Effectiveness, Cost-Utility, and Safety of Neurofeedback Self-Regulating Training in Patients with Post-Traumatic Stress Disorder: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design, Institutional Review Board Approval, and Registration
2.2. Participants
2.2.1. Eligibility Criteria: Inclusion Criteria
2.2.2. Eligibility Criteria: Exclusion Criteria
2.3. Randomization, Allocation Concealment, and Blinding
2.4. Intervention
2.4.1. Study Schedule
2.4.2. Neurofeedback Procedure
2.4.3. Control Group Intervention
2.4.4. Concomitant Treatment
2.5. Primary Outcome Measures: Korean Version of the PTSD Checklist-5 (PCL-5-K) Score
2.6. Secondary Outcome Measures
2.6.1. Impact of the Event Scale-Revised Korean Version (IES-R-K)
2.6.2. Clinical Global Impression-Improvement Scale (CGI-I)
2.6.3. Beck Anxiety Inventory (BAI)
2.6.4. Beck Depression Inventory (BDI)
2.6.5. Insomnia Severity Index (ISI)
2.6.6. Hwa-Byung Scale (HBS)
2.6.7. Core Seven Emotions Inventory Short Form (CSEI-S)
2.6.8. Mentalizing the Rooms of Mind (MRM)
2.6.9. QEEG Analysis
2.6.10. Safety
2.7. Quality of Life (QOL)
2.7.1. Short Form Health Survey-36 (SF-36)
2.7.2. EuroQoL-5 Dimension (EQ-5D-5L)
2.8. Cost Outcomes
2.9. Sample Size Calculation
2.10. Statistical Analysis
2.11. Economic Analysis (Cost-Utility Analysis)
3. Results
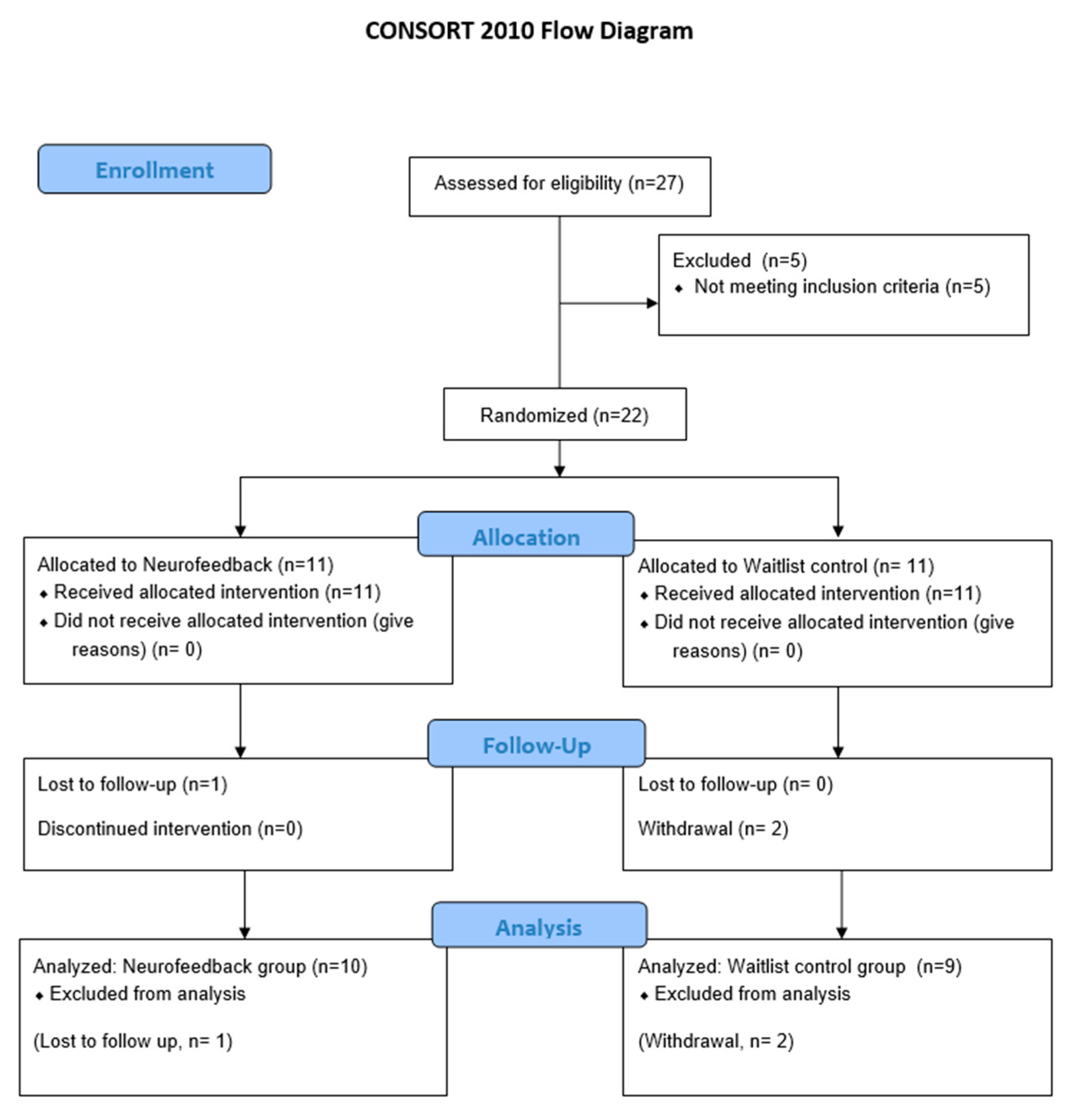
3.1. Study Flow and Patient Characteristics
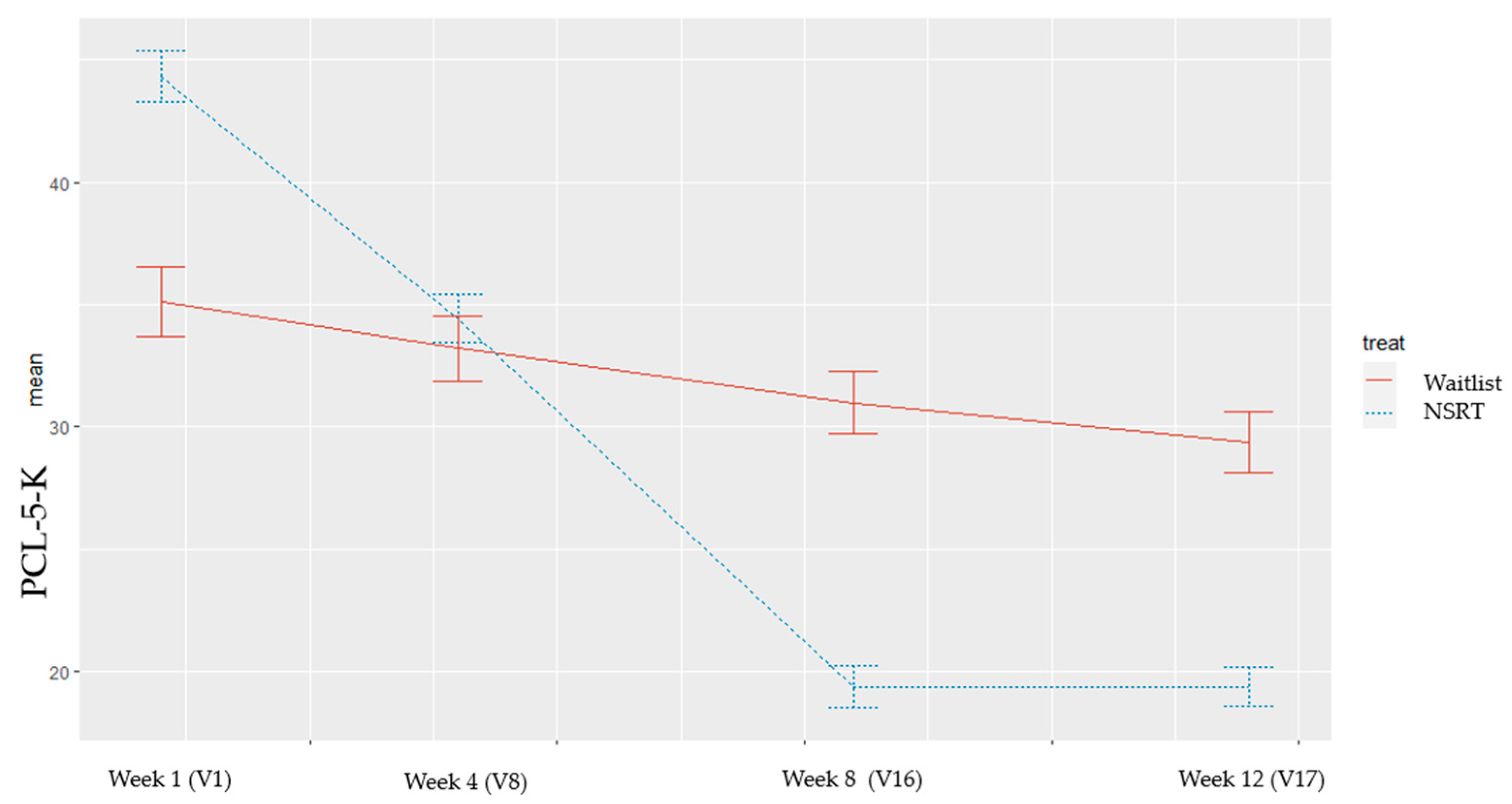
3.2. Primary Outcome
3.3. Secondary Outcome
3.4. AEs
3.5. QOL
3.6. Cost-Utility Analysis
4. Discussion
4.1. Summary of Findings
4.2. Debates
4.3. Strengths, Limitations, and Implications for Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aliev, G.; Beeraka, N.M.; Nikolenko, V.N.; Svistunov, A.A.; Rozhnova, T.; Kostyuk, S.; Cherkesov, I.; Gavryushova, L.V.; Chekhonatsky, A.A.; Mikhaleva, L.M.; et al. Neurophysiology and Psychopathology Underlying PTSD and Recent Insights into the PTSD Therapies—A Comprehensive Review. J. Clin. Med. 2020, 9, 2951. [Google Scholar] [CrossRef]
