Infection Control Improvement of a Negative-Pressurized Pediatric Intensive Care Unit
Abstract
:1. Introduction
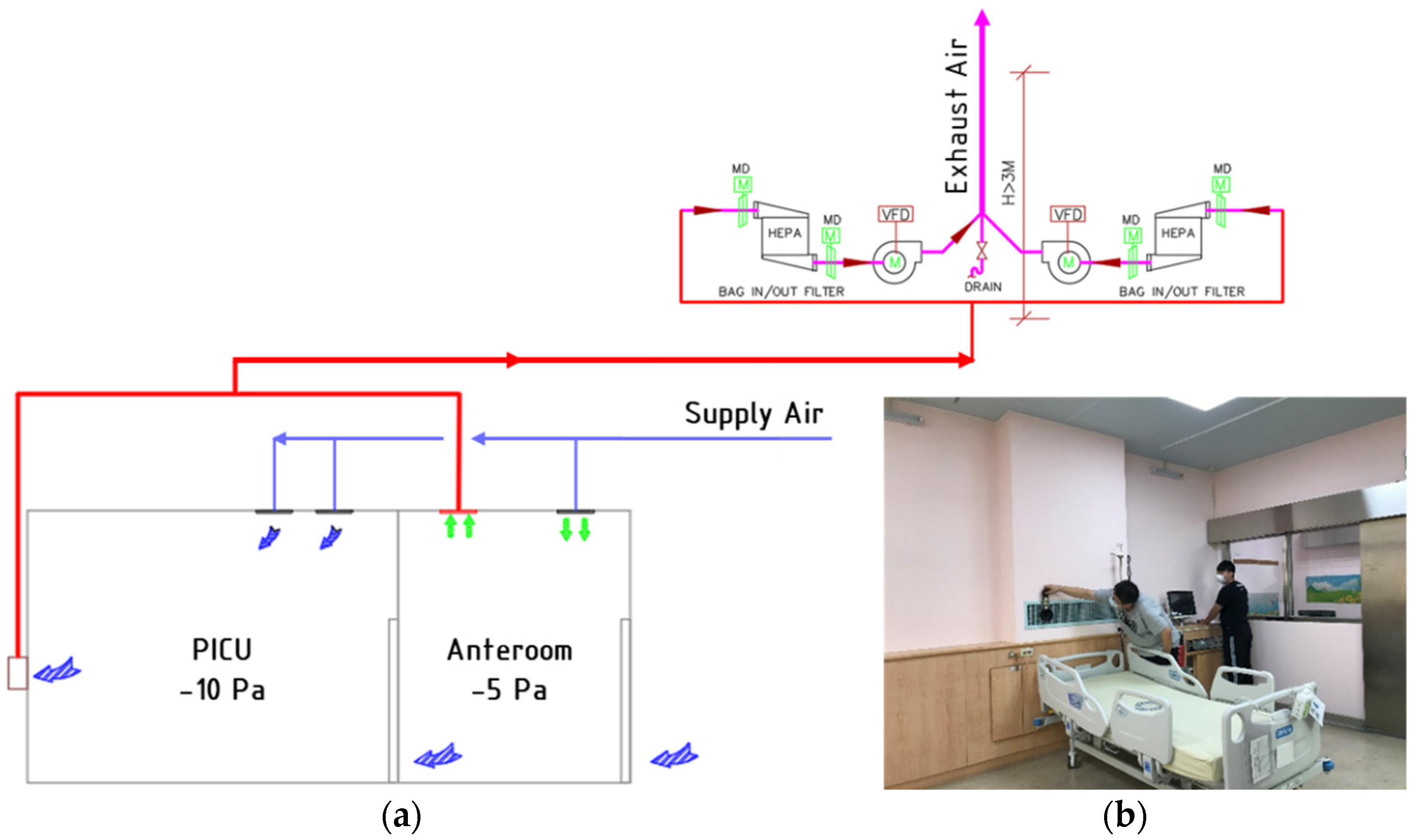
2. Negative-Pressurized Pediatric Intensive Care Unit
2.1. System Description
2.2. CFD Simulation and Improvement Strategy
- Case 1: baseline case (Figure 2a).
- Case 2: occupied by two patients (Figure 2b).
- Case 3: two patients with additional air-jet curtain ceiling mounted, placed between the patient. The corresponding velocity was 0.5 m/s (Figure 2c).
- Case 4: case 3 with a new arrangement ventilation system based on an additional exhaust air grille identical with the original design placed right besides patient 2’s head along with one HEPA placed for each patient (Figure 2d).
