Change Management and Digital Innovations in Hospitals of Five European Countries
Abstract
1. Introduction
Setting the Scene of Change Management Analysis in Healthcare
2. Materials and Methods
2.1. Questionnaire Survey
2.2. Systematic Review
3. Results
3.1. Questionnaire
3.1.1. Finding 1 (Research Question No. 1)
3.1.2. Finding 2 (Research Question No. 2)
3.1.3. Finding 3 (Research Question No. 3)
3.1.4. Finding 4 (Research Question No. 4)
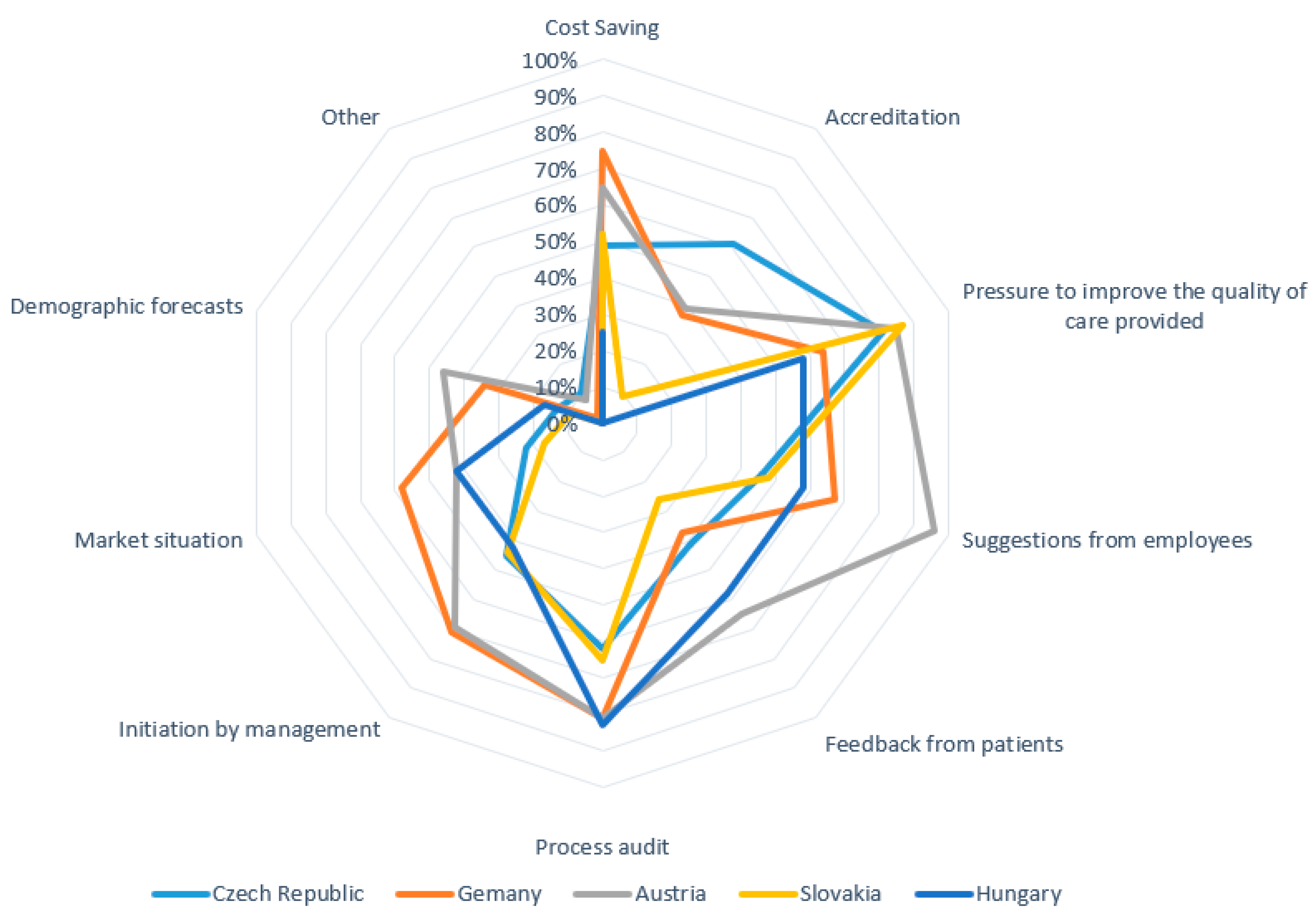
3.1.5. Finding 5 (Research Question No. 5)
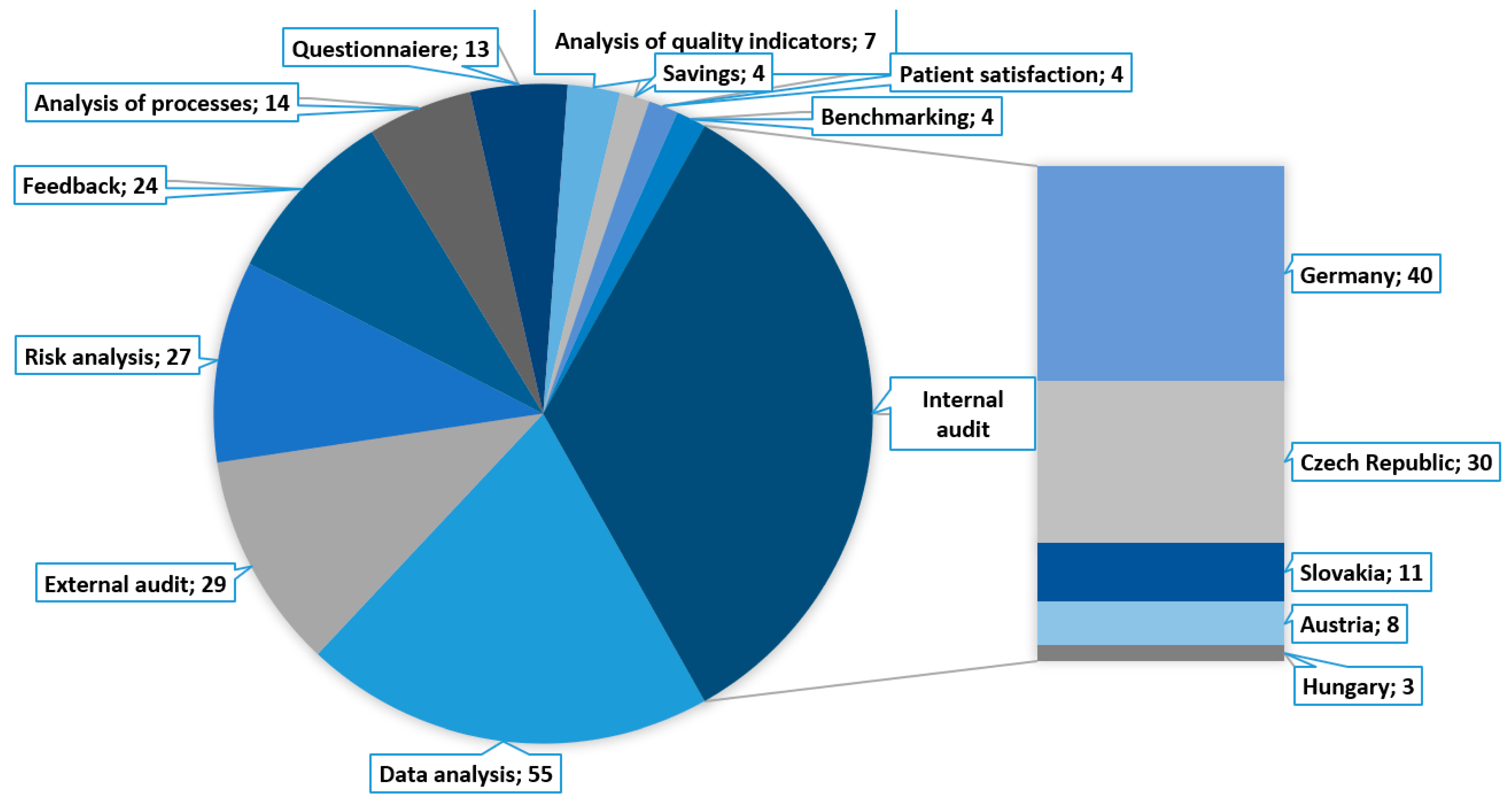
3.1.6. Finding 6 (Research Question No. 6)
3.2. Systematic Review
Finding 7 (Research Question No. 7)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nilsen, P.; Seing, I.; Ericsson, C.; Birken, S.A.; Schildmeijer, K. Characteristics of successful changes in health care organizations: An interview study with physicians, registered nurses and assistant nurses. BMC Health Serv. Res. 2020, 20, 147. [Google Scholar] [CrossRef]
- Moran, J.W.; Brightman, B.K. Leading organizational change. J. Workplace Learn. 2000, 12, 66–74. [Google Scholar] [CrossRef]
- Burnes, B. Emergent change and planned change-Competitors or allies? The case of XYZ construction. Int. J. Oper. Prod. Manag. 2004, 24, 886–902. [Google Scholar] [CrossRef]
- Lexa, F.J. Profiles in Leadership: What Does it Mean to Be a Great Leader. In Leadership Lessons for Health Care Providers, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 8–12. [Google Scholar]
- Lexa, F.J. Leading Change in an Organization. In Leadership Lessons for Health Care Providers, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 100–108. [Google Scholar]
- World Health Organization. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. Available online: https://www.who.int/reproductivehealth/publications/digital-interventions-health-system-strengthening/en/ (accessed on 20 October 2021).
