State of the Art in Hepatic Dysfunction in Pregnancy
Abstract
:1. Introduction
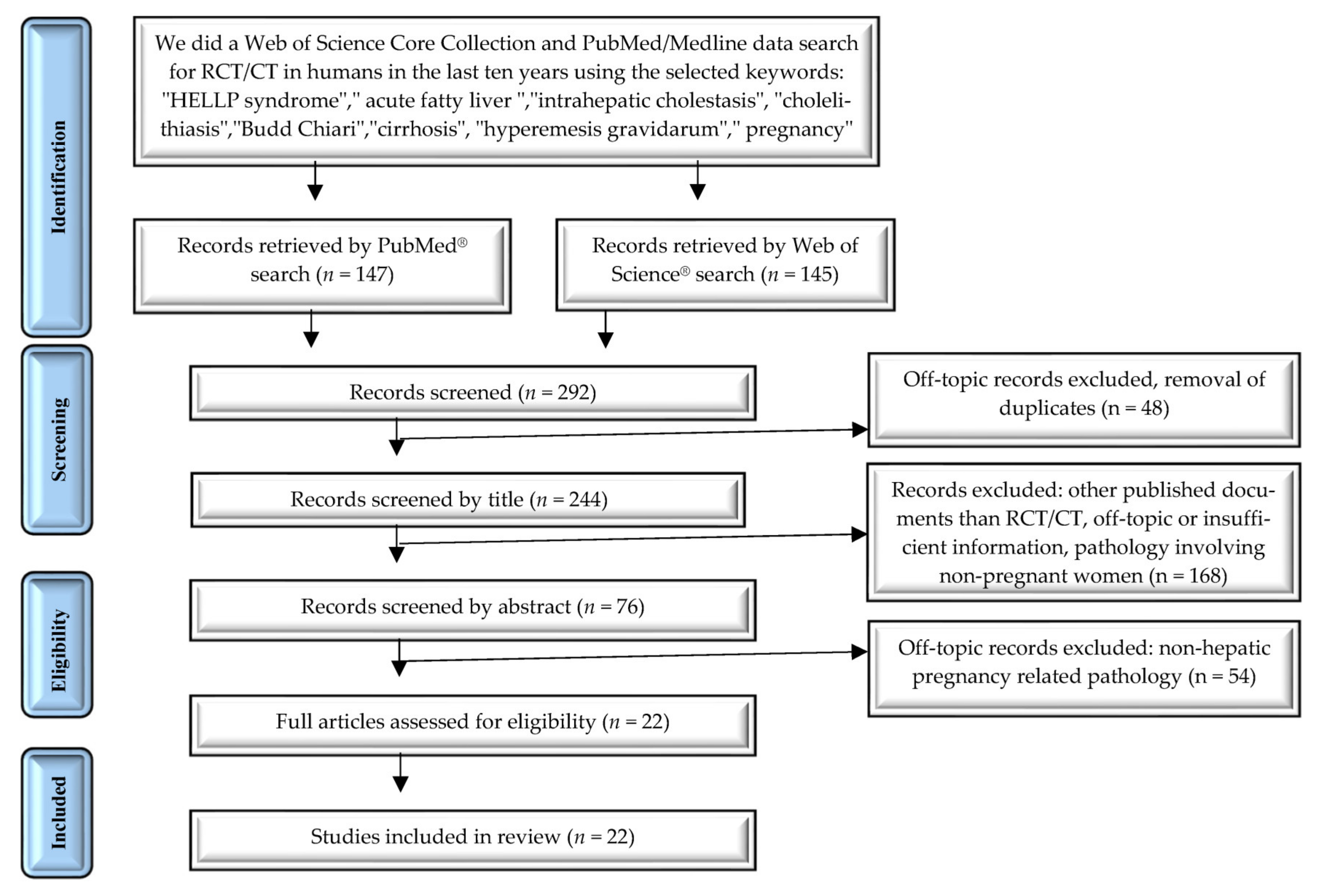
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Synthesis
3. Results
Study Characteristics
4. Hyperemesis Gravidarum and Liver Dysfunction
5. HELLP Syndrome
6. Acute Fatty Liver of Pregnancy
7. Intrahepatic Cholestasis of Pregnancy
8. Cholelithiasis
- Asymptomatic women, in whom gallstones are discovered incidentally on ultrasound examination.
- Women with typical biliary symptoms and the presence of gallstones on ultrasound examination.
- Women with atypical biliary symptoms and the presence of gallstones on ultrasound examination.
9. Budd–Chiari Syndrome
- Thrombophilia: factor V Leiden G1691A mutation, prothrombin gene G20210A mutation, protein C deficiency, protein S deficiency, antithrombin deficiency, antiphospholipid antibodies syndrome, hyperhomocysteinemia, and paroxysmal nocturnal hemoglobinuria.
- JAK2V617F mutation.
- Myeloproliferative disorders, such as polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis.
- Hormonal factors: oral contraceptive use, pregnancy.
- Connective tissue disease: inflammatory bowel disease, Behçet disease, sarcoidosis, and vasculitis.
- Dehydration [105].
- Anticoagulant treatment with vitamin K antagonists is not recommended in pregnancy.
- Pregnancy and childbirth can be performed safely if the patient receives appropriate supportive and anticoagulant treatment.
- Liver transplantation can be used as a life-saving therapeutic method [110].
