Hazardous Effect of Low-Dose Aspirin in Patients with Predialysis Advanced Chronic Kidney Disease Assessed by Machine Learning Method Feature Selection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Research Samples
2.2. Study Population and Exclusion Criteria
2.3. Clinical Outcomes
2.4. Feature Selection
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Nephrotoxcitiy of Aspirin in Patients with Predialysis Advanced CKD
3.3. Aspirin Effect on Other Clinical Outcomes in Patients with Predialysis Advanced CKD
3.4. Subgroup Analysis
3.5. Feature Selection of Important Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, M.H.; Hsu, C.Y.; Lin, M.Y.; Yen, M.F.; Chen, H.H.; Chiu, Y.H.; Hwang, S.J. Incidence, Prevalence, and Duration of Chronic Kidney Disease in Taiwan: Results from a Community-Based Screening Program of 106,094 Individuals. Nephron 2018, 140, 175–184. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Provenzano, M.; Coppolino, G.; Faga, T.; Garofalo, C.; Serra, R.; Andreucci, M. Epidemiology of cardiovascular risk in chronic kidney disease patients: The real silent killer. Rev. Cardiovasc. Med. 2019, 20, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Major, R.W.; Cheng, M.R.I.; Grant, R.A.; Shantikumar, S.; Xu, G.; Oozeerally, I.; Brunskill, N.J.; Gray, L.J. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192895. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Hansson, L.; Zanchetti, A.; Carruthers, S.G.; Dahlof, B.; Elmfeldt, D.; Julius, S.; Menard, J.; Rahn, K.H.; Wedel, H.; Westerling, S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998, 351, 1755–1762. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Salvetti, A.; Jamerson, K.; Hansson, L.; Warnold, I.; Wedel, H.; Zanchetti, A. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J. Am. Soc. Nephrol. 2001, 12, 218–225. [Google Scholar] [CrossRef]
- Jardine, M.J.; Ninomiya, T.; Perkovic, V.; Cass, A.; Turnbull, F.; Gallagher, M.P.; Zoungas, S.; Lambers Heerspink, H.J.; Chalmers, J.; Zanchetti, A. Aspirin is beneficial in hypertensive patients with chronic kidney disease: A post-hoc subgroup analysis of a randomized controlled trial. J. Am. Coll. Cardiol. 2010, 56, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Yan, B.; Wang, L.; Lv, J.; Cheng, H.; Chen, Y. Effect of antiplatelet therapy on cardiovascular and kidney outcomes in patients with chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2019, 20, 309. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Perazella, M.A. NSAIDs in CKD: Are They Safe? Am. J. Kidney Dis. 2020, 76, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [Green Version]