- Reiter, K.; Andersen, S.B.; Carlsson, J. Neurofeedback Treatment and Posttraumatic Stress Disorder: Effectiveness of Neurofeedback on Posttraumatic Stress Disorder and the Optimal Choice of Protocol. J. Nerv. Ment. Dis. 2016, 204, 69–77. [Google Scholar] [CrossRef]
- Watkins, L.E.; Sprang, K.R.; Rothbaum, B.O. Treating PTSD: A Review of Evidence-Based Psychotherapy Interventions. Front. Behav. Neurosci. 2018, 12, 258. [Google Scholar] [CrossRef]
- Atwoli, L.; Stein, D.J.; Koenen, K.C.; McLaughlin, K.A. Epidemiology of Posttraumatic Stress Disorder: Prevalence, Correlates and Consequences. Curr. Opin. Psychiatry 2015, 28, 307–311. [Google Scholar] [CrossRef]
- Scott, K.M.; Koenen, K.C.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; Bruffaerts, R.; Caldas-de-Almeida, J.M.; de Girolamo, G.; Florescu, S.; et al. Associations between Lifetime Traumatic Events and Subsequent Chronic Physical Conditions: A Cross-National, Cross-Sectional Study. PLoS ONE 2013, 8, e80573. [Google Scholar] [CrossRef]
- Gradus, J.L.; Qin, P.; Lincoln, A.K.; Miller, M.; Lawler, E.; Sørensen, H.T.; Lash, T.L. Posttraumatic Stress Disorder and Completed Suicide. Am. J. Epidemiol. 2010, 171, 721–727. [Google Scholar] [CrossRef]
- Bothe, T.; Jacob, J.; Kröger, C.; Walker, J. How Expensive Are Post-Traumatic Stress Disorders? Estimating Incremental Health Care and Economic Costs on Anonymised Claims Data. Eur. J. Health Econ. 2020, 21, 917–930. [Google Scholar] [CrossRef]
