Acute Effects of Dermal Suction on Passive Muscle and Joint Stiffness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
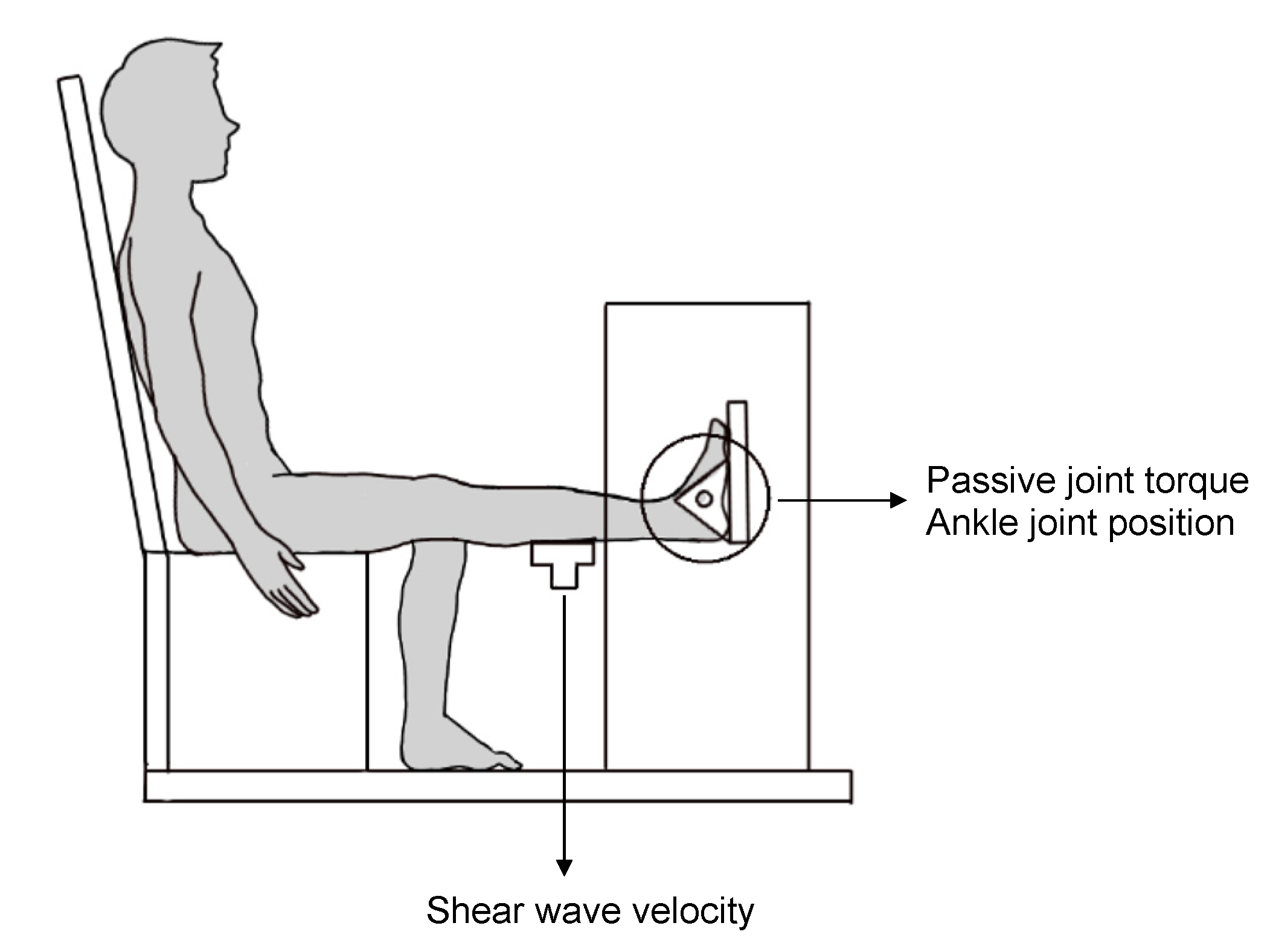
2.2. Procedure
2.3. Passive Ankle Joint Stiffness
2.4. Passive Muscle Stiffness
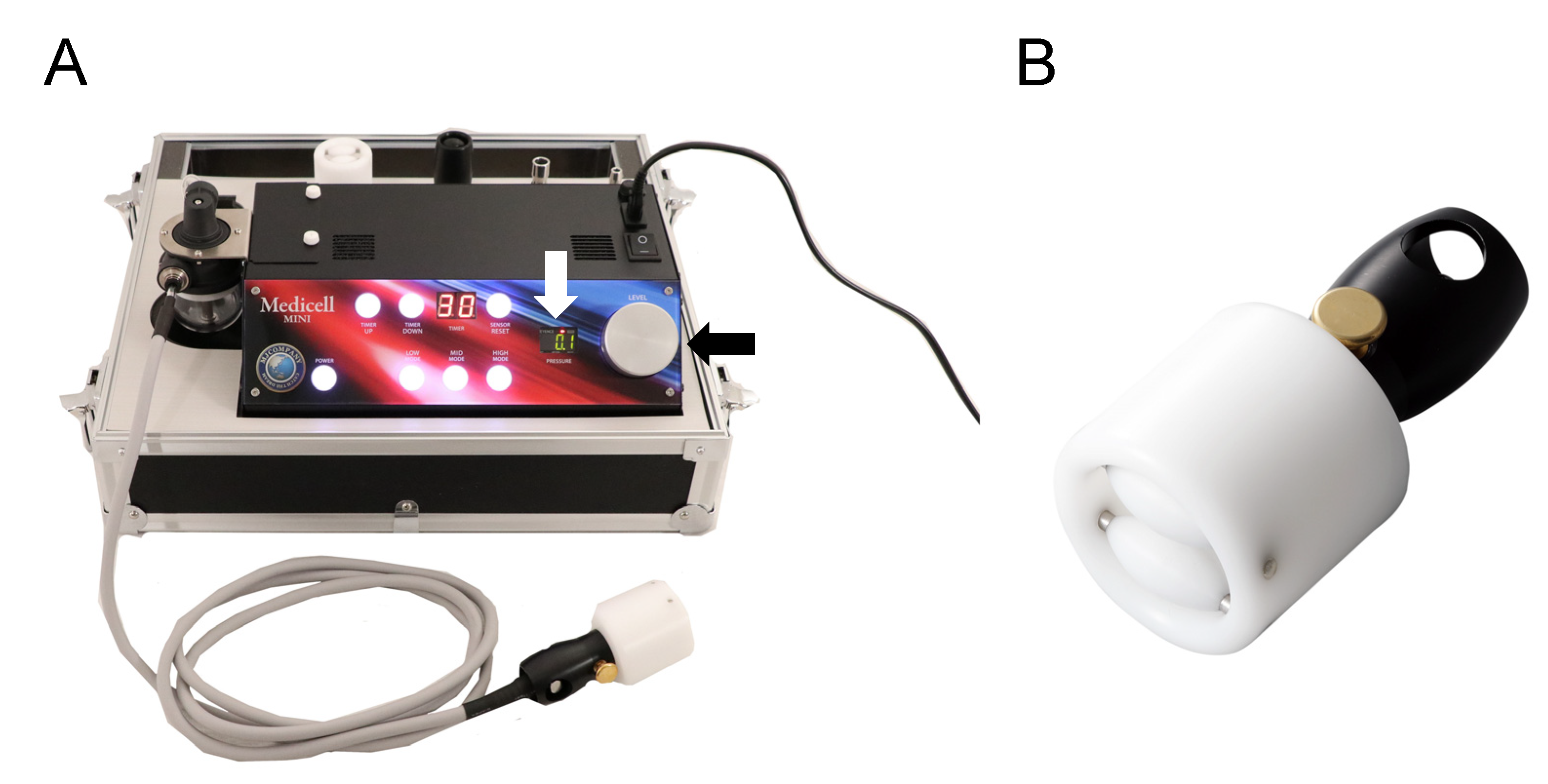
2.5. Dermal Suction
2.6. Statistics
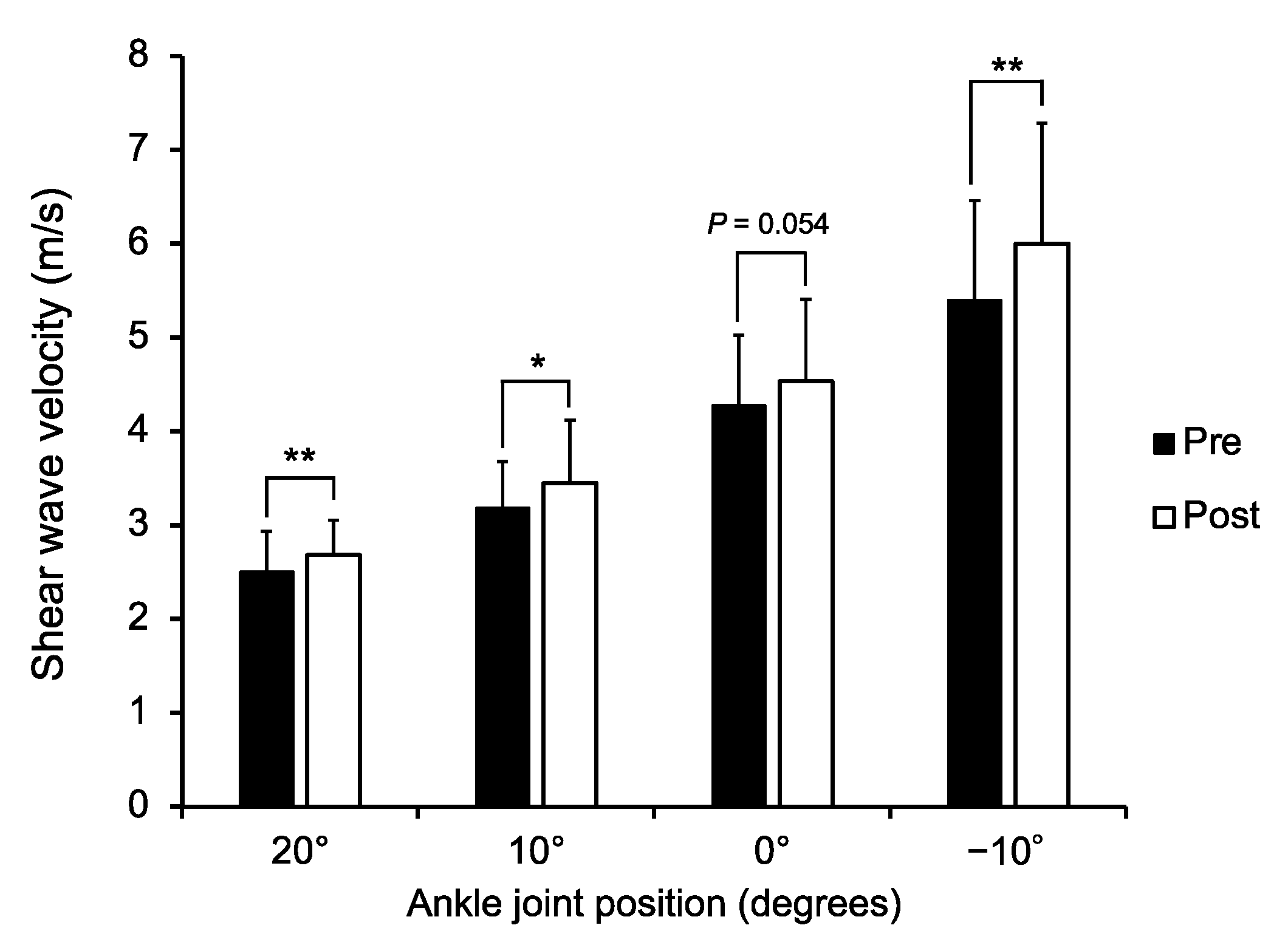
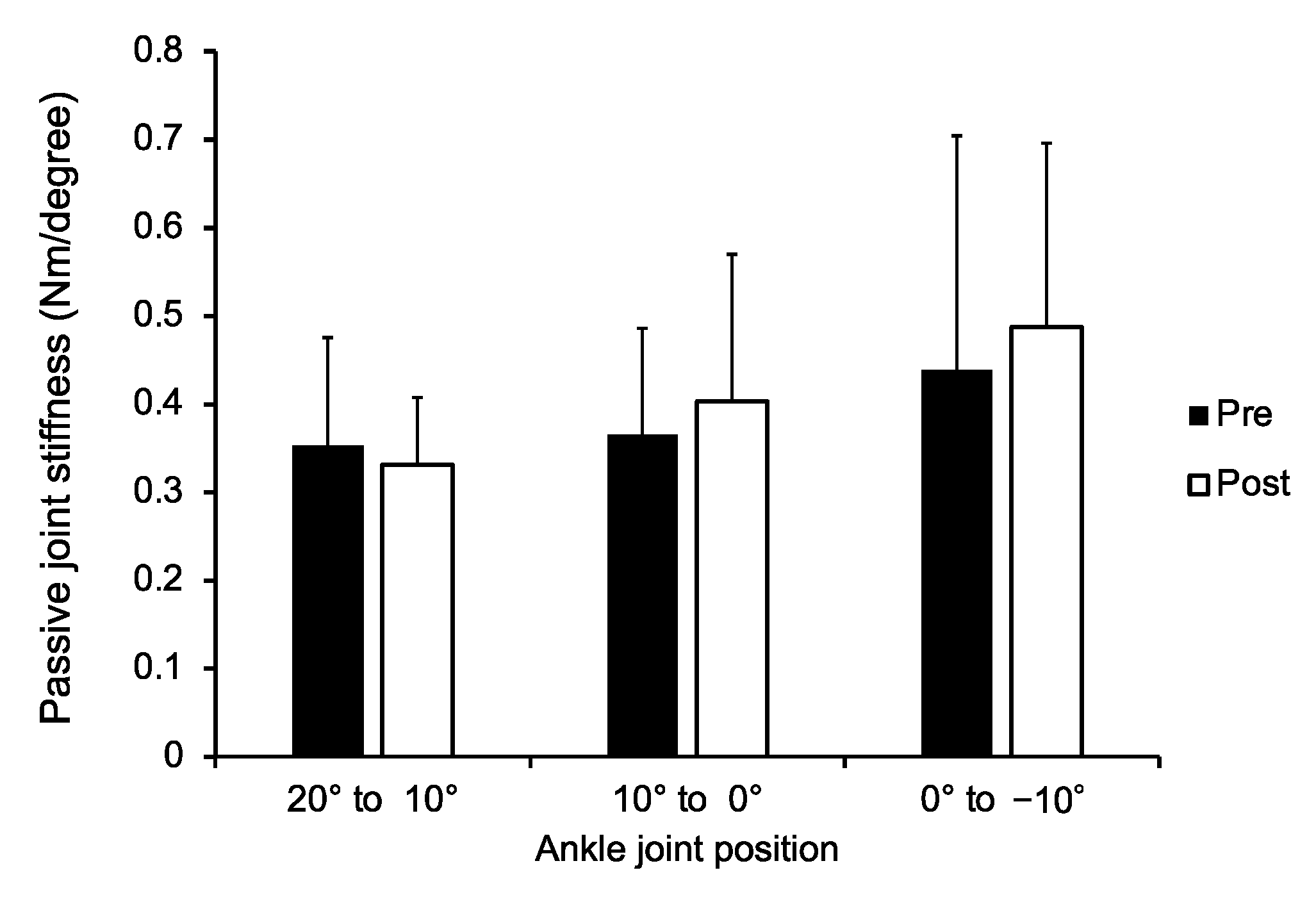
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alter, M.J. Science of Flexibility, 3rd ed.; Human Kinetics Publishers: Champaign, IL, USA, 2004. [Google Scholar]
- Tham, L.M.; Lee, H.P.; Lu, C. Cupping: From a biomechanical perspective. J. Biomech. 2006, 39, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, X.; Liu, J. An updated review of the efficacy of cupping therapy. PLoS ONE 2012, 7, e31793. [Google Scholar] [CrossRef]
- Cao, H.; Han, M.; Li, X.; Dong, S.; Shang, Y.; Wang, Q.; Xu, S.; Liu, J. Clinical research evidence of cupping therapy in China: A systematic literature review. BMC Complement. Altern. Med. 2010, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-E.; Cho, J.-E.; Do, K.-S.; Lim, S.-Y.; Kim, H.-J.; Yim, J.-E. Effect of Cupping Therapy on Range of Motion, Pain Threshold, and Muscle Activity of the Hamstring Muscle Compared to Passive Stretching. J. Korean Soc. Phys. Med. 2017, 12, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Markowski, A.; Sanford, S.; Pikowski, J.; Fauvell, D.; Cimino, D.; Caplan, S. A pilot study analyzing the effects of