- Baigent, C.; Landray, M.; Leaper, C.; Altmann, P.; Armitage, J.; Baxter, A.; Cairns, H.S.; Collins, R.; Foley, R.N.; Frighi, V.; et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: Biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am. J. Kidney Dis. 2005, 45, 473–484. [Google Scholar] [CrossRef]
- Hsiao, K.C.; Huang, J.Y.; Lee, C.T.; Hung, T.W.; Liaw, Y.P.; Chang, H.R. Different impact of aspirin on renal progression in patients with predialysis advanced chronic kidney disease with or without previous stroke. Eur. J. Intern. Med. 2017, 39, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.W.; Liu, J.S.; Hung, S.C.; Kuo, K.L.; Chang, Y.K.; Chen, Y.C.; Hsu, C.C.; Tarng, D.C. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern. Med. 2014, 174, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, Y.H.; Hsieh, T.F.; Chuang, S.Y.; Wu, M.J. Prediction of Mortality in Incident Hemodialysis Patients: A Validation and Comparison of CHADS2, CHA2DS2, and CCI Scores. PLoS ONE 2016, 11, e0154627. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Li, S.J.; Chen, M.; Lee, T.S.; Chien, Y.N. Machine-Learning Techniques for Feature Selection and Prediction of Mortality in Elderly CABG Patients. Healthcare 2021, 9, 547. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, S.J.; Chen, M.; Lee, T.S. The Prediction Model of Medical Expenditure Appling Machine Learning Algorithm in CABG Patients. Healthcare 2021, 9, 710. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H. Renal effects of prostaglandins and cyclooxygenase-2 inhibitors. Electrolyte Blood Press. 2008, 6, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrono, C.; Dunn, M.J. The clinical significance of inhibition of renal prostaglandin synthesis. Kidney Int. 1987, 32, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, G.N.C.; Leitao, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.F.; Silva Junior, G.B.D. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J. Bras. Nephrol. 2019, 41, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornelas, A.; Zacharias-Millward, N.; Menter, D.G.; Davis, J.S.; Lichtenberger, L.; Hawke, D.; Hawk, E.; Vilar, E.; Bhattacharya, P.; Millward, S. Beyond COX-1: The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017, 36, 289–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goicoechea, M.; de Vinuesa, S.G.; Quiroga, B.; Verde, E.; Bernis, C.; Morales, E.; Fernandez-Juarez, G.; de Sequera, P.; Verdalles, U.; Delgado, R.; et al. Aspirin for Primary Prevention of Cardiovascular Disease and Renal Disease Progression in Chronic Kidney Disease Patients: A Multicenter Randomized Clinical Trial (AASER Study). Cardiovasc. Drugs Ther. 2018, 32, 255–263. [Google Scholar] [CrossRef]