- Lancaster, C.L.; Teeters, J.B.; Gros, D.F.; Back, S.E. Posttraumatic Stress Disorder: Overview of Evidence-Based Assessment and Treatment. J. Clin. Med. 2016, 5, 105. [Google Scholar] [CrossRef]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Lewis, C.; Roberts, N.P.; Gibson, S.; Bisson, J.I. Dropout from Psychological Therapies for Post-Traumatic Stress Disorder (PTSD) in Adults: Systematic Review and Meta-Analysis. Eur. J. Psychotraumatol. 2020, 11, 1709709. [Google Scholar] [CrossRef]
- Steingrimsson, S.; Bilonic, G.; Ekelund, A.-C.; Larson, T.; Stadig, I.; Svensson, M.; Vukovic, I.S.; Wartenberg, C.; Wrede, O.; Bernhardsson, S. Electroencephalography-Based Neurofeedback as Treatment for Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Eur. Psychiatry 2020, 63, e7. [Google Scholar] [CrossRef]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A Comprehensive Review on System Design, Methodology and Clinical Applications. Basic. Clin. Neurosci. 2016, 7, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Kanazawa, T.; Koizumi, A.; Ide, K.; Taschereau-Dumouchel, V.; Boku, S.; Hishimoto, A.; Shirakawa, M.; Sora, I.; Lau, H.; et al. Current Status of Neurofeedback for Post-Traumatic Stress Disorder: A Systematic Review and the Possibility of Decoded Neurofeedback. Front. Hum. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Rabarison, K.M.; Bish, C.L.; Massoudi, M.S.; Giles, W.H. Economic Evaluation Enhances Public Health Decision Making. Front. Public Health 2015, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Leem, J.; Cheong, M.J.; Yoon, S.-H.; Kim, H.; Jo, H.-G.; Lee, H.; Kim, J.; Kim, H.Y.; Kim, G.-W.; Kang, H.W. Neurofeedback Self-Regulating Training in Patients with Post Traumatic Stress Disorder: A Randomized Controlled Trial Study Protocol. Integr. Med. Res. 2020, 9, 100464. [Google Scholar] [CrossRef] [PubMed]
- First, M.B. Structured Clinical Interview for the DSM (SCID). Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118625392 (accessed on 29 December 2014).