10. Cirrhosis
11. Discussions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
| AFLP | acute fatty liver of pregnancy |
| HUS | hemolytic-uremic syndrome |
| ITP | immune thrombocytopenia |
| TTP | thrombotic thrombocytopenic purpura |
| AST | aspartate aminotransferase |
| LDH | lactate dehydrogenase |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| LCHAD | long-chain L-3 hydroxyacyl-CoA dehydrogenase |
| UDCA | ursodeoxycholic acid |
| ERCP | endoscopic retrograde cholangiopancreatography |
| MRCP | magnetic resonance cholangiopancreatography |
| HIDA | hepatobiliary iminodiacetic acid |
| BCS | Budd–Chiari syndrome |
| DIC | disseminated intravascular coagulation |
| ARDS | acute respiratory distress syndrome |
| NAFLD | nonalcoholic fatty liver disease |
References
- García-Romero, C.S.; Guzman, C.; Cervantes, A.; Cerbón, M. Liver Disease in Pregnancy: Medical Aspects and Their Implications for Mother and Child. Ann. Hepatol. 2019, 18, 553–562. [Google Scholar] [CrossRef]
- Goel, A.; Jamwal, K.D.; Ramachandran, A.; Balasubramanian, K.A.; Eapen, C.E. Pregnancy-Related Liver Disorders. J. Clin. Exp. Hepatol. 2014, 4, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; O’Donohue, J.; Wendon, J.; Williams, R. Maternal and Perinatal Outcome in Severe Pregnancy-Related Liver Disease. Hepatology 1997, 26, 1258–1262. [Google Scholar] [CrossRef]
- Casey, L.C.; Fontana, R.J.; Aday, A.; Nelson, D.B.; Rule, J.A.; Gottfried, M.; Tran, M.; Lee, W.M. Acute Liver Failure Study Group. Acute Liver Failure (ALF) in Pregnancy: How Much Is Pregnancy Related? Hepatolology 2020, 72, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.F.; Sousa, M.; Lourenço, I.; Martins, D.; Torres, J. Gastrointestinal Diseases during Pregnancy: What Does the Gastroenterologist Need to Know? Ann. Gastroenterol. 2018, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Filipec-Kanizaj, T.; Jakopcic, I.; Majurec, I.; Brncic-Fischer, A.; Sobocan, N.; Hrstic, I.; Stimac, T.; Stimac, D.; Milic, S. Liver Disease During Pregnancy: A Challenging Clinical Issue. Med. Sci. Monit. 2018, 24, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Dusheiko, G.; Williamson, C. Pregnancy and Liver Disease. J. Hepatol. 2016, 64, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Pericleous, M. Pregnancy-Associated Liver Disease: A Curriculum-Based Review. Frontline Gastroenterol. 2018, 9, 170–174. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, N.; Wright, D.; Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Study Protocol for the Randomised Controlled Trial: Combined Multimarker Screening and Randomised Patient Treatment with ASpirin for Evidence-Based PREeclampsia Prevention (ASPRE). BMJ Open 2016, 6, e011801. [Google Scholar] [CrossRef]
- Kozic, J.R.; Benton, S.J.; Hutcheon, J.A.; Payne, B.A.; Magee, L.A.; Von Dadelszen, P. PIERS (Preeclampsia Integrated Estimate of RiSk) Study Group. Abnormal Liver Function Tests as Predictors of Adverse Maternal Outcomes in Women with Preeclampsia. Int. J. Gynecol. Obstet. 2011, 33, 995–1004. [Google Scholar] [CrossRef]
- Hay, J.E. Liver Disease in Pregnancy. Hepatology 2008, 47, 1067–1076. [Google Scholar] [CrossRef]
- Brady, C.W. Liver Disease in Pregnancy: What’s New. Hepatol. Commun. 2020, 4, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Ellington, S.R.; Flowers, L.; Legardy-Williams, J.K.; Jamieson, D.J.; Kourtis, A.P. Recent trends in hepatic diseases during pregnancy in the United States, 2002–2010. Am. J. Obstet. Gynecol. 2015, 212, 524.e1–524.e7. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Saab, A.M.; Saab, S.; El-Kabany, M. A Systematic Approach to Pregnancy-Specific Liver Disorders. Gastroenterol. Hepatol. 2021, 17, 322–329. [Google Scholar]
- Simsek, Y.; Gul, M.; Celik, O.; Aydin, N.E.; Arda Düz, S.; Celik, E.; Ozerol, E.; Özerol, I.H.; Tanbek, K. Nuclear Transcription Factor-Kappa Beta-Dependent Ultrastructural Alterations within the Placenta and Systemic Inflammatory Activation in Pregnant Patients with Hemolysis, Elevated Liver Functions and Low Thrombocyte Count (HELLP) Syndrome: A Case-Control Study. Hypertens. Pregnancy 2013, 32, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Blois, S.M.; Meint, P.; Freitag, N.; Ernst, W.; Barrientos, G.; Conrad, M.L.; Rose, M.; Seelbach-Göbel, B. Elevated Systemic Galectin-1 Levels Characterize HELLP Syndrome. J. Reprod. Immunol. 2016, 114, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, L.; Sun, Q.; Wang, X.D. Expression of Urocortin and Corticotrophin-Releasing Hormone Receptor-2 in Patients with Intrahepatic Cholestasis of Pregnancy. Placenta 2014, 35, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Ozler, A.; Ucmak, D.; Evsen, M.S.; Kaplan, I.; Elbey, B.; Arica, M.; Kaya, M. Immune Mechanisms and the Role of Oxidative Stress in Intrahepatic Cholestasis of Pregnancy. Cent. Eur. J. Immunol. 2014, 39, 198–202. [Google Scholar] [CrossRef]
- Oztas, E.; Erkenekli, K.; Ozler, S.; Ersoy, A.O.; Kurt, M.; Oztas, E.; Uygur, D.; Danisman, N. Can Routine Laboratory Parameters Predict Adverse Pregnancy Outcomes in Intrahepatic Cholestasis of Pregnancy? J. Perinat. Med. 2015, 43, 667–674. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.-Q.; Zhou, D.-X.; Cui, Y.; Deng, L.-L.; Yang, T.; Shao, Y.; Ding, M. Feasibility of Urinary MicroRNA Profiling Detection in Intrahepatic Cholestasis of Pregnancy and Its Potential as a Non-Invasive Biomarker. Sci. Rep. 2016, 6, 31535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanhal, C.Y.; Daglar, K.; Kara, O.; Yılmaz, Z.V.; Turkmen, G.G.; Erel, O.; Uygur, D.; Yucel, A. An Alternative Method for Measuring Oxidative Stress in Intrahepatic Cholestasis of Pregnancy: Thiol/Disulphide Homeostasis. J. Matern.-Fetal Neonatal Med. 2018, 31, 1477–1482. [Google Scholar] [CrossRef]
- Zou, S.; Zhao, S.; Wang, J.; Dong, R.; Zou, P.; Liang, F.; Zhu, T.T.; Zhou, T.; Li, N.; Zhang, Y.; et al. Diagnostic and Prognostic Value of Long Noncoding RNAs as Potential Novel Biomarkers in Intrahepatic Cholestasis of Pregnancy. BioMed Res. Int. 2021, 2021, 8858326. [Google Scholar] [CrossRef]
- Katz, L.; Amorim, M.; Souza, J.P.; Haddad, S.M.; Cecatti, J.G. COHELLP Study Group. COHELLP: Collaborative Randomized Controlled Trial on Corticosteroids in HELLP Syndrome. Reprod. Health 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, N.; Irshad, B.; Sami, N.; Nadeem, D. Comparison of Dexamethasone versus Betamethasone for the Management of Females with HELLP Syndrome. Pak. J. Med. Health Sci. 2017, 11, 593–597. [Google Scholar]
- Fonseca, J.E.; Otero, J.C.; Messa, C. Dexamethasone for the Treatment of Class I HELLP Syndrome: A Double-Blind, Placebo-Controlled, Multicenter, Randomized Clinical Trial. Pregnancy Hypertens. 2019, 17, 158–164. [Google Scholar] [CrossRef]
- Takahashi, A.; Kita, N.; Tanaka, Y.; Tsuji, S.; One, T.; Ishiko, A.; Kimura, F.; Takahashi, K.; Murakami, T. Effects of High-Dose Dexamethasone in Postpartum Women with Class 1 Haemolysis, Elevated Liver Enzymes and Low Platelets (HELLP) Syndrome. J. Obstet. Gynaecol. 2019, 39, 335–339. [Google Scholar] [CrossRef]
