Left Ventricular Systolic Impairment after Pediatric Cardiac Surgery Assessed by STE Analysis
Abstract
:1. Introduction
2. Methods
2.1. Ethics
2.2. Surgical and Medical Management
2.3. Statistical Analysis
3. Results
3.1. Population
3.2. Feasibility
3.3. Preoperative LV Strain Values
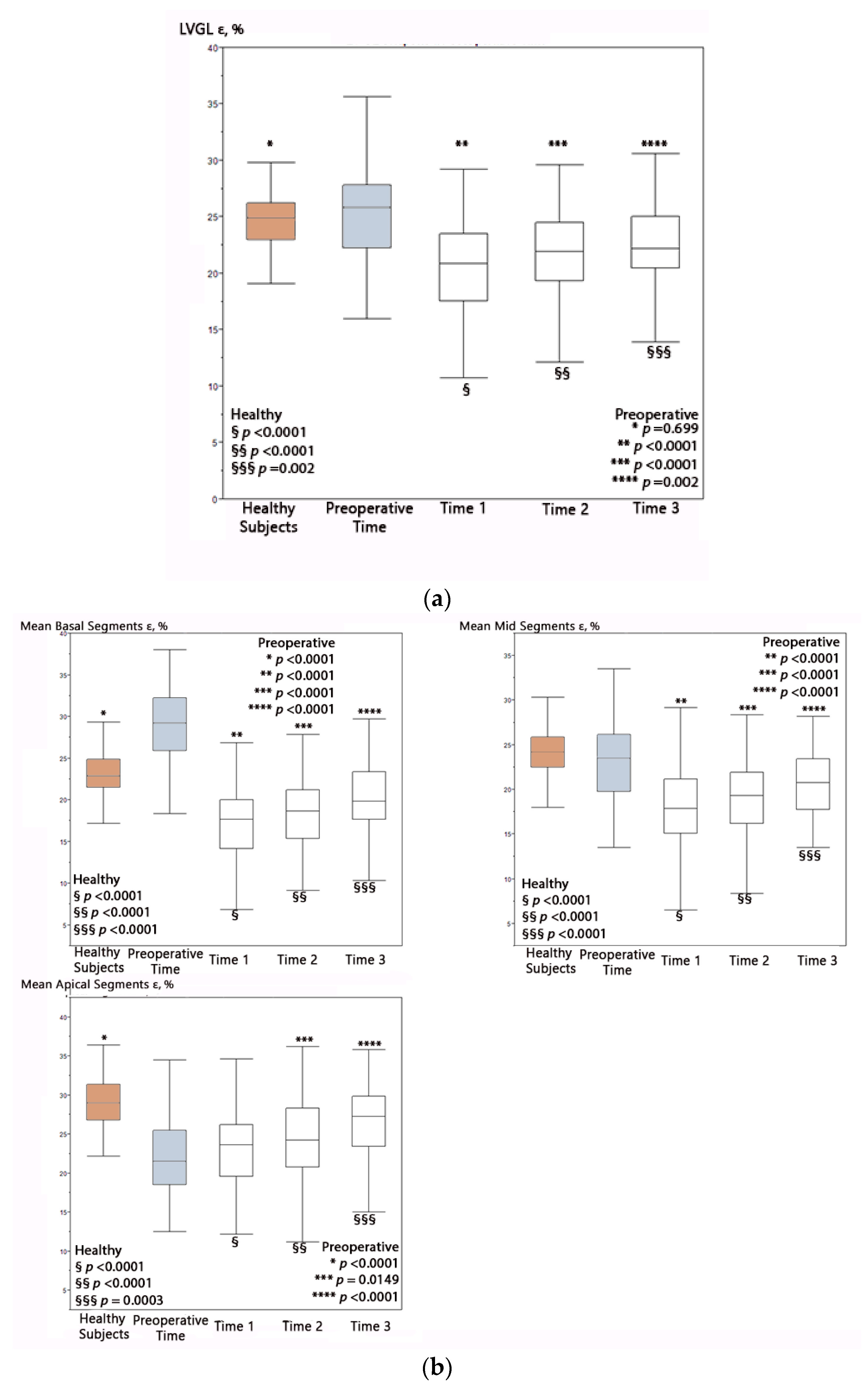
3.4. Progression of Global LV Strain Values as a Function of Postoperative Time
3.5. Progression of Segmental LV Strain Values as a Function of Postoperative Time
3.6. Differences of LV Ventricular Strain Values among Age Groups
3.7. Strain and Left Ventricular Ejection Fraction
3.8. Inotrope Data and Vasoactive–Inotropic Score
3.9. Correlation of Ventricular Strain with Conventional Risk Factors and Operative Data
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Postoperative Times | STE | Neonates | Infant | Older | Older vs. Infant | Older vs. Neonates | Neonates vs. Infant | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | STE | Mean | Dev std | Mean | Dev std | Mean | Dev std | p | p | p |
| Time 1 | LV 4 Ch ε | 22.37 | 4.03 | 20.74 | 5.53 | 20.14 | 4.84 | 0.6 | 0.048 * | 0.21 |
| LV 2 Ch ε | 22.54 | 3.85 | 21.93 | 6.63 | 19.91 | 4.72 | 0.09 | 0.03 * | 0.66 | |
| LV 3 Ch ε | 21.62 | 3.82 | 20.61 | 5.35 | 19.28 | 4.81 | 0.23 | 0.04 * | 0.44 | |
| LVGL ε | 22.11 | 3.36 | 20.15 | 5.92 | 19.71 | 4.4 | 0.7 | 0.03 * | 0.12 | |
| Time 2 | LV 4 Ch ε | 24.54 | 3.44 | 21.39 | 4.53 | 20.58 | 4.41 | 0.5 | 0.0004 * | 0.013 * |
| LV 2 Ch ε | 24.96 | 3.79 | 21.84 | 3.43 | 20.84 | 4.59 | 0.3 | 0.0002 * | 0.013 * | |
| LV 3 Ch ε | 23.02 | 3.6 | 21 | 4.59 | 19.87 | 4.43 | 0.31 | 0.0048 * | 0.11 | |
| LVGL ε | 24.22 | 3.25 | 21.3 | 3.68 | 20.38 | 4.21 | 0.36 | 0.0002 * | 0.013 * | |
| Time 3 | LV 4 Ch ε | 25.47 | 3.96 | 22.56 | 3.84 | 22.73 | 4.88 | 0.89 | 0.04 * | 0.05 |
| LV 2 Ch ε | 24.65 | 4.3 | 22.78 | 3.78 | 21.8 | 4.42 | 0.43 | 0.03 * | 0.2 | |
| LV 3 Ch ε | 23.95 | 3.34 | 22.9 | 3.71 | 21.69 | 4.18 | 0.3 | 0.06 | 0.42 | |
| LVGL ε | 24.66 | 3.29 | 22.68 | 3.42 | 22.02 | 3.9 | 0.54 | 0.02 * | 0.11 | |
| STE | Age | BSA | Aristotle Score | STAT | CPB | Cross Clamp | LVEF | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | beta | p | beta | p | p | p | beta | p | beta | p | beta | p |
| LV 4 Ch ε | −0.4 | 0.0012 * | −4.8 | 0.0002 * | 0.6 | 0.8 | −0.01 | 0.04 * | 0.2 | 1.34 | <0.0001 | |
| LV 2 Ch ε | −0.5 | <0.0001 * | −5.51 | <0.0001 * | 0.7 | 0.9 | 0.08 | 0.2 | 1.29 | <0.0001 | ||