- Okumura, M.; Imanishi, M.; Yamashita, T.; Yamamura, Y.; Kim, S.; Iwao, H.; Tanaka, S.; Fujii, S. Renal production of thromboxane and prostaglandins in a rat model of type 2 diabetes. Life Sci. 2000, 66, 371–377. [Google Scholar] [CrossRef]
- Boffa, J.J.; Just, A.; Coffman, T.M.; Arendshorst, W.J. Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J. Am. Soc. Nephrol. 2004, 15, 2358–2365. [Google Scholar] [CrossRef] [Green Version]
- Molnar, A.O.; Bota, S.E.; Garg, A.X.; Harel, Z.; Lam, N.; McArthur, E.; Nesrallah, G.; Perl, J.; Sood, M.M. The Risk of Major Hemorrhage with CKD. J. Am. Soc. Nephrol. 2016, 27, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Di Micco, L.; Razavian, M.; Craig, J.C.; Perkovic, V.; Pellegrini, F.; Jardine, M.J.; Webster, A.C.; Zoungas, S.; Strippoli, G.F. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst. Rev. 2013, CD008834. [Google Scholar] [CrossRef]
- Kim, A.J.; Lim, H.J.; Ro, H.; Ko, K.P.; Han, S.Y.; Chang, J.H.; Lee, H.H.; Chung, W.; Jung, J.Y. Low-dose aspirin for prevention of cardiovascular disease in patients with chronic kidney disease. PLoS ONE 2014, 9, e104179. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, A.J.; Ro, H.; Chang, J.H.; Lee, H.H.; Chung, W.; Hyun, Y.Y.; Lee, J.; Kim, Y.H.; Han, S.H.; et al. Low-dose aspirin was associated with an increased risk of cardiovascular events in patients with chronic kidney disease patients and low bodyweight: Results from KNOW-CKD study. Sci. Rep. 2021, 11, 6691. [Google Scholar] [CrossRef]
- Polzin, A.; Dannenberg, L.; Sansone, R.; Levkau, B.; Kelm, M.; Hohlfeld, T.; Zeus, T. Antiplatelet effects of aspirin in chronic kidney disease patients. J. Thromb. Haemost. 2016, 14, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; McMurray, J.J.; Pfeffer, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M.; et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005, 352, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Penzes, K.; Hurjak, B.; Katona, E.; Becs, G.; Balla, J.; Muszbek, L. Terminal Phase Components of the Clotting Cascade in Patients with End-Stage Renal Disease Undergoing Hemodiafiltration or Hemodialysis Treatment. Int. J. Mol. Sci. 2020, 21, 8426. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.J.; Wei, R.B.; Wang, Y.; Su, T.Y.; Di, P.; Li, Q.P.; Yang, X.; Li, P.; Chen, X.M. Blood coagulation system in patients with chronic kidney disease: A prospective observational study. BMJ Open 2017, 7, e014294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriacou, D.N.; Lewis, R.J. Confounding by Indication in Clinical Research. JAMA 2016, 316, 1818–1819. [Google Scholar] [CrossRef] [PubMed]



| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Variables | Aspirin User (n = 3021) | Aspirin Nonuser (n = 88,723) | p Value | Aspirin User (n = 3021) | Aspirin Nonuser (n = 9063) | p Value |