chinese cupping as an adjunct treatment for patients with subacute low back pain on relieving pain, improving range of motion, and improving function. J. Altern. Complement. Med. 2014, 20, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Younis, A.; Wali, M. An investigation into the effect of Cupping Therapy as a treatment for Anterior Knee Pain and its potential role in Health Promotion, Internet. J. Altern. Med. 2006, 4, 1–9. [Google Scholar]
- Yim, J.; Park, J.; Kim, H.; Woo, J.; Joo, S.; Lee, S.; Song, J. Comparison of the effects of muscle stretching exercises and cupping therapy on pain thresholds, cervical range of motion and angle: A cross-over study. Phys. Ther. Rehabil. Sci. 2017, 6, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Schafer, M.D.; Tom, J.C.; Girouard, T.J.; Navalta, J.W.; Turner, C.L.; Radzak, K.N. Cupping therapy does not influence healthy adult’s hamstring range of motion compared to control or sham conditions. Int. J. Exerc. Sci. 2020, 13, 216–224. [Google Scholar] [PubMed]
- Johns, R.J.; Wright, V. Relative importance of various tissues in joint stiffness. J. Appl. Physiol. 1962, 17, 824–828. [Google Scholar] [CrossRef]
- Inami, T.; Kawakami, Y. Assessment of individual muscle hardness and stiffness using ultrasound elastography. J. Phys. Fit. Sports Med. 2016, 5, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Leonard, C.T.; Brown, J.S.; Price, T.R.; Queen, S.A.; Mikhailenok, E.L. Comparison of surface electromyography and myotonometric measurements during voluntary isometric contractions. J. Electromyogr. Kinesiol. 