- Kim, W.H.; Jung, Y.E.; Roh, D.; Kim, D.; Kang, S.H.; Chae, J.H.; Park, J.E. Reliability and Validity of the Korean Version of Clinician-Administered Posttraumatic Stress Disorder Scale for DSM-5. J. Korean Med. Sci. 2019, 34, e219. [Google Scholar] [CrossRef]
- Chae, J.H. Diagnosis and Pathophysiology of Posttraumatic Stress Disorder(PTSD). Korean J. Psychopharmacol. 2016, 15, 14–21. [Google Scholar]
- Peniston, E.G.; Kulkosky, P.J. Alpha-Theta Brainwave Neurofeedback for Vietnam Veterans with Combat-Related Post-Traumatic Stress Disorder. Med. Psychother. 1991, 4, 47–60. [Google Scholar]
- Smith, T.C.; Ryan, M.A.K.; Wingard, D.L.; Slymen, D.J.; Sallis, J.F.; Kritz-Silverstein, D. Millennium Cohort Study Team New Onset and Persistent Symptoms of Post-Traumatic Stress Disorder Self Reported after Deployment and Combat Exposures: Prospective Population Based US Military Cohort Study. BMJ 2008, 336, 366–371. [Google Scholar] [CrossRef]
- Letica-Crepulja, M.; Stevanović, A.; Protuđer, M.; Popović, B.; Salopek-Žiha, D.; Vondraček, S. Predictors of Sexual Dysfunction in Veterans with Post-Traumatic Stress Disorder. J. Clin. Med. 2019, 8, 432. [Google Scholar] [CrossRef]
- Kim, J.W.; Chung, H.G.; Choi, J.H.; So, H.S.; Kang, S.-H.; Kim, D.S.; Moon, J.Y.; Kim, T.Y. Psychometric Properties of the Korean version of the PTSD Checklist-5 in Elderly Korean Veterans of the Vietnam War. Anxiety Mood 2017, 13, 123–131. [Google Scholar] [CrossRef]
- Lim, H.-K.; Woo, J.-M.; Kim, T.-S.; Kim, T.-H.; Choi, K.-S.; Chung, S.-K.; Chee, I.-S.; Lee, K.-U.; Paik, K.C.; Seo, H.-J.; et al. Reliability and Validity of the Korean Version of the Impact of Event Scale-Revised. Compr. Psychiatry 2009, 50, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Eun, H.-J.; Kwon, T.-W.; Lee, S.-M.; Kim, T.-H.; Choi, M.-R.; Cho, S.-J. A Study on Reliability and Validity of the Korean Version of Impact of Event Scale-Revised. J. Korean Neuropsychiatr. Assoc. 2005, 44, 303–310. [Google Scholar]
- Ferguson, L.; Scheman, J. Patient Global Impression of Change Scores within the Context of a Chronic Pain Rehabilitation Program. J. Pain 2009, 10, S73. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Young-Ho Lee; Jong-Yong Song A Study of the Reliability and the Validity of the BDI, SDS, and MMPI-D Scales. Korean J. Clin. Psychol. 1991, 10, 98–113.
