Effects of Somatic, Depression Symptoms, and Sedentary Time on Sleep Quality in Middle-Aged Women with Risk Factors for Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Setting and Sample
2.3. Sample Size
2.4. Data Collection
2.5. General and Anthropometric Characteristics of the Participants
2.5.1. Quality of Sleep
2.5.2. Somatic Symptoms
2.5.3. Depression Symptoms
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Cardiovascular Disease-Related Characteristics of the Participants
3.3. Correlation between Sleep Quality and the Independent Variables
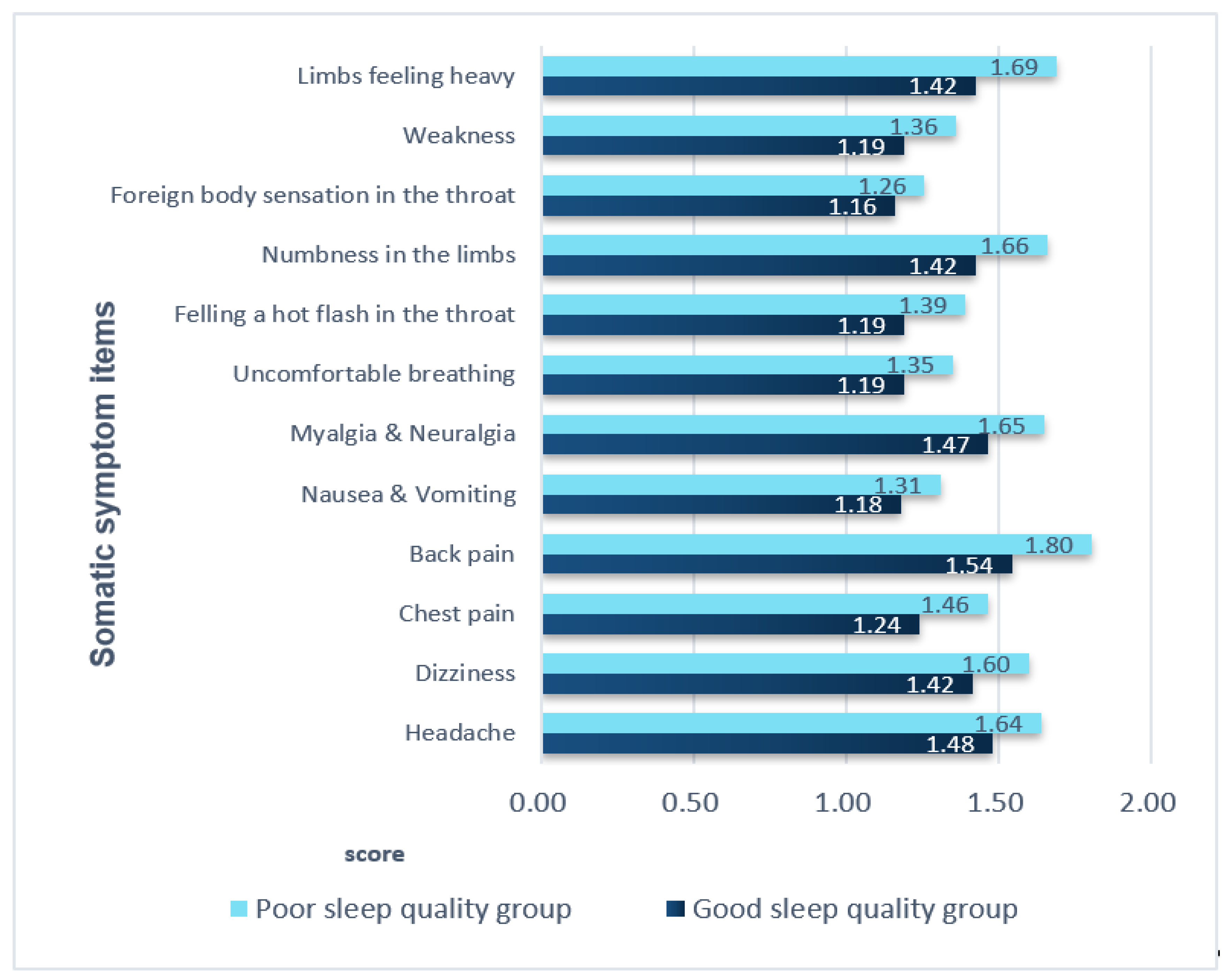
3.4. Comparison of Somatic Symptoms in the Two Groups
3.5. Predictors of Sleep Quality in Middle-Aged Women with CVD Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- CDC’s Public Health Efforts Related to Heart Disease. Available online: https://www.cdc.gov/heartdisease/women.htm (accessed on 31 January 2020).