| LV 3 Ch ε | −0.5 | <0.0001 * | −5.47 | <0.0001 * | 0.7 | 0.7 | −0.02 | 0.006 * | 0.05 | 1.49 | <0.0001 | |
| LVGL ε | −0.5 | 0.0002 * | −4.91 | <0.0001 | 0.9 | 0.9 | −0.02 | 0.0006 * | −0.03 | 0.007 * | 1.51 | <0.0001 |
| Mean Basal Segments | −0.3 | 0.0015 * | −3.77 | 0.0013 * | 0.5 | 0.7 | 0.13 | 0.4 | 1.04 | <0.0001 | ||
| Mean Mid Segments | −0.2 | 0.05 | −2.31 | 0.06 | 0.8 | 0.05 | −0.02 | 0.03 * | 0.1 | 0.9 | <0.0001 | |
| Mean Apical Segments | −0.6 | <0.0001 * | −6.94 | <0.0001 * | 0.2 | 0.9 | 0.1 | 0.3 | 0.93 | <0.0001 | ||
| VISmax Category | Neonates | Infant | Older | All | ||||
|---|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | % | N | |
| 0−5 | 42.31% | 11 | 61.29% | 19 | 73.33% | 44 | 63.25% | 74 |
| 5−15 | 46.15% | 12 | 38.71% | 12 | 25% | 15 | 33.30% | 39 |
| 15−30 | 11.54% | 3 | 0.0% | 0 | 1.67% | 1 | 3.42% | 4 |
| 30−45 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 |
| >45 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 |
| No Vasoactive–Inotropes Administration | 11.50% | 3 | 12.90% | 4 | 45% | 27 | 29.10% | 34 |
| STE | Inotropes-Group | No Inotropes-Group | Inotropes-Group | No Inotropes-Group | Comparison Inotropes vs. No Inotropes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| STE | Time 1 | Time 2 | Time 1 | Time 2 | ||||||
| Variables | Mean | Dev std | Mean | Dev std | Mean | Dev std | Mean | Dev std | p | p |
| LV 4 Ch ε | 21.15 | 4.83 | 19.91 | 5.08 | 22.25 | 4.47 | 21.25 | 4.5 | 0.23 | 0.29 |
| LV 2 Ch ε | 21.6 | 5.06 | 19.58 | 5.31 | 22.57 | 4.15 | 21.63 | 4.72 | 0.07 | 0.31 |
| LV 3 Ch ε | 20.69 | 4.46 | 18.82 | 5.43 | 21.13 | 4.27 | 20.72 | 4.62 | 0.07 | 0.66 |
| LVGL ε | 21 | 4.07 | 18.84 | 5.81 | 21.95 | 3.99 | 21.15 | 4.29 | 0.03 * | 0.35 |
| Mean Basal Segments | 17.75 | 3.94 | 16.53 | 5.16 | 18.07 | 3.46 | 18.22 | 4.29 | 0.17 | 0.85 |
| Mean Mid Segments | 17.93 | 4.1 | 18.17 | 5.64 | 18.84 | 4.11 | 18.91 | 4.73 | 0.8 | 0.9 |
| Mean Apical Segments | 23.3 | 5.14 | 21.67 | 7.16 | 24.74 | 5.04 | 23.48 | 5.59 | 0.17 | 0.26 |
References
- Karlsen, S.; Dahlslett, T.; Grenne, B.; Sjøli, B.; Smiseth, O.; Edvardsen, T.; Brunvand, H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc. Ultrasound 2019, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Klaeboe, L.G.; Edvardsen, T. Echocardiographic assessment of left ventricular systolic function. J. Echocardiogr. 2019, 17, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Perdreau, E.; Séguéla, P.-E.; Jalal, Z.; Perdreau, A.; Mouton, J.-B.; Nelson-Veniard, M.; Guillet, E.; Iriart, X.; Ouattara, A.; Roubertie, F.; et al. Postoperative assessment of left ventricular function by two-dimensional strain (speckle tracking) after paediatric cardiac surgery. Arch. Cardiovasc. Dis. 2016, 109, 599–606. [Google Scholar] [CrossRef]