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Min, S.K.; Suh, S.-Y.; Song, K.-J. Symptoms to Use for Diagnostic Criteria of Hwa-Byung, an Anger Syndrome. Psychiatry Investig. 2009, 6, 7–12. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Park, D.-K.; Min, S.K.; Kim, J.W.; Kwon, H.-I.; Lee, M.-S. Development and validation of the Hwa-Byung Scale. Korean J. Clin. Psychol. 2008, 27, 237–252. [Google Scholar] [CrossRef]
- Lee, G.-E.; Park, B.-Y.; Moon, K.; You, J.-M.; Kang, H.-W. A Study on the Development of the Core Emotional Assessment Questionnaire (CEAQ) Based on the Seven Emotions (七情). J. Orient. Neuropsychiatry 2015, 26, 143–160. [Google Scholar] [CrossRef][Green Version]
- Cheong, M.J.; Lee, G.-E.; Lee, Y.; Bae, K.-H.; Kang, Y.; Kim, J.-H.; Lyu, Y.-S.; Kang, H.W. Validation of the Core Seven-Emotions Inventory—Short Form. Integr. Med. Res. 2019, 8, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Sue, J.-H.; Lee, G.-E.; Kim, N.-K.; Choi, S.; Lyu, Y.; Kang, H.W. Development of Korean Medical Psychotherapy and Preliminary Clinical Trial for Post Traumatic Stress Disorder. Korean Soc. Orient. Neuropsychiatry 2015, 26, 49–61. [Google Scholar] [CrossRef]
- Parida, S. Clinical Causality Assessment for Adverse Drug Reactions. Indian J. Anaesth. 2013, 57, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in Orthopaedics: A Brief Guide. J. Bone Jt. Surg. Am. 2015, 97, 1628–1634. [Google Scholar] [CrossRef]
- Kim, M.-H.; Cho, Y.-S.; Uhm, W.-S.; Kim, S.; Bae, S.-C. Cross-Cultural Adaptation and Validation of the Korean Version of the EQ-5D in Patients with Rheumatic Diseases. Qual. Life Res. 2005, 14, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Anheyer, D.; Saha, F.J.; Dobos, G. Yoga for Posttraumatic Stress Disorder—A Systematic Review and Meta-Analysis. BMC Psychiatry 2018, 18, 72. [Google Scholar] [CrossRef]
- Brazier, J.; Ratcliffe, J.; Saloman, J.; Tsuchiya, A. Measuring and Valuing Health Benefits for Economic Evaluation; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-872592-3. [Google Scholar]
- Boutron, I.; Altman, D.G.; Moher, D.; Schulz, K.F.; Ravaud, P. CONSORT NPT Group CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 2017, 167, 40–47. [Google Scholar] [CrossRef]
- Ros, T.; Théberge, J.; Frewen, P.A.; Kluetsch, R.; Densmore, M.; Calhoun, V.D.; Lanius, R.A. Mind over Chatter: Plastic up-Regulation of the FMRI Salience Network Directly after EEG Neurofeedback. Neuroimage 2013, 65, 324–335. [Google Scholar] [CrossRef]
- Ghaziri, J.; Tucholka, A.; Larue, V.; Blanchette-Sylvestre, M.; Reyburn, G.; Gilbert, G.; Lévesque, J.; Beauregard, M. Neurofeedback Training Induces Changes in White and Gray Matter. Clin. EEG Neurosci. 2013, 44, 265–272. [Google Scholar] [CrossRef]
- Lang, A.J.; Wilkins, K.; Roy-Byrne, P.P.; Golinelli, D.; Chavira, D.; Sherbourne, C.; Rose, R.D.; Bystritsky, A.; Sullivan, G.; Craske, M.G.; et al. Abbreviated PTSD Checklist (PCL) as a Guide to Clinical Response. Gen. Hosp. Psychiatry 2012, 34, 332–338. [Google Scholar] [CrossRef]