- Statistics Korea. 2018 Annual Report on the Causes of Death Statistics. Available online: http://kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board?bmode=read&aSeq=377606&pageNo=&rowNum=10&amSeq=&sTarget=&sTxt= (accessed on 24 September 2019).
- Cardiovascular Diseases (CVDs), World Health Organization. Available online: https://www.who.int/news-room/factsheets/detail/cardiovascular-diseases-(cvds) (accessed on 21 June 2021).
- Galiuto, L.; Locorotondo, G. Gender differences in cardiovascular disease. J. Integr. Cardiol. 2015, 1, 20–22. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Sakson-Obada, O.; Wycisk, J. The body self and the frequency, intensity and acceptance of menopausal symptoms. Menopausal Rev. 2015, 2, 82–89. [Google Scholar] [CrossRef]
- Garrusi, B.; Danaei, M.; Aboosaeidi, R. The prevalence and predictive factors of somatization and its relationship with anxiety and depression in Iranian population. J. Prev. Med. Hyg. 2019, 60, E400–E406. [Google Scholar]
- Zheng, F.; Duan, Y.; Li, J.; Lai, L.; Zhong, Z.; Hu, M.; Ding, S. Somatic symptoms and their association with anxiety and depression in Chinese patients with cardiac neurosis. J. Int. Med. Res. 2019, 47, 4920–4928. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Grandner, M.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Lao, X.Q.; Liu, X.; Deng, H.-B.; Chan, T.-C.; Ho, K.F.; Wang, F.; Vermeulen, R.; Tam, T.; Wong, M.; Tse, L.A.; et al. Sleep Quality, Sleep Duration, and the Risk of Coronary Heart Disease: A Prospective Cohort Study With 60,586 Adults. J. Clin. Sleep Med. 2018, 14, 109–117. [Google Scholar] [CrossRef]
- Tamanna, S.; Geraci, S.A. Major Sleep Disorders Among Women. South. Med. J. 2013, 106, 470–478. [Google Scholar] [CrossRef]
- Worrall-Carter, L.; Ski, C.; Scruth, E.; Campbell, M.; Page, K. Systematic review of cardiovascular disease in women: Assessing the risk. Nurs. Health Sci. 2011, 13, 529–535. [Google Scholar] [CrossRef]
- Kling, J.M.; Miller, V.M.; Mankad, R.; Wilansky, S.; Wu, Q.; Zais, T.G.; Zarling, K.K.; Allison, T.G.; Mulvagh, S.L. Go Red for Women Cardiovascular Health–Screening Evaluation: The Dichotomy Between Awareness and Perception of Cardiovascular Risk in the Community. J. Women’s Health 2013, 22, 210–218. [Google Scholar] [CrossRef]
- Bushnell, C.; McCullough, L.D.; Awad, I.A.; Chireau, M.V.; Fedder, W.N.; Furie, K.L.; Howard, V.J.; Lichtman, J.H.; Lisabeth, L.D.; Piña, I.L.; et al. Guidelines for the Prevention of Stroke in Women. Stroke 2014, 45, 1545–1588. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.O.; Loria, A.S. Sex-specific effects of stress on metabolic and cardiovascular disease: Are women at higher risk? Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Nyström, M.B.; Hassmén, P.; Sörman, D.E.; Wigforss, T.; Andersson, G.; Carlbring, P. Are physical activity and sedentary behavior related to depression? Cogent Psychol. 2019, 6, 1633810. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and cardiovascular disease: The deep blue sea of women’s heart. Trends Cardiovasc. Med. 2020, 30, 170–176. [Google Scholar] [CrossRef]
- Mosca, L.; Benjamin, E.; Berra, K.; Bezanson, J.L.; Dolor, R.; Lloyd-Jones, D.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- Mørkedal, B.; Romundstad, P.R.; Vatten, L.J. Informativeness of indices of blood pressure, obesity and serum lipids in relation to ischaemic heart disease mortality: The HUNT-II study. Eur. J. Epidemiol. 2011, 26, 457–461. [Google Scholar] [CrossRef][Green Version]
- Kim, I.; Choi, H.; Kim, B. Psychometric Properties of Korean Version of Modified Leeds Sleep Evaluation Questionnaire (KMLSEQ). Korean J. Rehabil. Nurs. 2014, 17, 10–17. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Jun, J.; Lee, Y.-J.; Cho, S.-J. 2190–Effect of major depressive disorder and insomnia on somatization. Eur. Psychiatry 2013, 28, 84–88. [Google Scholar] [CrossRef]
- Jeon, J.A. Korean women’s mental health indicators. Health Welfare Forum. 2016, 235, 47–60. Available online: https://www.kihasa.re.kr/common/filedown.do?seq=35198 (accessed on 13 October 2021).