- Colquitt, J.L.; Pignatelli, R.H. Strain imaging: The emergence of speckle tracking echocardiography into clinical pediatric cardiology. Congenit. Heart Dis. 2016, 11, 199–207. [Google Scholar] [CrossRef]
- Pletzer, S.A.; Atz, A.M.; Chowdhury, S.M. The relationship between pre-operative left ventricular longitudinal strain and post-operative length of stay in patients undergoing arterial switch operation is age dependent. Pediatr. Cardiol. 2019, 40, 366–373. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.M.; Kuipers, I.M.; Klitsie, L.M.; Blom, N.A.; ten Harkel, A.D.J. Decreased biventricular longitudinal strain shortly after congenital heart defect surgery. Echocardiography 2017, 34, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Lv, Q.; Wang, J.; Yang, Y.; He, L.; Yuan, L.; Zhang, L.; Xie, M. Impact of surgical correction of tetralogy of Fallot on short-term right and left ventricular function as determined by 2-dimensional speckle tracking echocardiography. Medicine 2016, 95, e4426. [Google Scholar] [CrossRef]
- Karsenty, C.; Hadeed, K.; Dulac, Y.; Semet, F.; Alacoque, X.; Breinig, S.; Leobon, B.; Acar, P.; Hascoet, S. Two-dimensional right ventricular strain by speckle tracking for assessment of longitudinal right ventricular function after paediatric congenital heart disease surgery. Arch. Cardiovasc. Dis. 2017, 110, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rafieyian, S.; Roodpeyma, S.; Vahidshahi, K.; Moghadasi, A. Evaluation of regional myocardial function by strain and strain rate before and after surgical repair of congenital heart anomalies. J. Tehran. Heart Cent. 2018, 13, 18–23. [Google Scholar]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Franchi, E.; Assanta, N.; Marotta, M.; Viacava, C.; Molinaro, S.; Iervasi, G.; Santoro, G.; et al. Normative data for left and right ventricular systolic strain in healthy Caucasian Italian children by two-dimensional speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 712–720. [Google Scholar] [CrossRef]
- Lorch, S.M.; Ludomirsky, A.; Singh, G.K. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J. Am. Soc. Echocardiogr. 2008, 21, 1207–1215. [Google Scholar] [CrossRef]