- Stefanovics, E.A.; Rosenheck, R.A.; Jones, K.M.; Huang, G.; Krystal, J.H. Minimal Clinically Important Differences (MCID) in Assessing Outcomes of Post-Traumatic Stress Disorder. Psychiatr. Q. 2018, 89, 141–155. [Google Scholar] [CrossRef]
- Maher, M.J.; Rego, S.A.; Asnis, G.M. Sleep Disturbances in Patients with Post-Traumatic Stress Disorder: Epidemiology, Impact and Approaches to Management. CNS Drugs 2006, 20, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.L.; Barrett, E.L.; Merz, S.; Rosenfeld, J.; Ewer, P.L.; Sannibale, C.; Baker, A.L.; Hopwood, S.; Back, S.E.; Brady, K.T.; et al. Integrated Exposure-Based Therapy for Co-Occurring Post Traumatic Stress Disorder (PTSD) and Substance Dependence: Predictors of Change in PTSD Symptom Severity. J. Clin. Med. 2016, 5, 101. [Google Scholar] [CrossRef]
- Kelson, C.Y. The Impact of EEG Biofeedback on Veterans’ Symptoms of Posttraumatic Stress Disorder (PTSD)—ProQuest. Ph.D. Thesis, The Chicago School of Professional Psychology, Chicago, IL, USA, 2013. [Google Scholar]
- Van der Kolk, B.A.; Hodgdon, H.; Gapen, M.; Musicaro, R.; Suvak, M.K.; Hamlin, E.; Spinazzola, J. A Randomized Controlled Study of Neurofeedback for Chronic PTSD. PLoS ONE 2016, 11, e0166752. [Google Scholar] [CrossRef] [PubMed]
- Noohi, S.; Miraghaie, A.M.; Arabi, A.; Nooripour, R. Effectiveness of Neuro-Feedback Treatment with Alpha/Theta Method on PTSD Symptoms and Their Executing Function. Biomed. Res.-India 2017, 28, 2019–2027. [Google Scholar]
- Cho, E.; Yoo, S.-L.; Kang, Y.; Lee, J.H. Reimbursement and Pricing of Regenerative Medicine in South Korea: Key Factors for Achieving Reimbursement. Regen. Med. 2020, 15, 1550–1560. [Google Scholar] [CrossRef] [PubMed]

| Assessment | Enrollment | Treatment Phase | Follow-Up Phase | ||
|---|---|---|---|---|---|
| Screening | Before V1 (Week One) | After V8 (Week Four) | After V16 (Week Eight) | V17 (Week 12) | |
| Informed consent | X | ||||
| Demographic characteristics | X | ||||
| Medical history | X | ||||
| Vital signs and physical examination | X | Every visit before the intervention | |||
| EKG and X-ray | X | ||||
| Blood * and urine test | X | ||||
| SCID-5 | X | ||||
| Inclusion/exclusion criteria | X | ||||
| Jing Ji and Zheng Chong | X | ||||
| Mibyeong | X | ||||
| KS-15 | X | ||||
| KSRI-SF | X | ||||
| PCL-5-K (primary outcome) | X | X | X # | X | |
| IES-R-K | X | X | X | X | |
| CGI-I | X | X | X | X | |
| BAI | X | X | X | X | |
| BDI | X | X | X | X | |
| ISI | X | X | X | X | |
| Hwa-byung | X | X | X | X | |
| SF-36 | X | X | X | X | |
| EQ-5D, EQ-VAS | X | X | X | X | |
| Cost | X | X | X | X | |
| CSEI-S | Every visit before and after the intervention | ||||
| MRM | Every visit before and after the intervention | ||||
| QEEG | X | X | X | ||
| Safety assessment | During the trial, including waiting and follow-up period | ||||
| Characteristic | Experiment (N = 10) | Wait-Control (N = 9) | p-Value |
|---|---|---|---|
| Sex (male) | 1 (10%) | 1 (11.1%) | 0.93 |
| Age (years) | 44.40 ± 13.61 | 43.56 ± 19.10 | 0.26 |
| Height (centimeter) | 160.20 ± 7.41 | 160.56 ± 6.21 | 0.35 |
| Weight (kilogram) | 60.66 ± 11.68 | 56.67 ± 8.73 | 0.31 |
| Pulse (beat per min) | 79.20 ± 7.96 | 79.78 ± 12.51 | 0.66 |