- Sereda, Y.; Dembitskyi, S. Validity assessment of the symptom checklist SCL-90-R and shortened versions for the general population in Ukraine. BMC Psychiatry 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.L.; Wadden, T.; Lavenberg, J.; Moore, R.H.; Volger, S.; Perez, J.L.; Sarwer, D.B.; Tsai, A.G. Relation of health-related quality of life to metabolic syndrome, obesity, depression and comorbid illnesses. Int. J. Obes. 2011, 35, 1087–1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beutel, M.E.; Wiltink, J.; Kerahrodi, J.G.; Tibubos, A.N.; Brähler, E.; Schulz, A.; Wild, P.; Münzel, T.; Lackner, K.; König, J.; et al. Somatic symptom load in men and women from middle to high age in the Gutenberg Health Study—association with psychosocial and somatic factors. Sci. Rep. 2019, 9, 4610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lam, S.-P.; Li, S.X.; Tang, N.L.; Yu, M.W.; Li, A.M.; Wing, Y.-K. Insomnia, sleep quality, pain, and somatic symptoms: Sex differences and shared genetic components. Pain 2012, 153, 666–673. [Google Scholar] [CrossRef]
- Kim, Y.S. Physical Activity and Mental Health. Hanyang. Med. Rev. 2014, 34, 60–65. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, X.; Kaminga, A.C.; Zhu, T.; Nie, Y.; Xu, H. Association between poor sleep quality and depression symptoms among the elderly in nursing homes in Hunan province, China: A cross-sectional study. BMJ Open 2020, 10, e036401. [Google Scholar] [CrossRef]
- Lim, J.T.; Oh, M.K.; Kim, H.K.; Lee, J.H.; Lee, B.S.; Park, S.Y. The relationship between the sleep duration and health-related quality of life (HRQL) in Korea-using data from the Korea national health and nutrition examination survey 2012. Korean J. Fam. Pract. 2015, 5, 283–290. [Google Scholar]
- Mohan, J.; Xiaofan, G.; Yingxian, S. Association between sleep time and depression: A cross-sectional study from countries in rural Northeastern China. J. Int. Med. Res. 2017, 45, 984–992. [Google Scholar] [CrossRef]
- Wassertheil-Smoller, S.; Shumaker, S.A.; Ockene, J.K.; Talavera, G.A.; Greenland, P.; Cochrane, B.B.; Robbins, J.; Aragaki, A.K.; Dunbar-Jacob, J. Depression and Cardiovascular Sequelae in Postmenopausal Women. Arch. Intern. Med. 2004, 164, 289–298. [Google Scholar] [CrossRef]
- Yazdanpanah, M.H.; Homayounfar, R.; Khademi, A.; Zarei, F.; Shahidi, A.; Farjam, M. Short sleep is associated with higher prevalence and increased predicted risk of cardiovascular diseases in an Iranian population: Fasa PERSIAN Cohort Study. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Creasy, S.A.; Crane, T.E.; Garcia, D.O.; Thomson, C.A.; Kohler, L.N.; Wertheim, B.C.; Baker, L.D.; Coday, M.; Hale, L.; Womack, C.R.; et al. Higher amounts of sedentary time are associated with short sleep duration and poor sleep quality in postmenopausal women. Sleep 2019, 42, 093. [Google Scholar] [CrossRef]
- Buman, M.P.; Kline, C.E.; Youngstedt, S.D.; Phillips, B.; de Mello, M.T.; Hirshkowitz, M. Sitting and Television Viewing. Chest 2015, 147, 728–734. [Google Scholar] [CrossRef]
- Grøntved, A. Television Viewing and Risk of Type 2 Diabetes, Cardiovascular Disease, and All-Cause Mortality. JAMA 2011, 305, 2448–2455. [Google Scholar] [CrossRef]