- Marcus, K.A.; Mavinkurve-Groothuis, A.M.C.; Barends, M.; van Dijk, A.; Feuth, T.; de Korte, C.; Kapusta, L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J. Am. Soc. Echocardiogr. 2011, 24, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, J.; Xie, M.; Yin, P.; Liu, W.; Li, Y.; Klas, B.; Sun, J.; Balluz, R.; Ge, S. Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: Feasibility, reproducibility, maturational changes, and normal ranges. J. Am. Soc. Echocardiogr. 2013, 26, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Mejia, A.A.S.; Machefsky, A.; Fowler, S.; Holland, M.R.; Singh, G.K. Normal ranges of right ventricular systolic and diastolic strain measures in children: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 2014, 27, 549–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutty, S.; Padiyath, A.; Li, L.; Peng, Q.; Rangamani, S.; Schuster, A.; Danford, D.A. Functional maturation of left and right atrial systolic and diastolic performance in infants, children, and adolescents. J. Am. Soc. Echocardiogr. 2013, 26, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Molinaro, S.; Della Pina, F.; Storti, S.; Arcieri, L.; Murzi, B.; Marotta, M.; Pak, V.; et al. Prognostic role of BNP in children undergoing surgery for congenital heart disease: Analysis of prediction models incorporating standard risk factors. Clin. Chem. Lab. Med. 2015, 53, 1839–1846. [Google Scholar] [CrossRef]
- Koponen, T.; Karttunen, J.; Musualowicz, T.; Pietiläinen, L.; Uusaro, A.; Lahtinen, P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br. J. Anaesth. 2019, 122, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.U.; Walters, H.L., III; Delius, R.; Aggarwal, S. Vasoactive inotropic score (VIS) as biomarker of short-term outcomes in adolescents after cardiothoracic surgery. Pediatr. Cardiol. 2016, 37, 271–277. [Google Scholar] [CrossRef]
- Adamson, G.T.; Arunamata, A.; Tacy, T.A.; Silverman, N.H.; Ma, M.; Maskatia, S.A.; Punn, R. Postoperative recovery of left ventricular function following repair of large ventricular septal defects in infants. J. Am. Soc. Echocardiogr. 2020, 33, 368–377. [Google Scholar] [CrossRef]