2004, 14, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Murayama, M.; Nosaka, K.; Yoneda, T.; Minamitani, K. Changes in hardness of the human elbow flexor muscles after eccentric exercise. Eur. J. Appl. Physiol. 2000, 82, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Prado-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, A.S. Ultrasound elastography: Compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef]
- Jan, Y.-K.; Hou, X.; He, X.; Guo, C.; Jain, S.; Bleakney, A. Using elastographic ultrasound to assess the effect of cupping size of cupping therapy on stiffness of triceps muscle. Am. J. Phys. Med. Rehabil. 2021, 100, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, S.; Oda, T.; Kaga, M. Relationship between the morphological and mechanical properties of muscle-tendon units and sprint performance in prepubescent sprinters. Transl. Sports Med. 2019, 2, 263–269. [Google Scholar] [CrossRef]
- Muraoka, T.; Muramatsu, T.; Fukunaga, T.; Kanehisa, H. Elastic properties of human Achilles tendon are correlated to muscle strength. J. Appl. Physiol. 2005, 99, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Kato, E.; Oda, T.; Chino, K.; Kurihara, T. Musculotendinous Factors Influencing Difference in Ankle Joint Flexibility between Women and Men. Int. J. Sport Health. Sci. 2005, 3, 218–225. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Takai, Y.; Kanehisa, H.; Shinohara, M. Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve 2014, 50, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.N. Validation of shear wave elastography in skeletal muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef] [Green Version]
- Lima, K.M.M.E.; Costa Júnior, J.F.S.; de Pereira, W.C.A.; de Oliveira, L.F. Assessment of the mechanical properties of the muscle-tendon unit by supersonic shear wave imaging elastography: A review. Ultrasonography 2018, 37, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.A.; Jameel, S.S.; Jafri, K.; Khan, S.A.; Ahmad, E. Ilaj bil hijamah (Cupping therapy) in unani system of medicine: Anecdotal practice to evidence based therapy. AMHA-Acta Medico-Historica Adriat. 2016, 14, 81–94. [Google Scholar]
- Starbuck, C.; Bramah, C.; Herrington, L.; Jones, R. The effect of speed on Achilles tendon forces and patellofemoral joint stresses in high-performing endurance runners. Scand. J. Med. Sci. Sport 2021, 31, 1657–1665. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications Ltd.: London, UK, 2009. [Google Scholar]
- Enomoto, S.; Tsushima, A.; Oda, T.; Kaga, M. The characteristics of the muscle-tendon unit in children affected by Osgood-Schlatter disease. Transl. Sports Med. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Miyamoto, N.; Taniguchi, K.; Katayose, M.; Kanehisa, H. The Skin Acts to Maintain Muscle Shear Modulus. Ultrasound Med. Biol. 2016, 42, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, E.; Jung, G.; Lee, S.; Kim, J.G. The hemodynamic changes during cupping therapy monitored by using an optical sensor embedded cup. J. Biophotonics 2019, 12, 6–12. [Google Scholar] [CrossRef]