| SBP (mmHg) | 121.0 ± 13.1 | 123.3 ± 14.4 | 0.63 |
| DBP (mmHg) | 77.4 ± 10.7 | 77.9 ± 15.3 | 0.90 |
| Origin of PTSD | Domestic violence (8 participants) Traffic accident (2 participants) | Domestic violence (8 participants) School violence (1 participant) | NA |
| IES-R-K | 47.50 ± 16.78 | 34.56 ± 16.90 | 0.16 |
| PCL-5-K total score | 44.30 ± 10.87 | 35.11 ± 18.54 | 0.52 |
| Outcome Variables | NSRT Group (N = 10) | Waitlist Control Group (N = 9) | p-Value |
|---|---|---|---|
| PCL-5-K (# Primary outcome, V16) | |||
| V1 (Baseline) | 44.3 ± 10.8 | 35.1 ± 18.5 | 0.31 |
| V8 (week 4) | 34.43 ± 9.51 | 33.2 ± 16.35 | |
| Difference (V1–V8) | −9.9 ± 9.84 | −1.89 ± 7.39 | 0.10 |
| V16 (week 8) | 19.4 ± 7.75 | 31.0 ± 14.92 | |
| Difference (V1–V16) # | −24.90 ± 13.13 | −4.11 ± 9.03 | <0.01 * |
| V17 (week 12) | 19.4 ± 6.52 | 29.4 ± 13.99 | |
| Difference (V1–V17) | −24.90 ± 14.50 | −5.67 ± 12.91 | 0.01 * |
| IES-R-K | |||
| V1 (Baseline) | 47.50 ± 16.78 | 34.56 ± 16.90 | 0.16 |
| V8 (week 4) | 40.30 ± 13.99 | 33.44 ± 19.74 | |
| Difference (V1–V8) | 7.20 ± 22.26 | 1.12 ± 8.55 | 0.21 |
| V16 (week 8) | 25.60 ± 10.47 | 31.11 ± 16.61 | |
| Difference (V1–V16) | 21.90 ± 22.48 | 3.45 ± 12.03 | 0.05 |
| V17 (week 12) | 23.50 ± 11.77 | 28.22 ± 18.89 | |
| Difference (V1–V17) | 24.00 ± 26.60 | 6.34 ± 11.73 | 0.10 |
| CGI-I | |||
| V8 (week 4) | 3.10 ± 0.0.32 | 3.89 ± 0.33 | <0.01 * |
| V16 (week 8) | 2.70 ± 0.67 | 4.22 ± 0.44 | <0.01* |
| V17 (week 12) | 2.30 ± 0.48 | 3.78 ± 0.67 | <0.01 * |
| BAI | |||
| V1 (Baseline) | 48.80 ± 8.74 | 43.22 ± 16.24 | 0.55 |
| V8 (week 4) | 42.20 ± 10.22 | 41.22 ± 9.68 | |
| Difference (V1–V8) | 6.60 ± 6.06 | 2.00 ± 8.28 | 0.24 |
| V16 (week 8) | 32.20 ± 8.50 | 39.44 ± 9.66 | |
| Difference (V1–V16) | 16.60 ± 7.20 | 3.78 ± 9.67 | 0.00 * |
| V17 (week 12) | 32.20 ± 5.73 | 40.22 ± 10.22 | |
| Difference (V1–V17) | 16.60 ± 6.83 | 3.00 ± 10.11 | 0.00 * |
| BDI | |||
| V1 (Baseline) | 26.10 ± 7.87 | 16.89 ± 11.13 | 0.03 * |
| V8 (week 4) | 17.00 ± 5.50 | 15.56 ± 8.80 | |
| Difference (V1–V8) | 9.10 ± 4.61 | 1.33 ± 7.89 | 0.02 * |
| V16 (week 8) | 10.40 ± 5.72 | 14.89 ± 10.95 | |
| Difference (V1–V16) | 15.70 ± 7.47 | 2.00 ± 3.94 | 0.00 * |
| V17 (week 12) | 11.00 ± 5.16 | 15.89 ± 10.37 | |
| Difference (V1–V17) | 15.10 ± 8.89 | 1.00 ± 5.20 | 0.00 * |
| ISI | |||
| V1 (Baseline) | 17.30 ± 5.96 | 12.11 ± 6.89 | 0.11 |
| V8 (week 4) | 14.60 ± 4.84 | 11.44 ± 5.64 | |
| Difference (V1–V8) | 2.70 ± 5.70 | 0.78 ± 3.42 | 0.28 |
| V16 (week 8) | 9.40 ± 4.58 | 11.78 ± 5.56 | |
| Difference (V1–V16) | 7.90 ± 7.43 | 0.44 ± 3.97 | 0.02 * |
| V17 (week 12) | 9.60 ± 5.99 | 9.89 ± 6.99 | |
| Difference (V1–V17) | 7.70 ± 5.96 | 2.33 ± 4.27 | 0.05 |
| HBS | |||
| V1 (Baseline) | 70.2 ± 11.1 | 55.0 ± 23.4 | 0.10 |
| V8 (week 4) | 62.9 ± 9.48 | 57.78 ± 26.63 | |
| Difference (V1–V8) | −7.30 ± 9.17 | 2.78 ± 9.82 | 0.08 |
| V16 (week 8) | 52.1 ± 13.2 | 53.78 ± 21.71 | |
| Difference (V1–V16) | −18.10 ± 14.65 | −1.22 ± 6.55 | 0.00 * |
| V17 (week 12) | 52.3 ± 11.0 | 51.22 ± 19.29 | |
| Difference (V1–V17) | −17.90 ± 12.57 | −3.78 ± 10.22 | 0.03 * |
| EQ-5D | |||
| V1 (Baseline) | 0.61 ± 0.21 | 0.76 ± 0.09 | 0.75 |