- Klitsie, L.M.; Kuipers, I.M.; Roest, A.A.W.; Van Der Hulst, A.E.; Stijnen, T.; Hazekamp, M.G.; Blom, N.A.; Harkel, A.D.T. Disparity in right vs. left ventricular recovery during follow-up after ventricular septal defect correction in children. Eur. J. Cardio-Thorac. Surg. 2013, 44, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Klitsie, L.M.; Roest, A.A.W.; Kuipers, I.M.; Hazekamp, M.G.; Blom, N.A.; Ten Harkel, A.D.J. Left and right ventricular performance after arterial switch operation. J. Thorac. Cardiovasc. Surg. 2014, 147, 1561–1567. [Google Scholar] [CrossRef] [Green Version]
- Howard-Quijano, K.; Methangkool, E.; Scovotti, J.C.; Mazor, E.; Grogan, T.R.; Kratzert, W.B.; Mahajan, A. Regional left ventricular myocardial dysfunction after cardiac surgery characterized by 3-dimensional strain. Anesth. Analg. 2019, 128, 854–886. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, T.; Bayram, H.; Bayram, N.; Sari, C.; Keles, T.; Bastug, S.; Bozkurt, E. Effect of coronary artery bypass surgery on left ventricular function as assessed by strain and strain rate imaging. Perfusion 2014, 29, 425–433. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Neonates | Infant | Older | |||
|---|---|---|---|---|---|---|
| Variables | Mean (Median) | Dev std (IQR) | Mean (Median) | Dev std (IQR) | Mean (Median) | Dev std (IQR) |
| Age (years) | 0.027 (0.02) | 0.01 (0.02–0.03) | 0.25 (0.25) | 0.13 (0.12–0.38) | 4.37 (1.92) | 4.63 (0.95–6.8) |
| Age (months) | 0.33 (0.3) | 0.19 (0.22–0.38) | 3.12 (3.07) | 1.57 (1.5–4.63) | 53.16 (23.33) | 56.29 (11.5–82.76) |
| Age (days) | 9.88 (9) | 5.58 (6.5–11.5) | 93.74 (92) | 46.95 (45–139) | 1594.89 (700) | 1688.57 (345–248.75) |
| Weight (Kg) | 3.25 (3.29) | 0.62 (2.98–3.57) | 5.03 (4.92) | 1.64 (3.95–5.93) | 17.27 (11.03) | 14.18 (6.75–24) |
| Height (cm) | 49.92 (51) | 5.26 (48.5–52.5) | 60.47 (59) | 10.50 (53.5–64.25) | 95.12 (83) | 34.61 (67–124) |
| BSA (m2) | 0.21 (0.21) | 0.03 (0.21–0.23) | 0.29 (0.29) | 0.06 (0.25–0.35) | 0.66 (0.5) | 0.38 (0.36–0.9) |
| CPB (min) | 142.74 (177) | 103.13 (0–219) | 102.48 (93) | 63.68 (63–134) | 101.74 (99.5) | 47.51 (69.5–120) |
| Cross Clamp (min) | 93.38 (99.5) | 70.62 (27–128.75) | 62.00 (62) | 43.11 (36.5–87) | 65.36 (61.5) | 35.94 (40–85) |
| Aristotle score | 8.77 (10) | 1.95 (6–10) | 6.89 (6.3) | 1.37 (6–8) | 6.38 (6.3) | 2.33 (6–8) |
| STAT | 2.11 (3) | 0.99 (1–3) | 1.46 (1) | 0.81 (1–2) | 1.45 (1) | 0.67 (1–2) |
| Classification | Neonates | Infant | Older | |||
| LVVO | 1.00 | 16.00 | 16.00 | |||
| RVPO | 0.00 | 5.00 | 16.00 | |||
| LVPO | 8.00 | 2.00 | 10.00 | |||
| TGA | 16.00 | 3.00 | 0.00 | |||
| RVVO | 0.00 | 1.00 | 14.00 | |||
| AVSD | 1.00 | 2.00 | 4.00 | |||
| Other | 0.00 | 2.00 | 0.00 | |||