- Marciniak, B.; Kimber-Trojnar, Z.; Leszczyńska-Gorzelak, B.; Patro-Małysza, J.; Trojnar, M.; Oleszczuk, J. Treatment of obstetric cholestasis with polyunsaturated phosphatidylcholine and ursodeoxycholic acid. Ginekol. Pol. 2011, 82, 26–31. [Google Scholar] [PubMed]
- Chappell, L.C.; Gurung, V.; Seed, P.T.; Chambers, J.; Williamson, C.; Thornton, J.G.; PITCH Study Consortium. Ursodeoxycholic Acid versus Placebo, and Early Term Delivery versus Expectant Management, in Women with Intrahepatic Cholestasis of Pregnancy: Semifactorial Randomised Clinical Trial. BMJ 2012, 344, e3799. [Google Scholar] [CrossRef] [Green Version]
- Joutsiniemi, T.; Timonen, S.; Leino, R.; Palo, P.; Ekblad, U. Ursodeoxycholic Acid in the Treatment of Intrahepatic Cholestasis of Pregnancy: A Randomized Controlled Trial. Arch. Gynecol. Obstet. 2014, 289, 541–547. [Google Scholar] [CrossRef]
- Jain, R.; Suri, V.; Chopra, S.; Chawla, Y.K.; Kohli, K.K. Obstetric Cholestasis: Outcome with Active Management. J. Obstet. Gynaecol. Res. 2013, 39, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.-H.; Qi, H.-B.; Li, Z.; Fu, X.-D.; Chen, L.; Shao, Y. Ursodeoxycholic Acid and S-Adenosylmethionine in the Treatment of Intrahepatic Cholestasis of Pregnancy: A Multi-Centered Randomized Controlled Trial. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3770–3776. [Google Scholar]
- Grymowicz, M.; Czajkowski, K.; Smolarczyk, R. Pregnancy Course in Patients with Intrahepatic Cholestasis of Pregnancy Treated with Very Low Doses of Ursodeoxycholic Acid. Scand. J. Gastroenterol. 2016, 51, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Parízek, A.; Simják, P.; Cerný, A.; Sestinová, A.; Zdenková, A.; Hill, M.; Dusková, M.; Vlk, R.; Kokrdová, Z.; Koucký, M.; et al. Efficacy and Safety of Ursodeoxycholic Acid in Patients with Intrahepatic Cholestasis of Pregnancy. Ann. Hepatol. 2016, 15, 757–761. [Google Scholar] [CrossRef]
- Chappell, L.C.; Bell, J.L.; Smith, A.; Linsell, L.; Juszczak, E.; Dixon, P.H.; Chambers, J.; Hunter, R.; Dorling, J.; Williamson, C.; et al. Ursodeoxycholic Acid versus Placebo in Women with Intrahepatic Cholestasis of Pregnancy (PITCHES): A Randomised Controlled Trial. Lancet 2019, 394, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, N.; Mahey, R.; Kulshrestha, V.; Kriplani, A.; Saraya, A.; Sashdev, V. Serum Bile Acids in Intrahepatic Cholestasis of Pregnancy (ICP), Versus Pregnant and Nonpregnant Controls in Asian Indian Women and a Proposed Scoring to Optimize Management in ICP. J. Obstet. Gynecol. India 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Meng, M.; Zeng, J.; Zhou, H.-Y.; Jiang, J.-J.; Ren, H.-S.; Zhang, J.-C.; Zhu, W.-Y.; Wang, C.-T. Effectiveness of Combining Plasma Exchange with Continuous Hemodiafiltration on Acute Fatty Liver of Pregnancy Complicated by Multiple Organ Dysfunction. Artif. Organs 2012, 36, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Jueckstock, J.K.; Kaestner, R.; Mylonas, I. Managing Hyperemesis Gravidarum: A Multimodal Challenge. BMC Med. 2010, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- London, V.; Grube, S.; Sherer, D.M.; Abulafia, O. Hyperemesis Gravidarum: A Review of Recent Literature. Pharmacology 2017, 100, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Kuwata, T.; Kamozawa, C.; Sakamoto, Y.; Suzuki, M.; Tamada, K. Connection between Hyperemesis Gravidarum, Jaundice or Liver Dysfunction, and Biliary Sludge. J. Obstet. Gynaecol. Res. 2012, 38, 446–448. [Google Scholar] [CrossRef]
- Fiaschi, L.; Nelson-Piercy, C.; Deb, S.; King, R.; Tata, L.J. Clinical Management of Nausea and Vomiting in Pregnancy and Hyperemesis Gravidarum across Primary and Secondary Care: A Population-Based Study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1201–1211. [Google Scholar] [CrossRef]
- Reichmann, J.P.; Kirkbride, M.S. Reviewing the Evidence for Using Continuous Subcutaneous Metoclopramide and Ondansetron to Treat Nausea & Vomiting during Pregnancy. Manag. Care 2012, 21, 44–47. [Google Scholar]
- Broadhurst, D.; Cooke, M.; Sriram, D.; Gray, B. Subcutaneous Hydration and Medications Infusions (Effectiveness, Safety, Acceptability): A Systematic Review of Systematic Reviews. PLoS ONE 2020, 15, e0237572. [Google Scholar] [CrossRef]
- El Allani, L.; Benlamkaddem, S.; Berdai, M.A.; Harandou, M. A Case of Massive Hepatic Infarction in Severe Preeclampsia as Part of the HELLP Syndrome. Pan Afr. Med. J. 2020, 36, 78. [Google Scholar] [CrossRef] [PubMed]
- Haddad, B.; Barton, J.R.; Livingston, J.C.; Chahine, R.; Sibai, B.M. Risk Factors for Adverse Maternal Outcomes among Women with HELLP (Hemolysis, Elevated Liver Enzymes, and Low Platelet Count) Syndrome. Am. J. Obstet. Gynecol. 2000, 183, 444–448. [Google Scholar] [CrossRef]
- Collins, S.; Arulkumaran, S.; Hayes, K.; Jackson, S.; Impey, L. Oxford Handbook of Obstetrics and Gynaecology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Martin, J.N.; Brewer, J.M.; Wallace, K.; Sunesara, I.; Canizaro, A.; Blake, P.G.; Lamarca, B.; Owens, M.Y. Hellp Syndrome and Composite Major Maternal Morbidity: Importance of Mississippi Classification System. J. Matern.-Fetal Neonatal Med. 2013, 26, 1201–1206. [Google Scholar] [CrossRef]
- Ang, S.X.; Chen, C.-P.; Sun, F.-J.; Chen, C.-Y. Comparison of Maternal and Neonatal Outcomes between Acute Fatty Liver of Pregnancy and Hemolysis, Elevated Liver Enzymes and Low Platelets Syndrome: A Retrospective Cohort Study. BMC Pregnancy Childbirth 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Sasamori, Y.; Tanaka, A.; Ayabe, T. Liver Disease in Pregnancy. Hepatol. Res. 2020, 50, 1015–1023. [Google Scholar] [CrossRef]
- Tran, T.T.; Ahn, J.; Reau, N.S. ACG Clinical Guideline: Liver Disease and Pregnancy. Am. J. Gastroenterol. 2016, 111, 176–194. [Google Scholar] [CrossRef]
- Wilson, S.G.; White, A.D.; Young, A.L.; Davies, M.H.; Pollard, S.G. The Management of the Surgical Complications of HELLP Syndrome. Ann. R. Coll. Surg. Engl. 2014, 96, 512–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchill, D.; Duley, L.; Thornton, J.G.; Moussa, M.; Ali, H.S.; Walker, K.F. Interventionist versus Expectant Care for Severe Pre-Eclampsia between 24 and 34 Weeks’ Gestation. Cochrane Database Syst. Rev. 2018, 10, CD003106. [Google Scholar] [CrossRef]
- Duley, L.; Meher, S.; Jones, L. Drugs for Treatment of Very High Blood Pressure during Pregnancy. Cochrane Database Syst. Rev. 2013, 7, CD001449. [Google Scholar] [CrossRef]
- Duley, L.; Gülmezoglu, A.M.; Henderson-Smart, D.J.; Chou, D. Magnesium Sulphate and Other Anticonvulsants for Women with Pre-Eclampsia. Cochrane Database Syst. Rev. 2010, 11, CD000025. [Google Scholar] [CrossRef]
- Sankaran, S. Creasy and Resnik’s Maternal—Fetal Medicine: Principles and Practice Sixth Edition. Obstet. Med. 2012, 5, 88–89. [Google Scholar] [CrossRef]