| Gender | ||||||
| Male (%) | 1716 (56.8) | 45,893 (51.7) | <0.001 | 1716 (56.8) | 5148 (56.8) | 1 |
| Female (%) | 1305 (43.2) | 42,830 (48.3) | 1305 (43.2) | 3915 (43.2) | ||
| Age | 65.8 ± 12.9 | 64.6 ± 13.8 | <0.001 | 65.8 ± 12.9 | 65.5 ± 13.1 | 0.284 |
| Age group | <0.001 | 0.976 | ||||
| <50 (%) | 995 (32.9) | 29,231 (33.0) | 995 (33.0) | 2988 (33.0) | ||
| 50–64 (%) | 345 (11.4) | 12,518 (14.1) | 345 (11.4) | 1022 (11.3) | ||
| ≥65 (%) | 1681 (55.6) | 46,974 (53.0) | 1681 (55.6) | 5053 (55.8) | ||
| Comorbidities | ||||||
| Hypertension (%) | 609 (20.) | 17,177 (19.4) | 0.274 | 609 (20.2) | 1979 (21.8) | 0.051 |
| Diabetes mellitus (%) | 1685 (55.8) | 44,498 (50.2) | <0.001 | 1685 (55.8) | 5133 (56.6) | 0.408 |
| Hyperlipidemia (%) | 1313 (43.5) | 37,650 (42.4) | 0.261 | 1313 (43.5) | 4307 (47.5) | <0.001 |
| CAD (%) | 977 (32.3) | 22,711 (25.6) | <0.001 | 977 (32.3) | 2740 (30.2) | 0.029 |
| CHF (%) | 1234 (40.9) | 27,283 (30.8) | <0.001 | 1234 (40.9) | 3708 (40.9) | 0.948 |
| Stroke (%) | 655 (21.3) | 17,615 (19.9) | 0.047 | 655 (21.3) | 2041 (22.5) | 0.168 |
| PVD (%) | 297 (9.8) | 8361 (9.4) | 0.451 | 297 (9.8) | 966 (10.7) | 0.197 |
| COPD (%) | 576 (19.1) | 17,083 (19.3) | 0.796 | 576 (19.1) | 1945 (21.5) | 0.005 |
| Cancer (%) | 297 (9.8) | 9468 (10.7) | 0.14 | 297 (9.8) | 951 (10.5) | 0.3 |
| Atrial fibrillation (%) | 143 (4.7) | 3528 (4.0) | 0.036 | 143 (4.7) | 409 (4.5) | 0.614 |
| CCIS | 4.98 ± 3.1 | 4.69 ± 3.2 | <0.001 | 4.98 ± 3.1 | 4.91 ± 3.1 | 0.23 |
| Hospital area | ||||||
| Central (%) | 740 (24.5) | 21,066 (23.7) | 0.021 | 740 (24.5) | 2193 (24.2) | 0.175 |
| Northern (%) | 1329 (44.0) | 37,313 (42.1) | 1329 (44.0) | 3836 (42.3) | ||
| Southern (%) | 890 (29.5) | 28,253 (31.8) | 890 (29.5) | 2811 (31.0) | ||
| Eastern (%) | 62 (2.1) | 2091 (2.4) | 62 (2.1) | 223 (2.5) | ||
| Medications | ||||||
| ACEI/ARB (%) | 304 (10.1) | 5732 (6.5) | <0.001 | 304 (10.1) | 901 (9.9) | 0.847 |
| β blockers (%) | 1701 (56.3) | 41,354 (46.6) | <0.001 | 1701 (56.3) | 5100 (56.3) | 0.974 |
| CCB (%) | 2171 (71.9) | 62,618 (70.6) | 0.126 | 2171 (71.9) | 6663 (73.5) | 0.075 |
| Potassium diuretic (%) | 112 (3.7) | 2596 (2.9) | 0.012 | 112 (3.7) | 282 (3.1) | 0.11 |
| Metformin (%) | 27 (0.9) | 478 (0.5) | 0.009 | 27 (0.9) | 52 (0.6) | 0.058 |
| Insulin (%) | 897 (29.7) | 20,640 (23.3) | <0.001 | 897 (29.7) | 2689 (29.7) | 0.981 |
| Lipid-lowering agents (%) | 927 (30.7) | 22,080 (24.9) | <0.001 | 927 (30.7) | 2782 (30.7) | 0.99 |
| Nonselective NSAID (%) | 683 (22.6) | 17,208 (19.4) | <0.001 | 683 (22.6) | 2052 (22.6) | 0.97 |
| Selective NSAID (%) | 231 (7.7) | 5736 (6.5) | 0.009 | 231 (7.7) | 638 (7.0) | 0.263 |
| Acetaminophen (%) | 1568 (51.9) | 40,452 (45.6) | <0.001 | 1568 (51.9) | 4706 (51.9) | 0.983 |