- Pereira, S.P.; Ki, M.; Power, C. Sedentary Behaviour and Biomarkers for Cardiovascular Disease and Diabetes in Mid-Life: The Role of Television-Viewing and Sitting at Work. PLoS ONE 2012, 7, e31132. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, L.; Apostolopoulos, V.; Polman, R.; Borkoles, E. To exercise, or, not to exercise, during menopause and beyond. Maturitas 2014, 77, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Ward, H.; Norat, T.; Luan, J.; May, A.M.; Weiderpass, E.; Sharp, S.J.; Overvad, K.; Østergaard, J.N.; Tjønneland, A.; et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: The European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am. J. Clin. Nutr. 2015, 101, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Zaccardi, F.; Gillies, C.; Dhalwani, N.; Yates, T.; Rowlands, A.V.; Davies, M.; Khunti, K.K. Leisure-time physical activity and life expectancy in people with cardiometabolic multimorbidity and depression. J. Intern. Med. 2019, 287, 87–99. [Google Scholar] [CrossRef]
- Mynarski, W.; Rozpara, M.; Nawrocka, A.; Borek, Z.; Powerska, A.; Garbaciak, W. Physical activity of middle-age adults aged 50–65 years in view of health recommendations. Eur. Rev. Aging Phys. Act. 2014, 11, 141–147. [Google Scholar] [CrossRef]
- Kim, K.A.; Hwang, S.Y. Impact of Physical Activity, Central Obesity and Depression on the Quality of Life according to the Presence of Cardiovascular Risk among Menopausal Middle-aged Women: Secondary Data Analysis. Korean J. Adult Nurs. 2017, 29, 382–392. [Google Scholar] [CrossRef]
- Nelson, C.L. The Relationship between Sleep and Sedentary Time, and the Impact of Varying Sleep Patterns. Master’s Thesis, Iowa State University, Ames, Iowa, 2017. [Google Scholar]
| Variables | Categories | Good Sleep Quality Group (n = 106) | Poor Sleep Quality Group (n = 97) | t or x2 | p |
|---|---|---|---|---|---|
| n (%) or Mean ± SD | n (%) or Mean ± SD | ||||
| Age (yr) | 55.74 ± 6.33 | 53.67 ± 6.93 | −2.21 | 0.028 | |
| 40∼50 51~60 61~65 | 27(25.7) 50(47.6) 28(26.7) | 36(27.0) 43(44.4) 18(18.6) | 6.06 | 0.195 | |
| Menopause age | 50.92 ± 4.10 | 50.79 ± 3.09 | −0.20 | 0.843 | |
| Presence of menopause | Yes No | 75(71.4) 30(28.6) | 59(62.8) 35(37.2) | 1.69 | 0.193 |
| Education level | ≤Middle school High school ≥College | 38(36.2) 52(49.5) 15(14.3) | 27(28.1) 47(49.0) 22(22.9) | 3.04 | 0.219 |
| Living with | Spouse or children Alone Living with someone outside the family | 83(79.0) 44(10.5) 11(11.0) | 77(79.4) 10(10.3) 10(10.4) | 1.15 | 0.765 |
| Occupation | Managerial/Official Service/Sales Professional Laborer Unemployed/Housewife | 7(6.6) 7(6.6) 12(11.3) 63(52.7) 17(16.0) | 11(11.6) 21(22.1) 9(9.5) 29(27.4) 17(19.9) | 14.52 | 0.006 |
| Perceived health status | Good Moderate Poor | 20(18.7) 63(60.0) 22(21.0) | 16(16.5) 56(57.7) 25(25.8) | 0.73 | 0.693 |
| Variables | Categories | Good Sleep Quality Group (n = 106) | Poor Sleep Quality Group (n = 97) | t or x2 | p |
|---|---|---|---|---|---|
| n (%) or Mean ± SD | n (%) or Mean ± SD | ||||
| Height (cm) | 156.99 ± 5.31 | 157.70 ± 5.18 | 0.97 | 0.336 | |
| Weight (cm) | 61.00 ± 8.98 | 60.49 ± 8.01 | −0.43 | 0.671 | |
| Waist (cm) | 84.89 ± 9.10 | 83.54 ± 9.31 | −1.04 | 0.299 | |
| Hip (cm) | 104.69 ± 84.67 | 95.47 ± 8.32 | −1.12 | 0.299 | |
| Waist-to-hip ratio | 0.87 ± 0.10 | 0.87 ± 0.09 | −1.02 | 0.308 | |
| <0.8 ≥0.8 | 14(13.2) 92(86.8) | 6(6.0) 91(93.0) | 0.48 | 0.634 | |