- Shames, B.D.; Fernandez, L.A.; Sollinger, H.W.; Chin, L.T.; D’Alessandro, A.M.; Knechtle, S.J.; Lucey, M.R.; Hafez, R.; Musat, A.I.; Kalayoglu, M. Liver Transplantation for HELLP Syndrome. Liver Transplant. 2005, 11, 224–228. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Farmer, D.G.; Ghobrial, R.M.; Lipshutz, G.S.; Gu, Y.; Hiatt, J.R.; Busuttil, R.W. Liver Transplantation for HELLP Syndrome. Am. Surg. 2007, 73, 1013–1016. [Google Scholar] [CrossRef]
- Sibai, B.M.; Spinnato, J.A.; Watson, D.L.; Hill, G.A.; Anderson, G.D. Pregnancy Outcome in 303 Cases with Severe Preeclampsia. Obstet. Gynecol. 1984, 64, 319–325. [Google Scholar] [CrossRef]
- Yücesoy, G.; Ozkan, S.; Bodur, H.; Tan, T.; Calişkan, E.; Vural, B.; Corakçi, A. Maternal and Perinatal Outcome in Pregnancies Complicated with Hypertensive Disorder of Pregnancy: A Seven Year Experience of a Tertiary Care Center. Arch. Gynecol. Obstet. 2005, 273, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.N.; Arian, S.E.; Sibai, B.M. Management of Hypertensive Disorders in Pregnancy. Womens Health 2014, 10, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ling, G.-J.; Zhang, S.-Q.; Zhai, W.-Q.; Chen, Y.-J. Effect of HELLP Syndrome on Acute Kidney Injury in Pregnancy and Pregnancy Outcomes: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2020, 20, 657. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Fassett, M.J.; Reynolds, T.B.; Shaw, K.J.; Goodwin, T.M. Reversible Peripartum Liver Failure: A New Perspective on the Diagnosis, Treatment, and Cause of Acute Fatty Liver of Pregnancy, Based on 28 Consecutive Cases. Am. J. Obstet. Gynecol. 1999, 181, 389–395. [Google Scholar] [CrossRef]
- Knight, M.; Nelson-Piercy, C.; Kurinczuk, J.J.; Spark, P.; Brocklehurst, P. A Prospective National Study of Acute Fatty Liver of Pregnancy in the UK. Gut 2008, 57, 951–956. [Google Scholar] [CrossRef] [Green Version]
- Joueidi, Y.; Peoc’h, K.; Le Lous, M.; Bouzille, G.; Rousseau, C.; Bardou-Jacquet, E.; Bendavid, C.; Damaj, L.; Fromenty, B.; Lavoué, V.; et al. Maternal and Neonatal Outcomes and Prognostic Factors in Acute Fatty Liver of Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, P.; Gong, Y.; Wan, J.; Zou, W. Treating Acute Fatty Liver of Pregnancy with Artificial Liver Support Therapy: Systematic Review. Medicine 2018, 97, e12473. [Google Scholar] [CrossRef]
- Nelson, D.B.; Yost, N.P.; Cunningham, F.G. Acute Fatty Liver of Pregnancy: Clinical Outcomes and Expected Duration of Recovery. Am. J. Obstet. Gynecol. 2013, 209, 456.e1–456.e7. [Google Scholar] [CrossRef]
- Dwivedi, S.; Runmei, M. Retrospective Study of Seven Cases with Acute Fatty Liver of Pregnancy. ISRN Obstet. Gynecol. 2013, 2013, 730569. [Google Scholar] [CrossRef]
- Naoum, E.E.; Leffert, L.R.; Chitilian, H.V.; Gray, K.J.; Bateman, B.T. Acute Fatty Liver of Pregnancy: Pathophysiology, Anesthetic Implications, and Obstetrical Management. Anesthesiology 2019, 130, 446–461. [Google Scholar] [CrossRef]
- Ko, H.H.; Yoshida, E.M. Acute Fatty Liver of Pregnancy. Can. J. Gastroenterol. 2006, 20, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.B.; Byrne, J.J.; Cunningham, F.G. Acute Fatty Liver of Pregnancy. Clin. Obstet. Gynecol. 2020, 63, 152–164. [Google Scholar] [CrossRef]
- Seyyed Majidi, M.R.; Vafaeimanesh, J. Plasmapheresis in Acute Fatty Liver of Pregnancy: An Effective Treatment. Case Rep. Obstet. Gynecol. 2013, 2013, 615975. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.D.; Rood, K.M. Intrahepatic Cholestasis of Pregnancy. Clin. Obstet. Gynecol. 2020, 63, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Livingston, E.G.; Hughes, B.L.; Kuller, J.A. Intrahepatic Cholestasis of Pregnancy: A Review of Diagnosis and Management. Obstet. Gynecol. Surv. 2018, 73, 103–109. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Z.; Song, Y.; Sun, Y.; Shi, H.; Chen, D.; Zhang, Y. Molecular Pathogenesis of Intrahepatic Cholestasis of Pregnancy. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6679322. [Google Scholar] [CrossRef]
- Sticova, E.; Jirsa, M.; Pawłowska, J. New Insights in Genetic Cholestasis: From Molecular Mechanisms to Clinical Implications. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2313675. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, P. Intrahepatic Cholestasis of Pregnancy: A Further Important Step in Dissecting Its Genetic Architecture. Dig. Liver Dis. 2013, 45, 266–267. [Google Scholar] [CrossRef]
- Donet, A.; Girault, A.; Pinton, A.; Lepercq, J. Intrahepatic Cholestasis of Pregnancy: Is a Screening for Differential Diagnoses Necessary? J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101907. [Google Scholar] [CrossRef]
- Germain, A.M.; Kato, S.; Carvajal, J.A.; Valenzuela, G.J.; Valdes, G.L.; Glasinovic, J.C. Bile Acids Increase Response and Expression of Human Myometrial Oxytocin Receptor. Am. J. Obstet. Gynecol. 2003, 189, 577–582. [Google Scholar] [CrossRef]
- Geenes, V.; Williamson, C. Intrahepatic Cholestasis of Pregnancy. World J. Gastroenterol. 2009, 15, 2049–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glantz, A.; Marschall, H.-U.; Mattsson, L.-A. Intrahepatic Cholestasis of Pregnancy: Relationships between Bile Acid Levels and Fetal Complication Rates. Hepatology 2004, 40, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, C.; Seed, P.T.; Sklavounos, A.; Geenes, V.; Di Ilio, C.; Chambers, J.; Kohari, K.; Bacq, Y.; Bozkurt, N.; Brun-Furrer, R.; et al. Association of Adverse Perinatal Outcomes of Intrahepatic Cholestasis of Pregnancy with Biochemical Markers: Results of Aggregate and Individual Patient Data Meta-Analyses. Lancet 2019, 393, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, L.; Koster, M.P.H.; Page-Christiaens, G.C.M.L.; Kemperman, H.; Boon, J.; Evers, I.M.; Bogte, A.; Oudijk, M.A. Intrahepatic Cholestasis of Pregnancy: Maternal and Fetal Outcomes Associated with Elevated Bile Acid Levels. Am. J. Obstet. Gynecol. 2015, 212, 100.e1–100.e7. [Google Scholar] [CrossRef] [PubMed]
- Bacq, Y.; Sentilhes, L.; Reyes, H.B.; Glantz, A.; Kondrackiene, J.; Binder, T.; Nicastri, P.L.; Locatelli, A.; Floreani, A.; Hernandez, I.; et al. Efficacy of Ursodeoxycholic Acid in Treating Intrahepatic Cholestasis of Pregnancy: A Meta-Analysis. Gastroenterology 2012, 143, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.C.; Bell, J.L.; Smith, A.; Rounding, C.; Bowler, U.; Linsell, L.; Juszczak, E.; Tohill, S.; Redford, A.; Dixon, P.H.; et al. Ursodeoxycholic Acid to Reduce Adverse Perinatal Outcomes for Intrahepatic Cholestasis of Pregnancy: The PITCHES RCT. Efficacy and Mechanism Evaluation; NIHR Journals Library: Southampton, UK, 2020. [Google Scholar]
- Walker, K.F.; Chappell, L.C.; Hague, W.M.; Middleton, P.; Thornton, J.G. Pharmacological Interventions for Treating Intrahepatic Cholestasis of Pregnancy. Cochrane Database Syst. Rev. 2020, 7, CD000493. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians & Gynaecologists. Obstetric Cholestasis (Green-Top Guideline No. 43). Available online: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg43/ (accessed on 29 July 2021).