- Ema, R.; Saito, I.; Akagi, R. Neuromuscular adaptations induced by adjacent joint training. Scand. J. Med. Sci. Sport 2018, 28, 947–960. [Google Scholar] [CrossRef] [Green Version]
- Ema, R.; Wakahara, T.; Miyamoto, N.; Kanehisa, H.; Kawakami, Y. Inhomogeneous architectural changes of the quadriceps femoris induced by resistance training. Eur. J. Appl. Physiol. 2013, 113, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Roostayi, M.M.; Norouzali, T.; Manshadi, F.D.; Abbasi, M.; Baghban, A.A. The Effects of Cupping Therapy on Skin’s Biomechanical Properties in Wistar Rats. Chin. Med. 2016, 07, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, N.; Hirata, K.; Inoue, K.; Hashimoto, T. Muscle Stiffness of the Vastus Lateralis in Sprinters and Long-Distance Runners. Med. Sci. Sports Exerc. 2019, 51, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Suzuki, Y. Positive relationship between passive muscle stiffness and rapid force production. Hum. Mov. Sci. 2019, 66, 285–291. [Google Scholar] [CrossRef]
- Ichihashi, N.; Ibuki, S.; Nakamura, M. Effects of static stretching on passive properties of muscle-tendon unit. J. Phys. Fit. Sports Med. 2014, 3, 1–10. [Google Scholar] [CrossRef]
- Toft, E.; Espersen, G.T.; Kålund, S.; Sinkjær, T.; Hornemann, B.C. Passive tension of the ankle before and after stretching. Am. J. Sports Med. 1989, 17, 489–494. [Google Scholar] [CrossRef] [PubMed]