| Body mass index (m2/kg) | 27.74 ± 3.33 | 24.30 ± 2.78 | −1.02 | 0.308 | |
| <25 ≥25 | 61(58.0) 45(42.0) | 67(69.1) 30(30.9) | 3.77 | 0.152 | |
| CVD risk factors † | Hypertension Diabetes Hyperlipidemia Angina and arrhythmia symptoms ∫ Chronic kidney disease Arthritis Fatty liver | 60(74.1) 21(25.9) 34(42.0) 5(6.1) 0(0.0) 4(4.1) 3(3.7) | 54(68.4) 10(12.7) 44(55.7) 3(3.8) 2(2.5) 11(13.9) 6(7.6) | 0.20 3.54 4.51 0.55 0.29 7.95 0.25 | 0.990 0.060 0.105 0.723 0.557 0.019 0.298 |
| CVD risk factors related to family history ‡ | Hypertension Diabetes Stroke, MI Cancer | 47(44.3) 31(29.2) 21(19.8) 23(21.7) | 45(46.4) 31(32.0) 20(20.6) 27(27.8) | 0.09 0.18 1.13 1.03 | 0.769 0.675 0.568 0.311 |
| Smoking | Never Ex-smoker Current smoker | 95(91.3) 8(7.7) 1(1.0) | 86(89.6) 3(3.1) 7(7.3) | 6.91 | 0.032 |
| Alcohol | <1/month 2~3/months ≥2~4/weeks | 71(71.9) 18(17.3) 15(14.4) | 61(64.2) 26(27.4) 8(8.4) | 5.33 | 0.256 |
| Menopause (yr) | Yes No Estrogen therapy (Yes) | 50.92 ± 4.10 75(71.4) 30(28.6) 0(0.0) | 50.79 ± 3.09 59(62.8) 35(37.2) 0(0.0) | −0.19 0.19 | 0.848 0.227 |
| Sedentary time (1 day) | 5.21 ± 3.14 | 7.24 ± 8.16 | 2.33 | 0.021 | |
| Sleeping hours (1 day) | Poor (<7 or >8) Good (7~8) | 57(55.3) 46(44.7) | 69(71.1) 28(28.9) | 5.35 | 0.021 |
| Quality of sleep (score) | 79.97 ± 8.53 | 53.52 ± 10.76 | −19.29 | <0.001 | |
| Depression symptoms (score) | 3.76 ± 4.16 | 6.32 ± 4.89 | 4.00 | <0.001 | |
| No (<10) Yes (≥10) | 96(90.6) 10(9.4) | 75(77.3) 22(22.7) | 6.70 | 0.010 | |
| Somatic symptoms (score) | 5.79 ± 5.46 | 9.72 ± 7.72 | 4.19 | <0.001 | |
| Variables. | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| R(P) | ||||
| (1) Quality of sleep (score) | 1 | |||
| (2) Somatic symptoms (score) | −0.47 **(<0.001) | 1 | ||
| (3) Depression symptoms (score) | −0.39 **(<0.001) | 0.57 **(<0.001) | 1 | |
| (4) Sedentary time (1 day) | −0.15 *(0.041) | 0.00(0.990) | 0.09(0.196) | 1 |
| Variables. | B | SE | β | t | p |
|---|---|---|---|---|---|
| (Constant) | 79.40 | 1.97 | 40.28 | <0.001 | |
| Somatic symptoms (score) | −0.92 | 0.19 | −0.36 | −4.65 | <0.001 |
| Depression symptoms (score) | −0.61 | 0.27 | −0.17 | −2.30 | 0.023 |
| Sedentary time (1 day) | −0.35 | 0.17 | −0.13 | −2.06 | 0.041 |
| R2 = 0.24, Adjusted R2 = 0.23, F = 19.80, p < 0.001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-S.; Kim, K.-A. Effects of Somatic, Depression Symptoms, and Sedentary Time on Sleep Quality in Middle-Aged Women with Risk Factors for Cardiovascular Disease. Healthcare 2021, 9, 1378. https://doi.org/10.3390/healthcare9101378
Choi H-S, Kim K-A. Effects of Somatic, Depression Symptoms, and Sedentary Time on Sleep Quality in Middle-Aged Women with Risk Factors for Cardiovascular Disease. Healthcare. 2021; 9(10):1378. https://doi.org/10.3390/healthcare9101378
Chicago/Turabian StyleChoi, Hyun-Sook, and Kyung-Ae Kim. 2021. "Effects of Somatic, Depression Symptoms, and Sedentary Time on Sleep Quality in Middle-Aged Women with Risk Factors for Cardiovascular Disease" Healthcare 9, no. 10: 1378. https://doi.org/10.3390/healthcare9101378
APA StyleChoi, H.-S., & Kim, K.-A. (2021). Effects of Somatic, Depression Symptoms, and Sedentary Time on Sleep Quality in Middle-Aged Women with Risk Factors for Cardiovascular Disease. Healthcare, 9(10), 1378. https://doi.org/10.3390/healthcare9101378