- Álvarez-Villaseñor, A.S.; Mascareño-Franco, H.L.; Agundez-Meza, J.J.; Cardoza-Macías, F.; Fuentes-Orozco, C.; Rendón-Félix, J.; Chávez-Tostado, M.; Irusteta-Jiménez, L.; García-Rentería, J.; Contreras-Hernández, G.I.; et al. Cholelithiasis during pregnancy and postpartum: Prevalence, presentation and consequences in a Referral Hospital in Baja California Sur. Gac. Med. De Mex. 2017, 153, 159–165. [Google Scholar]
- Ibiebele, I.; Schnitzler, M.; Nippita, T.; Ford, J.B. Outcomes of Gallstone Disease during Pregnancy: A Population-Based Data Linkage Study. Paediatr. Perinat. Epidemiol. 2017, 31, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Everson, G.T. Pregnancy and Gallstones. Hepatology 1993, 17, 159–161. [Google Scholar] [CrossRef]
- Maringhini, A.; Ciambra, M.; Baccelliere, P.; Raimondo, M.; Orlando, A.; Tinè, F.; Grasso, R.; Randazzo, M.A.; Barresi, L.; Gullo, D.; et al. Biliary Sludge and Gallstones in Pregnancy: Incidence, Risk Factors, and Natural History. Ann. Intern. Med. 1993, 119, 116–120. [Google Scholar] [CrossRef]
- Galyani Moghaddam, T.; Fakheri, H.; Abdi, R.; Khosh Bavar Rostami, F.; Bari, Z. The Incidence and Outcome of Pregnancy-Related Biliary Sludge/Stones and Potential Risk Factors. Arch. Iran. Med. 2013, 16, 12–16. [Google Scholar]
- Mendez-Sanchez, N.; Chavez-Tapia, N.C.; Uribe, M. Pregnancy and Gallbladder Disease. Ann. Hepatol. 2006, 5, 227–230. [Google Scholar] [CrossRef]
- Mathew, L.K.; Ko, C. Dietary Fat and Protein Intake Are Not Associated with Incident Biliary Sludge and Stones during Pregnancy. JPEN J. Parenter. Enter. Nutr. 2015, 39, 124–128. [Google Scholar] [CrossRef]
- Lindseth, G.; Bird-Baker, M.Y. Risk Factors for Cholelithiasis in Pregnancy. Res. Nurs. Health 2004, 27, 382–391. [Google Scholar] [CrossRef]
- Diehl, A.K.; Sugarek, N.J.; Todd, K.H. Clinical Evaluation for Gallstone Disease: Usefulness of Symptoms and Signs in Diagnosis. Am. J. Med. 1990, 89, 29–33. [Google Scholar] [CrossRef]
- Festi, D.; Sottili, S.; Colecchia, A.; Attili, A.; Mazzella, G.; Roda, E.; Romano, F. Clinical Manifestations of Gallstone Disease: Evidence from the Multicenter Italian Study on Cholelithiasis (MICOL). Hepatology 1999, 30, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D. ABC of the Upper Gastrointestinal Tract. Upper Abdominal Pain: Gall Bladder. BMJ 2001, 323, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Chen, T.-H.; Wang, S.-E.; Tsai, Y.-F.; Su, C.-H.; Wu, C.-W.; Lui, W.-Y.; Shyr, Y.-M. Biochemical Predictors for Absence of Common Bile Duct Stones in Patients Undergoing Laparoscopic Cholecystectomy. Surg. Endosc. 2008, 22, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Coakley, F.V.; Kaimal, A.; Laros, R.K. Guidelines for Computed Tomography and Magnetic Resonance Imaging Use during Pregnancy and Lactation. Obstet. Gynecol. 2008, 112 Pt 1, 333–340. [Google Scholar] [CrossRef]
- Gilo, N.B.; Amini, D.; Landy, H.J. Appendicitis and Cholecystitis in Pregnancy. Clin. Obstet. Gynecol. 2009, 52, 586–596. [Google Scholar] [CrossRef]
- Adelstein, S.J. Administered Radionuclides in Pregnancy. Teratology 1999, 59, 236–239. [Google Scholar] [CrossRef]
- Halkic, N.; Tempia-Caliera, A.A.; Ksontini, R.; Suter, M.; Delaloye, J.-F.; Vuilleumier, H. Laparoscopic Management of Appendicitis and Symptomatic Cholelithiasis during Pregnancy. Langenbeck’s Arch. Surg. 2006, 391, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Schwulst, S.J.; Son, M. Management of Gallstone Disease During Pregnancy. JAMA Surg. 2020, 155, 1162–1163. [Google Scholar] [CrossRef]
- Khan, F.; Rowe, I.; Martin, B.; Knox, E.; Johnston, T.; Elliot, C.; Lester, W.; Chen, F.; Olliff, S.; Mehrzad, H.; et al. Outcomes of Pregnancy in Patients with Known Budd-Chiari Syndrome. World J. Hepatol. 2017, 9, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; De Stefano, V.; Li, H.; Zheng, K.; Bai, Z.; Guo, X.; Qi, X. Epidemiology of Budd-Chiari Syndrome: A Systematic Review and Meta-Analysis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Aydinli, M.; Bayraktar, Y. Budd-Chiari Syndrome: Etiology, Pathogenesis and Diagnosis. World J. Gastroenterol. 2007, 13, 2693–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish Murad, S.; Plessier, A.; Hernandez-Guerra, M.; Fabris, F.; Eapen, C.E.; Bahr, M.J.; Trebicka, J.; Morard, I.; Lasser, L.; Heller, J.; et al. EN-Vie (European Network for Vascular Disorders of the Liver). Etiology, Management, and Outcome of the Budd-Chiari Syndrome. Ann. Intern. Med. 2009, 151, 167–175. [Google Scholar] [CrossRef]
- Rautou, P.-E.; Plessier, A.; Bernuau, J.; Denninger, M.-H.; Moucari, R.; Valla, D. Pregnancy: A Risk Factor for Budd-Chiari Syndrome? Gut 2009, 58, 606–608. [Google Scholar] [CrossRef]
- Ren, W.; Li, X.; Jia, J.; Xia, Y.; Hu, F.; Xu, Z. Prevalence of Budd-Chiari Syndrome during Pregnancy or Puerperium: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2015, 2015, 839875. [Google Scholar] [CrossRef] [PubMed]
- Ferral, H.; Behrens, G.; Lopera, J. Budd-Chiari Syndrome. Am. J. Roentgenol. 2012, 199, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Karaca, C.; Yilmaz, C.; Ferecov, R.; Iakobadze, Z.; Kilic, K.; Caglayan, L.; Aydogdu, S.; Kilic, M. Living-Donor Liver Transplantation for Budd-Chiari Syndrome: Case Series. Transplant. Proc. 2017, 49, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, J.; Durand, F.; De Raucourt, E.; Ceccaldi, P.-F.; Plessier, A.; Valla, D.; Rautou, P.-E. Pregnancy and Vascular Liver Disease. J. Clin. Exp. Hepatol. 2015, 5, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Surti, B.; Saab, S. Pregnancy and Cirrhosis. Liver Transplant. 2008, 14, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Afdhal, N.H. Liver Cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Shaheen, A.A.M.; Myers, R.P. The Outcomes of Pregnancy in Patients with Cirrhosis: A Population-Based Study. Liver Int. 2010, 30, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.A.; Mullin, M.; Lu, J.; Sarkar, M.A.; Djerboua, M.; Velez, M.P.; Brogly, S.; Terrault, N.A. Outcomes of Pregnant Women with Cirrhosis and Their Infants in a Population-Based Study. Gastroenterology 2020, 159, 1752–1762.e10. [Google Scholar] [CrossRef]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients with Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef]