| Sex | Mean | SD | 95% CI lb | 95% CI ub | |
|---|---|---|---|---|---|
| Age (years) | Men | 23.2 | 3.6 | 20.9 | 25.4 |
| Women | 22.8 | 9.3 | 16.9 | 28.7 | |
| Height (cm) | Men | 172.1 | 4.2 | 169.4 | 174.8 |
| Women | 159.0 | 4.9 | 155.9 | 162.1 | |
| Body mass (kg) | Men | 70.1 | 8.7 | 64.6 | 75.6 |
| Women | 54.4 | 5.2 | 51.0 | 57.7 | |
| BMI (kg/m2) | Men | 23.7 | 3.4 | 21.5 | 25.9 |
| Women | 21.5 | 1.9 | 20.3 | 22.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enomoto, S.; Shibutani, T.; Akihara, Y.; Nakatani, M.; Yamada, K.; Oda, T. Acute Effects of Dermal Suction on Passive Muscle and Joint Stiffness. Healthcare 2021, 9, 1483. https://doi.org/10.3390/healthcare9111483
Enomoto S, Shibutani T, Akihara Y, Nakatani M, Yamada K, Oda T. Acute Effects of Dermal Suction on Passive Muscle and Joint Stiffness. Healthcare. 2021; 9(11):1483. https://doi.org/10.3390/healthcare9111483
Chicago/Turabian StyleEnomoto, Shota, Tomonari Shibutani, Yu Akihara, Miyuki Nakatani, Kazunori Yamada, and Toshiaki Oda. 2021. "Acute Effects of Dermal Suction on Passive Muscle and Joint Stiffness" Healthcare 9, no. 11: 1483. https://doi.org/10.3390/healthcare9111483
APA StyleEnomoto, S., Shibutani, T., Akihara, Y., Nakatani, M., Yamada, K., & Oda, T. (2021). Acute Effects of Dermal Suction on Passive Muscle and Joint Stiffness. Healthcare, 9(11), 1483. https://doi.org/10.3390/healthcare9111483






