossRef]
- Horton, D.D.; Pollay, M.; Wilson, D.A.; Brandon, F.M.; Sexauer, C.L. Cranial Hemophilic Pseudotumor. Case Report. J. Neurosurg. 1993, 79, 936–938. [Google Scholar] [CrossRef]
- Sim, K.B.; Hong, S.K. Cranial Hemophilic Pseudotumor: Case Report. Neurosurgery 1996, 39, 1239–1242. [Google Scholar] [CrossRef]
- Issaivanan, M.; Shrikande, M.P.; Mahapatra, M.; Choudhry, V.P. Management of Hemophilic Pseudotumor of Thumb in a Child. J. Pediatr. Hematol. Oncol. 2004, 26, 128–132. [Google Scholar] [CrossRef]
- Lima, G.S.; Robaina, T.F.; de Queiroz Chaves Lourenço, S.; Dias, E.P. Maxillary Hemophilic Pseudotumor in a Patient with Mild Hemophilia A. J. Pediatr. Hematol. Oncol. 2008, 30, 605–607. [Google Scholar] [CrossRef]
- Simurda, T.; Kubisz, P.; Dobrotova, M.; Necas, L.; Stasko, J. Perioperative Coagulation Management in a Patient with Congenital Afibrinogenemia during Revision Total Hip Arthroplasty. Semin. Thromb. Hemost. 2016, 42, 689–692. [Google Scholar] [CrossRef]
- Espandar, R.; Heidari, P.; Rodriguez-Merchan, E.C. Management of Haemophilic Pseudotumours with Special Emphasis on Radiotherapy and Arterial Embolization. Haemoph. Off. J. World Fed. Hemoph. 2009, 15, 448–457. [Google Scholar] [CrossRef]
- Kashiwazaki, D.; Terasaka, S.; Kamoshima, Y.; Kubota, K.; Asano, T.; Houkin, K. Hemophilic Pseudotumor of the Temporal Bone with Conductive Hearing Loss—Case Report. Neurol. Med. Chir. 2012, 52, 745–747. [Google Scholar] [CrossRef][Green Version]
- Magallón, M.; Monteagudo, J.; Altisent, C.; Ibáñez, A.; Rodríguez-Pérez, A.; Riba, J.; Tusell, J.; Martín-Villar, J. Hemophilic Pseudotumor: Multicenter Experience over a 25-Year Period. Am. J. Hematol. 1994, 45, 103–108. [Google Scholar] [CrossRef]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the Management of Hemophilia. Haemoph. Off. J. World Fed. Hemoph. 2013, 19, e1–e47. [Google Scholar] [CrossRef]
- Dagli, M.; Kutlucan, A.; Abusoglu, S.; Basturk, A.; Sozen, M.; Kutlucan, L.; Unlu, A.; Yilmaz, F. Evaluation of Bone Mineral Density (BMD) and Indicators of Bone Turnover in Patients with Hemophilia. Bosn. J. Basic Med. Sci. 2018, 18, 206–210. [Google Scholar] [CrossRef]
- Kerr, R. Imaging of Musculoskeletal Complications of Hemophilia. Semin. Musculoskelet. Radiol. 2003, 7, 127–136. [Google Scholar] [CrossRef]
- Ahuja, S.P.; Sidonio, R.; Raj, A.B.; Bertolone, S.J.; Silverman, C.; Antekeier, D.P.; Fallat, M.E. Successful Combination Therapy of a Proximal Haemophilic Pseudotumour with Surgery, Radiation and Embolization in a Child with Mild Haemophilia A. Haemoph. Off. J. World Fed. Hemoph. 2007, 13, 209–212. [Google Scholar] [CrossRef]
- Pennekamp, P.H.; Strauss, A.C.; Klein, C.; Marx, A.; Goldmann, G.; Friedrich, M.; Marquardt, N.; Oldenburg, J. Giant Haemophilic Pseudotumour of the Pelvis: Case Report and Literature Review. Haemoph. Off. J. World Fed. Hemoph. 2015, 21, e484–e486. [Google Scholar] [CrossRef]
- Nassif, N.A.; Buchowski, J.M.; Osterman, K.; McDonald, D.J. Surgical Technique: Iliosacral Reconstruction With Minimal Spinal Instrumentation. Clin. Orthop. 2013, 471, 947–955. [Google Scholar] [CrossRef]
- Wu, X.-T.; Liu, Z.-Q.; Fu, W.-Q.; Zhao, S. Minimally Invasive Treatment of Unstable Pelvic Ring Injuries with Modified Pedicle Screw–Rod Fixator. J. Int. Med. Res. 2018, 46, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, Q.; Wu, J.; Zhou, F.; Zhang, F.; Liang, H.; Lyu, F.; Wang, J. Modified Pedicle Screw-Rod Fixation versus Anterior Pelvic External Fixation for the Management of Anterior Pelvic Ring Fractures: A Comparative Study. J. Orthop. Surg. 2017, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Shao, Y.; Lu, H.; Zhang, Z.; Lin, H.; Wang, S.; Li, B.; Li, H.; Wang, Z.; Lin, N.; et al. Pelvic Reconstruction with Different Rod-Screw Systems Following Enneking Type I/I + IV Resection: A Clinical Study. Oncotarget 2017, 8, 38978–38989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Yunus, A.; Tian, Z.; Chen, J.-T.; Wang, C.; Xu, L.-L.; Song, X.-H. The Pedicle Screw-Rod System Is an Acceptable Method of Reconstructive Surgery after Resection of Sacroiliac Joint Tumours. Współczesna Onkol. 2016, 1, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. 7—Medical, Dental, and Pharmaceutical Applications. In Biopolymers: Applications and Trends; Niaounakis, M., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 291–405. ISBN 978-0-323-35399-1. [Google Scholar]