- Palatnik, A.; Rinella, M.E. Medical and Obstetric Complications Among Pregnant Women with Liver Cirrhosis. Obstet. Gynecol. 2017, 129, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Höijer, J.; Marschall, H.-U.; Williamson, C.; Heneghan, M.A.; Westbrook, R.H.; Ludvigsson, J.F.; Stephansson, O. Outcomes of Pregnancy in Mothers with Cirrhosis: A National Population-Based Cohort Study of 1.3 Million Pregnancies. Hepatol. Commun. 2018, 2, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Lelei-Mailu, F.J.; Mariara, C.M. Pregnancy in a Patient with Portal Hypertension Secondary to Liver Cirrhosis. Case Rep. 2018, 2018, bcr-2017-223076. [Google Scholar] [CrossRef]
- Murthy, S.K.; Heathcote, E.J.; Nguyen, G.C. Impact of Cirrhosis and Liver Transplant on Maternal Health during Labor and Delivery. Clin. Gastroenterol. Hepatol. 2009, 7, 1367–1372. [Google Scholar] [CrossRef] [PubMed]



| Pregnancy-Related Liver Disease | Pregnancy Unrelated Liver Disease | |
|---|---|---|
| De Novo | Pre-Existing | |
| Pre-eclamptic liver dysfunction | Hepatitis | Acute viral hepatitis |
| Intrahepatic cholestasis of pregnancy | Cirrhosis | Cholelithiasis |
| Hyperemesis gravidarum | Autoimmune liver disease | Budd–Chiari syndrome |
| HELLP syndrome | Wilson’s disease | Metabolic disease |
| Acute fatty liver of pregnancy | Post liver transplantation | Liver tumors |
| Non-alcoholic fatty liver disease | Drug-induced hepatotoxicity | |
| Authors, Publ. Year | Study Design | Cases | Maternal Age | Gestational Weeks | Platelets | Proteinuria | ALT | AST | Biomarker | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Simsek, 2013 [15] | CCS | 50 20 (H) 30 (C) | 28.6 ± 6.8 (H) 28.3 ± 4.8 (C) | 33.4 ± 4.7 (H) 37.9 ± 1.2 (C) | 91 ± 24.2 (H) 217 ± 68 (C) | NR | 248.3 ± 296.8 (H) 12.5 ± 4.5 (C) | 352.5 ± 514 (H) 17.7 ± 4.3 (C) | Levels of p65/RelA expression of nuclear transcription factor-kappa beta (NF-kB) in paraffin-embedded placental tissue samples | p65/RelA immunoexpression and serum MPO and CRP levels were significantly higher in patients with HELLP; over-expression of placental NF-kB is correlated with elevation of serum inflammatory markers and placental ultrastructural changes |
| Schnabel, 2016 [16] | CCS | 107; 21 (H) 86 (C) | 34.3 ± 4.6 (*) 32.7 ± 4.0 (**) 32.6 ± 5.2 (C) | 34–41 (*); 26–33 (**) | 130,14 ± 65,08 (*); 109,85 ± 51,67 (**); 196.68 ± 61.60 (C) | 4.23 ± 5.18 (*); 4.24 ± 5.03(**); 0.36 ± 0.47 (C) | 140.0 ± 200.7 (*); 306.3 ± 213.7 (**); 45.03 ± 117.1 (C) | 176.2 ± 308.9 (*); 269.0 ± 268.2 (**); 68.56 ± 159.52 (C) | Galectin-1 (gal-1) | Increased circulating levels of gal-1 are found in HELLP syndrome |
| Authors, Publ. Year | Study Design | Cases | Maternal Age | Gestational Weeks | Total Bile Acids | Total Bilirubin | ALT | AST | Biomarker | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhou, 2014 [17] | CCS | 30; 16 * 14 ** | NR | 34 weeks–34 weeks and 6 days; | NR | NR | NR | NR | CRH expression in patients with ICP after UDCA | Maternal serum and placental CRH expression in ICP patients were up-regulated after treatment of UDCA |
| Ozler, 2014 [18] | CCS | 60; 30 * 30 ** | 29.7 ± 5.9* 29.9 ± 5.0 ** | 32.8 ± 3.1 * 31.6 ± 4.5 ** | NR | NR | 242.9 ± 237.8 * 14.9 ± 6.1 ** | 161.6 ± 140.9 * 12.8 ± 4.1 ** | IL-6, TNF-α and neopterin | There was no difference between the groups in IL-6 and TNF-α levels, but the mean neopterin level was significantly higher in group * |
| Oztas, 2015 [19] | CCS | 217; 117 *; 100 ** | 28 *; 27 ** | 34.7 ± 2.5 * 34.8 ± 2.5 ** | NR | 0.7 * 0.47 ** | 88 * 11.7 ** | 64 * 16.8 ** | Mean platelet volume, total bilirubin levels, increased postprandial total SBA levels | Increased MPV and total bilirubin levels are associated with preterm delivery, and increased postprandial total SBA levels are predictive for low APGAR in ICP patients |
| Ma, 2016 [20] | CCS | 90; 40 * 50 ** | 28.8 ± 4.1 * 28.9 ± 3.4 ** | 34.0 ± 2.1 * 32.3 ± 2.5 ** | 26.7 ± 21.1 * 2.6 ± 1.3 ** | 10.4 ± 5.6 * 7.6 ± 2.6 ** | 88.7 ± 99.7 * 15.9 ± 17.7 ** | 53.1 ± 49.2 * 18.7 ± 9.6 ** | Urinary miRNAs as non-invasive biomarkers for ICP | Urinary microRNA profiling detection is feasible and has the potential to be noninvasive biomarkers for the diagnosis of ICP |
| Sanhal, 2018 [21] | CCS | 107; 57 * 50 ** | 27.9 ± 5.3 * 27.3 ± 5.6 ** | 35 * 35 ** | NR | 0.5 * 0.37 ** | 92 * 9 ** | 66 * 16 ** | Thiol/disulfide to evaluate oxidative stress (OS). | Pregnant women with ICP had significantly lower serum levels of native total thiol and higher levels of disulfide; thiol/disulfide balance indicate OS in the pregnant woman with ICP; favorable diagnostic abilities of native thiol and total thiol in ICP |
| Zou, 2021 [22] | CCS | 108 | 28.9 ± 6.32 *; 26.1 ± 4.87 ** | 37.9 ± 0.9 * 38.9 ± 0.9 ** | 68.9 ± 50.3 * 5.3 ± 3.1 ** | NR | 108.6 ± 101.4 * 16.1 ± 7.6 ** | 106.7 ± 96.1 * 16.9 ± 8.3 ** | Long noncoding RNAs (lncRNAs) | The three lncRNAs serum level are potential biomarkers of ICP. Combining with TBA, alanine aminotransferase, and glycocholic acid, may improve the diagnosis of ICP. |
| Authors, Publ. Year | Study Design | Pathology | Cases | Maternal Age | Gestational Weeks | Total Bile Acids | Direct Bilirubin | ALT (Mean ± SD) | AST (Mean ± SD) | Treatment | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Katz, 2013 [23] | Triple blind RCT | HELLP class I syndrome | 400 | NI | NI | NI | NI | NI | NI | Eligible patients receive dexamethasone every 12 h for two days | Corticosteroids increase platelet counts significantly, with no clear evidence of the effect on clinical outcomes |