| V8 (week 4) | 0.71 ± 0.13 | 0.81 ± 0.18 | 0.19 |
| Difference (V1–V8) | 0.09 ± 0.18 | 0.05 ± 0.15 | 0.54 |
| V16 (week 8) | 0.75 ± 0.20 | 0.80 ± 0.15 | 0.56 |
| Difference (V1–V16) | 0.13 ± 0.13 | 0.04 ± 0.12 | 0.12 |
| V17 (week 12) | 0.74 ± 0.16 | 0.81 ± 0.13 | 0.35 |
| Difference (V1–V17) | 0.13 ± 0.13 | 0.05 ± 0.11 | 0.15 |
| EQ-VAS | |||
| V1 (Baseline) | 42.80 ± 18.70 | 64.67 ± 8.93 | 0.01 * |
| V8 (week 4) | 59.90 ± 15.37 | 60.33 ± 10.42 | 0.94 |
| Difference (V1–V8) | 17.10 ± 15.30 | −4.33 ± 9.53 | 0.00 * |
| V16 (week 8) | 66.80 ± 9.95 | 66.11 ± 12.44 | 0.90 |
| Difference (V1–V16) | 24.00 ± 20.11 | 1.44 ± 12.99 | 0.01 * |
| V17 (week 12) | 70.80 ± 13.57 | 68.33 ± 13.92 | 0.70 |
| Difference (V1–V17) | 28.00 ± 16.12 | 3.67 ± 12.64 | 0.00 * |
| SF-36 | |||
| V1 (Baseline) | 63.58 ± 5.88 | 78.11 ± 10.88 | 0.00 * |
| V8 (week 4) | 72.80 ± 11.25 | 83.65 ± 10.51 | 0.05 * |
| Difference (V1–V8) | 9.22 ± 9.77 | 5.54 ± 13.28 | 0.50 |
| V16 (week 8) | 85.12 ± 13.30 | 83.67 ± 9.41 | 0.79 |
| Difference (V1–V16) | 21.54 ± 13.17 | 5.56 ± 12.85 | 0.01 * |
| V17 (week 12) | 84.60 ± 11.43 | 87.11 ± 11.15 | 0.64 |
| Difference (V1–V17) | 21.02 ± 13.97 | 9.00 ± 12.16 | 0.06 |
| Neurofeedback Group (N = 10) | Waitlist Control Group (N = 9) | |
|---|---|---|
| Cost (KRW) | ||
| Neurofeedback training fee | 1,280,000 | 0 |
| Medical cost | 220,253 | 120,667 |
| Transportation expense | 67,462 | 67,462 |
| Loss of productivity | 116,545 | 151,926 |
| Total cost (8 weeks) | 1,684,260 (A) | 340,055 (B) |
| Area under the curve (AUC) of utility (EQ-5D) | ||
| Week 0–4 | 0.190 | 0.094 |
| Week 5–8 | 0.454 | 0.168 |
| Week 9–12 | 0.524 | 0.170 |
| Week 13–52 | 5.200 | 1.920 |
| Increased QALY | ||
| AUC total/one year (52 weeks) | 0.122 (C) | 0.045 (D) |
| ICUR: (A–B)/(C–D) | ||
| 17,457,208 (KRW/QALY) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leem, J.; Cheong, M.J.; Lee, H.; Cho, E.; Lee, S.Y.; Kim, G.-W.; Kang, H.W. Effectiveness, Cost-Utility, and Safety of Neurofeedback Self-Regulating Training in Patients with Post-Traumatic Stress Disorder: A Randomized Controlled Trial. Healthcare 2021, 9, 1351. https://doi.org/10.3390/healthcare9101351
Leem J, Cheong MJ, Lee H, Cho E, Lee SY, Kim G-W, Kang HW. Effectiveness, Cost-Utility, and Safety of Neurofeedback Self-Regulating Training in Patients with Post-Traumatic Stress Disorder: A Randomized Controlled Trial. Healthcare. 2021; 9(10):1351. https://doi.org/10.3390/healthcare9101351
Chicago/Turabian StyleLeem, Jungtae, Moon Joo Cheong, Hyeryun Lee, Eun Cho, So Young Lee, Geun-Woo Kim, and Hyung Won Kang. 2021. "Effectiveness, Cost-Utility, and Safety of Neurofeedback Self-Regulating Training in Patients with Post-Traumatic Stress Disorder: A Randomized Controlled Trial" Healthcare 9, no. 10: 1351. https://doi.org/10.3390/healthcare9101351
APA StyleLeem, J., Cheong, M. J., Lee, H., Cho, E., Lee, S. Y., Kim, G.-W., & Kang, H. W. (2021). Effectiveness, Cost-Utility, and Safety of Neurofeedback Self-Regulating Training in Patients with Post-Traumatic Stress Disorder: A Randomized Controlled Trial. Healthcare, 9(10), 1351. https://doi.org/10.3390/healthcare9101351