| STE | Neonates (%) | Infants (%) | Older (%) | Total (%) |
|---|---|---|---|---|
| LV 4 Ch ε | 98.5 | 97.4 | 96.2 | 97.1 |
| LV 2 Ch ε | 96.5 | 94.8 | 96.2 | 96.3 |
| LV 3 Ch ε | 97.5 | 94.8 | 96.2 | 96.3 |
| LVGL ε | 98.5 | 97.4 | 97.7 | 97.8 |
| STE | Before Surgery (Pre) | Time 1 | Time 2 | Time 3 | Healthy Subjects | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean | Dev std | Mean | Dev std | Mean | Dev std | Mean | Dev std | Mean | Dev std |
| LV 4 Ch ε | 24.98 | 4.22 | 20.81 | 4.91 | 21.76 | 4.49 | 23.4 | 4.49 | 24.93 | 2.77 |
| LV 2 Ch ε | 25.24 | 4.24 | 21.04 | 5.19 | 22.11 | 4.44 | 22.82 | 4.32 | 25.19 | 2.74 |
| LV 3 Ch ε | 25.46 | 4.24 | 20.17 | 4.8 | 20.93 | 4.43 | 22.61 | 3.91 | 23.8 | 3.48 |
| LVGL ε | 25.23 | 4.23 | 20.37 | 4.71 | 21.56 | 4.14 | 22.9 | 3.73 | 24.84 | 2.67 |
| Mean Basal Segments | 28.64 | 4.9 | 17.39 | 4.34 | 18.15 | 3.87 | 20.2 | 3.97 | 23.03 | 2.77 |
| Mean Mid Segments | 23.19 | 5.05 | 18 | 4.57 | 18.88 | 4.4 | 20.81 | 3.85 | 24.11 | 2.85 |
| Mean Apical Segments | 21.94 | 4.83 | 22.83 | 5.82 | 24.12 | 5.33 | 26.63 | 5.38 | 29.05 | 3.25 |
| STE | Pre vs. T1 | Pre vs. T2 | Pre vs. T3 | Pre vs. Controls | Control vs. Time 1 | Control vs. Time 2 | Control vs. Time 3 | Time 1 vs. Time 2 | Time 2 vs. Time 3 | Time 1 vs. Time 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | p | p | P | p | p | p | p | p | p | p |
| LV 4 Ch ε | <0.0001 * | <0.0001 * | 0.048 * | 0.9 | 0.0009 * | 0.0077 * | 0.005 * | 0.6 | 0.5 | 0.23 |
| LV 2 Ch ε | <0.0001 * | <0.0001 * | 0.003 * | 0.9 | <0.0001 * | <0.0001 * | 0.0004 * | 0.07 | 0.3 | 0.007 * |
| LV 3 Ch ε | <0.0001 * | <0.0001 * | 0.0002 * | 0.0084 * | <0.0001 * | <0.0001 * | 0.07 | 0.2 | 0.01 * | 0.0002 * |
| LVGL ε | <0.0001 * | <0.0001 * | 0.002 * | 0.47 | <0.0001 * | <0.0001 * | 0.002 * | 0.03 * | 0.03 * | <0.0001 * |
| Mean Basal Segments | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | 0.19 | 0.0013 * | <0.0001 * |
| Mean Mid Segments | <0.0001 * | <0.0001 * | 0.0042 * | 0.14 | <0.0001 * | <0.0001 * | <0.0001 * | 0.16 | 0.005 * | <0.0001 * |
| Mean Apical Segments | 0.33 | 0.0149 * | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | 0.0003 * | 0.09 | 0.004 * | <0.0001 * |
| Percentile | <5th | 5–10th | 10–25th | 25–50th | 50–75th | 75–90th | 90–95th | >95th |
|---|---|---|---|---|---|---|---|---|
| Preoperative | 14.8% | 5.6% | 7.4% | 22.2% | 16.7% | 13.0% | 5.6% | 14.7% |
| Time 1 | 61.40% | 5.26% | 14.04% | 9.65% | 3.51% | 5.26% | 0.00% | 0.88% |