| Shahzad, 2017 [24] | RCT | HELLP syndrome | 100 | 30.5 ± 5.8 | 39.6 ± 1.1 | NI | NI | NI | NI | Group A: 10 mg dexamethasone sodium phosphate IV every 12 h; Group B: 12 mg combination of betamethasone acetate and betamethasone sodium phosphate IM every 24 h | Decrease in mean arterial pressure with dexamethasone was significantly higher than that of betamethasone for management of females presenting with postpartum HELLP syndrome |
| Fonseca, 2019 [25] | RCT | HELLP class I syndrome | 87 | 25.7 ± 7.5 | 33.8 ± 4.8 | NR | NR | 188.4 | 337.4 | Pregnant women: 10 mg doses of dexamethasone sodium phosphate, IV, every 12 h until delivery; and 3 additional doses after delivery. Postpartum women: three 10-mg doses after delivery. | Failed to demonstrate the benefit of using dexamethasone in patients with class I HELLP syndrome |
| Takahashi, 2019 [26] | Retro- spective study | HELLP syndrome | 18 7 * 11 ** | 30.2 ± 4.3 * 30.3± 5.3 ** | 36.5 ± 5.3 * 37.1± 4.5 ** | NR | NR | NR | 177 ± 128 * 399± 228 ** | Group *: without dexamethasone Group **: with IV dexamethasone | AST levels were significantly higher in group *. No maternal postpartum complications between the groups. |
| Marciniak, 2011 [27] | CCS | ICP | 43 | NI | NI | NI | NI | NI | NI | Group 1: PPC Group 2: UDCA Group 3: a combination of these two drugs | Combined therapy with UDCA and PPC could be considered in ICP, especially in case of early-onset and/or severe course |
| Chappell, 2012 [28] | Semi-factorial RCT | ICP | 111 | 29.8 ± 5.7 | 34.2 ± 3 | 25.95 | 9 | 94 | 59.7 | UDCA (250 mg dose) or placebo capsules, two capsules twice a day, and if there was no improvement, the dose was increased in increments of two capsules per day every 3–14 days up to a maximum of 2 g/day | UDCA significantly reduces pruritus, but the size of the benefit may be too small for most doctors to recommend it, or for most women to want to take it |
| Joutsiniemi, 2013 [29] | Double blind RCT | ICP | 20 | NI | 32.6 | NI | NI | NI | NI | Random administration of 450 mg/day UDCA or placebo for a period of 14 days during the third trimester of pregnancy | UDCA significantly improves maternal pruritus, improves liver function tests and has no adverse effects on newborns |
| Jain, 2013 [30] | Prospective randomized study | ICP | 69 | 27.5 ± 4.3 | 35 | NI | NI | 165.6 ± 116.4 | 145.9 ± 102.6 | Group I was planned for delivery at 37 weeks. In Group II, pregnancy was carried to 38 weeks under surveillance. Fetal surveillance start at >34 weeks at diagnosis and included daily maternal records of fetal movements, biophysical profiles. Fetal monitoring was conducted weekly before 36 weeks and biweekly after that | With active intervention, pregnancies with obstetric cholestasis can be carried to a later gestation under surveillance |
| Zhang, 2015 [31] | RCT | ICP | 120 | 28.2 ± 3.9 | 31.1 ± 3.3 | 44.2 ± 40.3 | 22.4 ± 18.7 | 259.3 ± 173.7 | 187.5 ± 124.7 | Group 1: oral UDCA 4×250 mg daily until delivery. Group 2: IV SAMe 1000 mg daily until delivery. Group 3:a combination of these two drugs in the same dosage until delivery | UDCA and SAMe are safe and effective in ICP treatment. UDCA monotherapy should be used as the first-line therapy because it is more efficacious, cost-effective and convenient |
| Grymowicz, 2016 [32] | CCS | ICP | 303; 203 *: I = 46 (TBA < 10 mmol/l); II = 157 (TBA > 10 mmol/l) 100 ** | NR | 34.4 ± 3.4 | 9.5 (I) 21.8 (II) | 14.2 (I) 14.8 (II) | 158.96 (I) 214.36 (II) | 105.62 (I) 138.34 (II) | Only group A: UDCA (300–450 mg/day; 4–6 mg/kg/day) until delivery | Low doses of UDCA improved clinical symptoms and biochemical markers in almost 90% of patients |
| Parízek, 2016 [33] | Retro- spective multicentric study | ICP | 191 | 31.9 ± 4.6 | 37.4 | 20.5 | NR | 237 ± 204 | 145 ± 120 | UDCA was used in the range of 500–1500 mg/day, 750 mg/day in most cases (10 mg/kg/day). The average duration of therapy was 17 days | UDCA ameliorated liver dysfunction in the majority of the affected women (86.1%) |
| Chappell, 2019 [34] | RCT | ICP | 604 | 30.6 ± 5.4 | 34.4 | 27.5 | 8.23 | 64.75 | 55.42 | UDCA or placebo, given as two oral tablets a day at an equivalent dose of 500 mg twice a day (maximum of 4 tablets and a minimum of one tablet a day) from enrolment until the infant’s birth | Treatment with UDCA does not reduce adverse perinatal outcomes in women with ICP |
| Agarwal, 2021 [35] | Prospectivestudy | ICP | 121 71 A 50 B | 27.7 ± 3.8 A 27.2 ± 3.5 B | 30.4 ± 4.7 A 31.2 ± 3.3 B | 75.9 ± 39.5 29.2 ± 5.7 | 173.3 ± 139 28.9 ± 8.2 | NR | 173.3 ± 139 28.9 ± 8.2 | Group A-oral UDCA 300 mg thrice daily | In Asian/Indian patients, biliary acids (BA) levels are higher compared to the general population. In this case, it’s necessary to establish a higher BA cut-off of 30 μmol/L for diagnosing ICP. |
| Chu, 2012 [36] | CCS | AFLP | 11 | 26 ± 4.2 | 33 | - | - | - | - | Plasma exchange and continuous hemodiafiltration were used in ten patients who were cured and discharged from the hospital. The hospitalization average duration was 17 days | This is an effective treatment for patients with AFLP suffering multiple organ dysfunction. |
| Features | Hyperemesis Gravidarum | HELLP Syndrome | Acute Fatty Liver (AFLP) | Intrahepatic Cholestasis (ICP) | Cholelithiasis | Budd–Chiari Syndrome (BCS) | Cirrhosis |
|---|---|---|---|---|---|---|---|
| Epidemiology | 0.3–2% | 0.1%–0.6% | 0.01–0.02% | 0.2–2% with seasonal pattern | 5–12% no symptom gallstones Gallbladder disease 0.05–0.3% | 0–21.5% | 0.045% |
| Moment of appearance | First trimester | Late second trimester (25–38 weeks) to early postpartum | Third trimester (32–38 weeks) –postpartum | Second or third trimester (21–38 weeks) | - | - | - |