- Webb, J.C.J.; Spencer, R.F. The Role of Polymethylmethacrylate Bone Cement in Modern Orthopaedic Surgery. J. Bone Jt. Surg. Br. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- O’dowd-Booth, C.J.; White, J.; Smitham, P.; Khan, W.; Marsh, D.R. Bone Cement: Perioperative Issues, Orthopaedic Applications and Future Developments. J. Perioper. Pract. 2011, 21, 304–308. [Google Scholar] [CrossRef]
- Yang, H.; Zou, J. Filling Materials Used in Kyphoplasty and Vertebroplasty for Vertebral Compression Fracture: A Literature Review. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 87–91. [Google Scholar] [CrossRef]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone Cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Remedios, D.; Saifuddin, A.; Pringle, J. Radiological and Clinical Recurrence of Giant-Cell Tumour of Bone after the Use of Cement. J. Bone Jt. Surg. Br. 1997, 79, 26–30. [Google Scholar] [CrossRef]
- Zuo, D.; Zheng, L.; Sun, W.; Fu, D.; Hua, Y.; Cai, Z. Contemporary Adjuvant Polymethyl Methacrylate Cementation Optimally Limits Recurrence in Primary Giant Cell Tumor of Bone Patients Compared to Bone Grafting: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2013, 11, 156. [Google Scholar] [CrossRef]
- Mjöberg, B.; Pettersson, H.; Rosenqvist, R.; Rydholm, A. Bone Cement, Thermal Injury and the Radiolucent Zone. Acta Orthop. Scand. 1984, 55, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Chen, H.-R.; Chen, J.-X.; Huang, C.-G.; Han, X.; Lu, Y.-C.; Luo, C. Intracranial Haemophilic Pseudotumor Associated with Factor VIII Deficiency. Br. J. Neurosurg. 2009, 23, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Aljassir, F.; Beadel, G.P.; Turcotte, R.E.; Griffin, A.M.; Bell, R.S.; Wunder, J.S.; Isler, M.H. Outcome after Pelvic Sarcoma Resection Reconstructed with Saddle Prosthesis. Clin. Orthop. 2005, 438, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.J.; Back, D.L.; Austin, S. Characteristics and Management of the Haemophilia-Associated Pseudotumours. Haemoph. Off. J. World Fed. Hemoph. 2020, 26, 33–40. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasta, G.; Ruggieri, R.; Annunziata, S.; Gallese, A.; Gagliardi, V.P.; Cuzzocrea, F.; Ghiara, M.; Russo, M.; Preti, P.S.; Santi, R.M.; et al. Haemophilic Pelvic Pseudotumour: A New Surgical Option. Healthcare 2021, 9, 1269. https://doi.org/10.3390/healthcare9101269
Pasta G, Ruggieri R, Annunziata S, Gallese A, Gagliardi VP, Cuzzocrea F, Ghiara M, Russo M, Preti PS, Santi RM, et al. Haemophilic Pelvic Pseudotumour: A New Surgical Option. Healthcare. 2021; 9(10):1269. https://doi.org/10.3390/healthcare9101269
Chicago/Turabian StylePasta, Gianluigi, Roberta Ruggieri, Salvatore Annunziata, Alessandro Gallese, Vincenzo Pio Gagliardi, Fabrizio Cuzzocrea, Matteo Ghiara, Mariaconcetta Russo, Paola Stefania Preti, Roberto Mario Santi, and et al. 2021. "Haemophilic Pelvic Pseudotumour: A New Surgical Option" Healthcare 9, no. 10: 1269. https://doi.org/10.3390/healthcare9101269
APA StylePasta, G., Ruggieri, R., Annunziata, S., Gallese, A., Gagliardi, V. P., Cuzzocrea, F., Ghiara, M., Russo, M., Preti, P. S., Santi, R. M., Mosconi, M., & Benazzo, F. (2021). Haemophilic Pelvic Pseudotumour: A New Surgical Option. Healthcare, 9(10), 1269. https://doi.org/10.3390/healthcare9101269