2.3. Boundary Conditions and Initial Conditions
2.4. Field Measurement Test and Validation
3. Results and Discussion
3.1. Airflow Distribution
3.2. Temperature Distribution
3.3. Concentration Distribution and Concentration Decay
3.4. Particle Tranjectories
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Piscitelli, P.; Miani, A. Airborne transmission route of COVID-19: Why 2 meters/6 feet of inter-personal distance could not be enough. Int. J. Environ. Res. Public Health 2020, 17, 2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.W.; Li, Y.; Eames, I.; Chan, P.K.S.; Ridgway, G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006, 64, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.M.; Mindorff, C.; Patrick, M.L.; Gold, R.; Ford-Jones, E.L. Isolation usage in a pediatric hospital. Infect. Control. 1987, 8, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Blickle, M.; Schmidt, B.; Sievers, L.K.; Pfitzer, C. SARS-CoV-2 in Pediatric Inpatient Care: Management, Clinical Presentation and Utilization of Healthcare Capacity. Healthcare 2021, 9, 1190. [Google Scholar] [CrossRef] [PubMed]
- Abu-Taleb, A.-R.M. Paediatric Intensive Care Physical Environment. In Textbook of Clinical Pediatrics; Elzouki, A.Y., Harfi, H.A., Nazer, H.M., Stapleton, F.B., Oh, W., Whitley, R.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Health-Care Settings; Department of Health and Human Services: Atlanta, GA, USA, 2005.
- American Academy of Pediatrics (AAP). Guidelines for pediatric intensive care units. Pediatrics 1983, 72, 364–371. [Google Scholar]
- ANSI/ASHRAE/ASHE Standard-170. Ventilation of Health Care Facilities; American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 2017. [Google Scholar]
- World Health Organization (WHO). Natural Ventilation for Infection Control in Healthcare Settings; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Institute of Labor, Occupational Safety and Health (ILOSH). Recommended Guidelines of Isolation Ward for SARS Patients; ILOSH: Taipei, Taiwan, 2016. Available online: https://www.ilosh.gov.tw/1261/1274/1276/8875/ (accessed on 5 June 2021).
- Subhash, S.S.; Baracco, G.; Fennelly, K.P.; Hodgson, M.; Radonovich, L.J. Isolation anterooms: Important components of airborne infection control. Am. J. Infect. Control. 2013, 41, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020, 144, 106039. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Infection Prevention and Control of Epidemic and Pandemic—Prone Acute Respiratory Infections in Health Care; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Liu, T.; Guo, Y.; Hao, X.; Wang, M.; He, S.; Lin, Z.; Zhou, R. Evaluation of an innovative pediatric isolation (PI) bed using fluid dynamics simulation and aerosol isolation efficacy. Build. Simul. 2021, 14, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Borro, L.; Mazzei, L.; Raponi, M.; Piscitelli, P.; Miani, A.; Secinaro, A. The role of air conditioning in the diffusion of SARS-CoV-2 in indoor environments: A first computational fluid dynamic model, based on investigations performed at the Vatican State Children’s hospital. Environ. Res. 2021, 193, 110343. [Google Scholar] [CrossRef]
- Wang, F.; Chaerasari, C.; Rakshit, D.; Permana, I.; Kusnandar. Performance Improvement of a Negative-Pressurized Isolation Room for Infection Control. Healthcare 2021, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Investigation on the contaminant distribution with improved ventilation system in hospital isolation rooms: Effect of supply and exhaust air diffuser configurations. Appl. Therm. Eng. 2019, 148, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Yadav, S.S.; Sikarwar, B.S. Design and simulation of isolation room for a hospital. Adv. Fluid Therm. Eng. 2019, 1, 75–93. [Google Scholar]
- Khankari, K. Airflow Path Matters: Patient Room HVAC. ASHRAE J. 2016, 58, 16–26. [Google Scholar]
- Cao, G.; Liu, S.; Boor, B.E.; Novoselac, A. Dynamic interaction of a downward plane jet and a cough jet with respect to particle transmission: An analytical and experimental study. J. Occup. Environ. Hyg. 2017, 14, 618–631. [Google Scholar] [CrossRef] [PubMed]
- ANSYS. Ansys Fluent, Workbench 2020 R2. Available online: https://www.scribd.com/document/478910198/ANSYS-FluentTutorial-Guide-2020-R2-pdf (accessed on 6 March 2021).