| Time 2 | 53.26% | 2.17% | 17.39% | 15.22% | 6.52% | 4.35% | 1.09% | 0.00% |
| Time 3 | 43.75% | 4.69% | 14.06% | 18.75% | 6.25% | 7.81% | 0.00% | 4.69% |
| Age Group | Time of Examination | Ejection Fraction (%) | |||||
|---|---|---|---|---|---|---|---|
| Normal (>50%) | Mildly Decreased (40–50%) | Reduced (<40%) | |||||
| Newborn | Before Surgery | 100.00% | 0.00% | 0.00% | |||
| Time 1 | 92.31% | 7.69% | 0.00% | ||||
| Time 2 | 100.00% | 0.00% | 0.00% | ||||
| Time 3 | 100.00% | 0.00% | 0.00% | ||||
| Infant | Before Surgery | 100.00% | 0.00% | 0.00% | |||
| Time 1 | 78.57% | 14.29% | 7.14% | ||||
| Time 2 | 85.71% | 4.76% | 9.52% | ||||
| Time 3 | 100.00% | 0.00% | 0.00% | ||||
| Older | Before Surgery | 100.00% | 0.00% | 0.00% | |||
| Time 1 | 81.36% | 13.56% | 5.08% | ||||
| Time 2 | 82.61% | 10.87% | 6.52% | ||||
| Time 3 | 93.10% | 6.90% | 0.00% | ||||
| All | Before Surgery | 100.00% | 0.00% | 0.00% | |||
| Time 1 | 83.2% | 12.39% | 4.42% | ||||
| Time 2 | 87.78% | 6.67% | 5.56% | ||||
| Time 3 | 96.83% | 3.17% | 0.00% | ||||
| Ejection Fraction over time | |||||||
| Ejection Fraction (%) | Mean ± dev std | 63.7 ± 5.1 | 58.6 ± 9.7 | 61.6 ± 10.7 | 63.8 ± 7.9 | ||
| Ejection Fraction | Before Surgery to Time 1 | Before Surgery to Time 2 | Before Surgery to Time 3 | Time 1 to Time 2 | Time 2 to Time 3 | Time 1 to Time 3 | |
| Ejection Fraction (%) | p | 0.0004 * | 0.17 | 0.9 | 0.041 * | 0.17 | 0.0004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantinotti, M.; Marchese, P.; Scalese, M.; Medino, P.; Jani, V.; Franchi, E.; Vitali, P.; Santoro, G.; Viacava, C.; Assanta, N.; et al. Left Ventricular Systolic Impairment after Pediatric Cardiac Surgery Assessed by STE Analysis. Healthcare 2021, 9, 1338. https://doi.org/10.3390/healthcare9101338
Cantinotti M, Marchese P, Scalese M, Medino P, Jani V, Franchi E, Vitali P, Santoro G, Viacava C, Assanta N, et al. Left Ventricular Systolic Impairment after Pediatric Cardiac Surgery Assessed by STE Analysis. Healthcare. 2021; 9(10):1338. https://doi.org/10.3390/healthcare9101338
Chicago/Turabian StyleCantinotti, Massimiliano, Pietro Marchese, Marco Scalese, Paola Medino, Vivek Jani, Eliana Franchi, Pak Vitali, Giuseppe Santoro, Cecilia Viacava, Nadia Assanta, and et al. 2021. "Left Ventricular Systolic Impairment after Pediatric Cardiac Surgery Assessed by STE Analysis" Healthcare 9, no. 10: 1338. https://doi.org/10.3390/healthcare9101338