| Clinical findings | Intense nausea, vomiting, nutritional deficiency, weight loss | Abdominal pain, nausea/vomiting, overlap with preeclampsia, hypertension, and proteinuria | Abdominal pain, nausea/vomiting, malaise, anorexia, jaundice, hypoglycemia, signs of liver failure, ascites | Pruritus, dark urine, jaundice, loss of appetite, fatigue, nausea, steatorrhea, abdominal pain | Stabbing pain or colicky in the right upper quadrant and/or epigastric area, anorexia, nausea, vomiting, dyspepsia, low-grade fever, tachycardia, and fatty food intolerance | Abdominal pain, hepatomegaly, ascites, variceal bleeding | Variceal bleeding (20%–25%), especially during the second trimester or during labor; jaundice, pruritus, nausea, vomiting |
| Imagistic findings | No biliary obstruction | Hepatic hematomas, infarcts, possible rupture | Bright liver secondary liver fatty infiltration | Exclusion diagnosis with cholelithiasis | Ultrasound 95% effective | MRI and ultrasound are most effective | Endoscopy: esophageal and gastric varices |
| Histologic findings | - | Variable periportal necrosis | Microvesicular fatty infiltration | Dilated bile canaliculi | Biliary sludge-plate-like cholesterol crystals and calcium bilirubinate granules embedded in strands of mucin gel | Zone 3 hemorrhage into liver cell plates and ischemic necrosis leading to veno-centric cirrhosis | Diffuse disruption in the architecture of the entire liver (loss of the normal central–portal relationship) |
| Laboratory findings | ALT 1–2 x (50%) | ALT up to 2–30 fold Total bilirubin up to 1.5–10 fold s LDH >600 IU/mL | ALT up to 3–15 fold bilirubin up to 3–15 fold, uric acid (>340 μmol/L), glucose; antithrombin III creatinine > 150 μmol/L; DIC; s | ALT up to 2–10 fold total bile acid up 10–100 fold; GGT up to 0–4 fold; Alkaline phosphatase up to 7–10 fold | bilirubin ALT AST | Hypercoagulability blood volume expansion and hypoproteinemia | AST and ALT usually moderately elevated Other parameters depend on etiology |
| Therapeutic management | Rehydratation, antiemetic drugs, vitamins (C, B1, B6, B12) | Rapid delivery (34 weeks or before 24–34 weeks with corticoids for fetal lung maturation | Rapid delivery, plasmapheresis, liver transplantation | UDCA (10–20 mg/kg/day) | Discontinuation of oral intake, IV fluid replacement, analgesia, and administration of antibiotics when signs of infection are present laparoscopic cholecystectomy (I/II trimester) or ERCP | Prophylaxis of variceal hemorrhage; large or ‘at-risk’ varices should be eradicated with endoscopic band ligation. Preferable mode of delivery: assisted vaginal delivery with adequate analgesia:; Caesarean section reserved for obstetric indications | Active management of varices. Preferable vaginal deliveries. Cesarean in case of large varices. Correction of coagulopathy and prophylactic antibiotics to reduce postpartum hemorrhage and bacterial infections. |
| Prognosis | Remission in the first part of the second trimester (18 wks) | Usually resolves by the first part of the second trimester | Small risk of recurrence; maternal mortality has decreased to <10% in most recent series | Good after delivery | Good | Favorable in patients with treated and stabilized BCS | Increased maternal and fetal problems |
| Fetal outcome | Not associated with adverse pregnancy outcomes | Fetal bradycardia, fetal loss, fetal distress, Premature birth | Premature birth, newborn asphyxia, mortality rate: 23% | Preterm birth, sudden intra-uterine death, meconium-stained amniotic fluid, NICU admission | Risk of preterm birth and neonatal morbidity | Fetal outcomes beyond 20 weeks gestation are good | Related to the severity of the maternal liver disease (MELD score: the risk of decompensating of the maternal liver) |
| HELLP Class | Mississippi Classification | ||
|---|---|---|---|
| Platelet Count | AST/ALT | LDH | |
| 1–mild | 100,000–150,000/L | >40 IU/L | >600 IU/L |
| 2–moderate | 50,000–100,000/L | >70 IU/L | |
| 3-severe | <50,000/L | >70 IU/L | |
| HELLP class | Tennessee classification | ||
| Severe preeclampsia | Complete (3 criteria) | Platelet count <100 × 109/L | |
| AST >70 IU/L and LDH >600 IU/L | |||
| Bilirubin ≥1.2 mg/dL | |||
| Incomplete/partial | One or two 2 criteria | ||
| Clinical Findings | Laboratory Findings |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Clinical Features | Laboratory Features | Imaging Features |
|---|---|---|
|
|
|
| Clinical Features | Laboratory Features | Imaging Features |
|---|---|---|
|
|
|
| Clinical Features | Laboratory Features | Imaging Features |
|---|---|---|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlas, V.N.; Bohîlțea, R.; Gheorghe, G.; Bostan, G.; Angelescu, G.A.; Penes, O.N.; Bors, R.G.; Cloțea, E.; Bacalbasa, N.; Diaconu, C.C. State of the Art in Hepatic Dysfunction in Pregnancy. Healthcare 2021, 9, 1481. https://doi.org/10.3390/healthcare9111481
Varlas VN, Bohîlțea R, Gheorghe G, Bostan G, Angelescu GA, Penes ON, Bors RG, Cloțea E, Bacalbasa N, Diaconu CC. State of the Art in Hepatic Dysfunction in Pregnancy. Healthcare. 2021; 9(11):1481. https://doi.org/10.3390/healthcare9111481
Chicago/Turabian StyleVarlas, Valentin Nicolae, Roxana Bohîlțea, Gina Gheorghe, Georgiana Bostan, Gabriela Anca Angelescu, Ovidiu Nicolae Penes, Roxana Georgiana Bors, Eliza Cloțea, Nicolae Bacalbasa, and Camelia Cristina Diaconu. 2021. "State of the Art in Hepatic Dysfunction in Pregnancy" Healthcare 9, no. 11: 1481. https://doi.org/10.3390/healthcare9111481
APA StyleVarlas, V. N., Bohîlțea, R., Gheorghe, G., Bostan, G., Angelescu, G. A., Penes, O. N., Bors, R. G., Cloțea, E., Bacalbasa, N., & Diaconu, C. C. (2021). State of the Art in Hepatic Dysfunction in Pregnancy. Healthcare, 9(11), 1481. https://doi.org/10.3390/healthcare9111481