- Karthikeyan, C.P.; Samuel, A.A. CO2-dispersion studies in an operation theatre under transient conditions. Energy Build. 2008, 40, 231–239. [Google Scholar] [CrossRef]
- Spagnoli, G.; Tranfo, G.; Moccaldi, R. Air Quality in Operating Theatres: The Occupational Point of View; Maroni, M., Ed.; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Schell, M.; Hinthout, D. Demand control ventilation using CO2. ASHRAE J. 2001, 43, 18–29. [Google Scholar]
- Minnesota Department of Health United States. Carbon Dioxide (CO2). Available online: https://www.health.state.mn.us/communities/environment/air/toxins/co2.html (accessed on 15 June 2021).
- Tsega, E.G. Computational Fluid Dynamics Modeling of Respiratory Airflow in Tracheobronchial Airways of Infant, Child, and Adult. Comput. Math. Methods Med. 2018, 2018, 9603451. [Google Scholar] [CrossRef] [PubMed]
- ASHRAE GRP 158. Cooling and Heating Calculation Manuals; American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 1979. [Google Scholar]
- Shaheed, R.; Mohammadian, A.; Gildeh, H.K. A comparison of standard k–ε and realizable k–ε turbulence models in curved and confluent channels. Environ. Fluid Mech. 2019, 19, 543–568. [Google Scholar] [CrossRef]
- Bombač, M.; Novak, G.; Mlačnik, J.; Četina, M. Extensive field measurements of flow in vertical slot fishway as data for validation of numerical simulations. Ecol. Eng. 2015, 84, 476–484. [Google Scholar] [CrossRef]







| Parameter | Type | Value |
|---|---|---|
| Supply air | Velocity inlet | Isolation room HEPA: 0.49 m/s Anteroom HEPA: 0.47 m/s Temperature: 22.2 °C Concentration: 400 ppm |
| Exhaust air | Pressure outlet | Temperature: 24 °C Isolation room pressure: −11.4 Pa Anteroom pressure: −6.12 Pa |
| CO2 concentration | Velocity inlet | Velocity inlet: 1.12 m/s Temperature: 35 °C Patient’s exhale: 38,000 ppm |
| Patient | Wall | Heatflux: 34.87 W/m2 |
| Lightings | Wall | Heatflux: 33.3 W/m2 |
| Windows | Wall | Heat transfer coefficient: 3.18 W/m2K |
| Walls | Wall | Heat transfer coefficient: 5.60 W/m2K |
| Apparatus Model | Parameters | Operative Range | Accuracy |
|---|---|---|---|
| TSI-8380 | Velocity Pressure | 0.125–12.5 (m/s) Diff ± 3735 Pa | ± 3% ± 2% |
| TSI TA465P | Temperature Humidity | −10~60 °C 5~95% RH | ± 0.3 °C ± 1% |
| Room Name | Volume (m3) | Inlet/Outlet | Airflow Rate (CMH) | Pressure (Pa) | Air Changes Hour (ACH) |
|---|---|---|---|---|---|
| Anteroom | 19.15 | HEPA 1 EA 1 | 123 315 | −6.12 | 6.42 |
| Isolation room | 69.5 | HEPA 2 HEPA 3 EA 2 | 331 317 1318 | −11.4 | 9.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Permana, I.; Chaerasari, C.; Panigrahi, B.; Rakshit, D. Infection Control Improvement of a Negative-Pressurized Pediatric Intensive Care Unit. Healthcare 2021, 9, 1500. https://doi.org/10.3390/healthcare9111500
Wang F, Permana I, Chaerasari C, Panigrahi B, Rakshit D. Infection Control Improvement of a Negative-Pressurized Pediatric Intensive Care Unit. Healthcare. 2021; 9(11):1500. https://doi.org/10.3390/healthcare9111500
Chicago/Turabian StyleWang, Fujen, Indra Permana, Citra Chaerasari, Bivas Panigrahi, and Dibakar Rakshit. 2021. "Infection Control Improvement of a Negative-Pressurized Pediatric Intensive Care Unit" Healthcare 9, no. 11: 1500. https://doi.org/10.3390/healthcare9111500
APA StyleWang, F., Permana, I., Chaerasari, C., Panigrahi, B., & Rakshit, D. (2021). Infection Control Improvement of a Negative-Pressurized Pediatric Intensive Care Unit. Healthcare, 9(11), 1500. https://doi.org/10.3390/healthcare9111500