| Clinical Outcomes | Before Matching | After Matching | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin Users (n = 3021) | Aspirin Nonusers (n = 88,723) | Aspirin Users vs. Nonusrs | Aspirin Users (n = 3021) | Aspirin Nonuser (n = 9063) | Aspirin Users vs. Nonusrs | |||||||
| Events | IR | Events | IR | Crude HR (95%CI) | p Value | Events | IR | Events | IR | HR (95%CI) | p Value | |
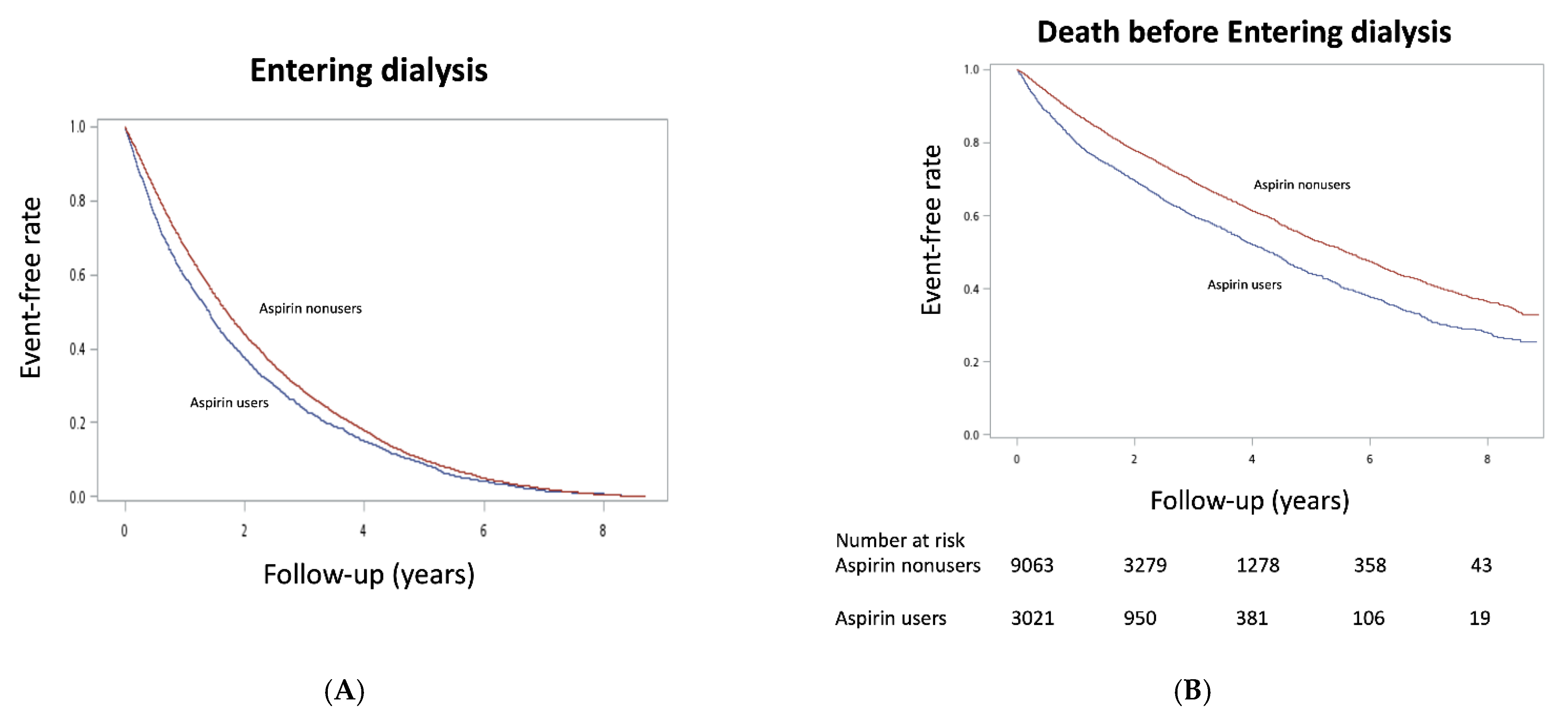
| Dialysis | 2616 | 64.0 | 74,777 | 49.8 | 1.23 (1.18–1.28) | <0.001 | 2616 | 64.0 | 7769 | 53.2 | 1.15 (1.10–1.21) | <0.001 |
| Dead before dialysis | 228 | 5.6 | 5344 | 3.6 | 1.49 (1.30–1.70) | <0.001 | 228 | 5.6 | 535 | 3.7 | 1.46 (1.25–1.71) | <0.001 |
| All-cause Hospitalization | 2427 | 37.5 | 70,969 | 33.7 | 1.12 (1.07–1.16) | <0.001 | 2427 | 37.5 | 7373 | 35.1 | 1.06 (1.02–1.11) | 0.009 |
| GI bleeding | 640 | 8.9 | 19,683 | 8.1 | 1.10 (1.01–1.19) | 0.021 | 640 | 8.9 | 2027 | 8.5 | 1.05 (0.96–1.15) | 0.280 |
| Ischemic stroke | 100 | 1.2 | 2905 | 1.0 | 1.16 (0.95–1.42) | 0.141 | 100 | 1.2 | 290 | 1.0 | 1.15 (0.92–1.45) | 0.217 |
| ICH | 102 | 1.2 | 2883 | 1.0 | 1.20 (0.98–1.46) | 0.073 | 102 | 1.2 | 278 | 1.0 | 1.23 (0.98–1.55) | 0.071 |
| All-cause mortality | 1460 | 19.0 | 32,706 | 13.2 | 1.51 (1.43–1.59) | <0.001 | 1460 | 19.0 | 3557 | 12.4 | 1.37 (1.29–1.45) | <0.001 |
| MACE | 2351 | 27.9 | 58,956 | 20.7 | 1.29 (1.24–1.35) | <0.001 | 2351 | 27.9 | 6613 | 26.6 | 1.15 (1.10–1.20) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-H.; Liou, H.-H.; Huang, Y.-C.; Lee, T.-S.; Chen, M.; Fang, Y.-W. Hazardous Effect of Low-Dose Aspirin in Patients with Predialysis Advanced Chronic Kidney Disease Assessed by Machine Learning Method Feature Selection. Healthcare 2021, 9, 1484. https://doi.org/10.3390/healthcare9111484
Tsai M-H, Liou H-H, Huang Y-C, Lee T-S, Chen M, Fang Y-W. Hazardous Effect of Low-Dose Aspirin in Patients with Predialysis Advanced Chronic Kidney Disease Assessed by Machine Learning Method Feature Selection. Healthcare. 2021; 9(11):1484. https://doi.org/10.3390/healthcare9111484
Chicago/Turabian StyleTsai, Ming-Hsien, Hung-Hsiang Liou, Yen-Chun Huang, Tian-Shyug Lee, Mingchih Chen, and Yu-Wei Fang. 2021. "Hazardous Effect of Low-Dose Aspirin in Patients with Predialysis Advanced Chronic Kidney Disease Assessed by Machine Learning Method Feature Selection" Healthcare 9, no. 11: 1484. https://doi.org/10.3390/healthcare9111484
APA StyleTsai, M.-H., Liou, H.-H., Huang, Y.-C., Lee, T.-S., Chen, M., & Fang, Y.-W. (2021). Hazardous Effect of Low-Dose Aspirin in Patients with Predialysis Advanced Chronic Kidney Disease Assessed by Machine Learning Method Feature Selection. Healthcare, 9(11), 1484. https://doi.org/10.3390/healthcare9111484