- Organization for Economic Co-Operation and Development (OECD). OECD Observatory of Public Sector Innovation. Available online: https://www.oecd.org/media/oecdorg/satellitesites/opsi/contents/files/SystemsApproachesDraft.pdf (accessed on 20 October 2021).
- Bodenheimer, T.; Sinsky, C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann. Fam. Med. 2014, 12, 573–576. [Google Scholar] [CrossRef]
- Barry, S.; Dalton, R.; Eustace-Cook, J. Understanding Change in Complex Health Systems—A Review of the Literature on Change Management in Health and Social Care 2007–2017; Organisation Development and Design Services: Kells, Ireland, 2018. [Google Scholar]
- Birken, S.A.; Lee, S.Y.D.; Weiner, B.J.; Chin, M.H.; Chiu, M.; Schaefer, C.T. From strategy to action: How top managers’ support increases middle managers’ commitment to innovation implementation in health care organizations. Health Care Manag. Rev. 2015, 40, 159–168. [Google Scholar] [CrossRef]
- KPMG. Medical Devices 2030. Available online: https://assets.kpmg/content/dam/kpmg/xx/pdf/2017/12/medical-devices-2030.pdf (accessed on 20 October 2021).
- Deloitte. The Digital Era in the MedTech Industry. Available online: https://www2.deloitte.com/content/dam/Deloitte/us/Documents/process-and-operations/us-the-digital-era-in-the-medtech-industry.pdf (accessed on 20 October 2021).
- Ernst and Young. Pulse of the Industry: Medical Technology Report. 2020. Available online: https://assets.ey.com/content/dam/ey-sites/ey-com/en_gl/topics/health/ey-pulse-medical-technology-report.pdf (accessed on 20 October 2021).
- World Health Organization (WHO). Global Strategy on Digital Health 2020–2025. Available online: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf (accessed on 20 October 2021).
- Labrique, A.B.; Vasudevan, L.; Kochi, E.; Fabricant, R.; Mehl, G. mHealth innovations as health system strengthening tools: 12 common applications and a visual framework. Glob. Health Sci. Pract. August 2013, 1, 160–171. [Google Scholar] [CrossRef]
- Small, A.; Gist, D.; Souza, D.; Dalton, J.; Magny-Normilus, C.; David, D. Using Kotter’s change model for implementing bedside handoff. J. Nurs. Care Qual. 2016, 31, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kotter, J.P. Leading Change, With a New Preface by the Author, 1st ed.; Harvard Business Review Press: Boston, MA, USA, 2012. [Google Scholar]
- Maclean, D.F.W.; Vannet, N. Improving trauma imaging in Wales through Kotter’s theory of change. Clin. Radiol. 2016, 71, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Dolansky, M.A.; Hitch, J.A.; Piña, I.L.; Boxer, R.S. Improving Heart Failure Disease Management in Skilled Nursing Facilities: Lessons Learned. Clin. Nurs. Res. 2013, 22, 432–447. [Google Scholar] [CrossRef]
- Ellsbury, D.L.; Clark, R.H.; Ursprung, R.; Handler, D.L.; Dodd, E.D.; Spitzer, A.R. A multifaceted approach to improving outcomes in the NICU: The pediatrix 100 000 babies campaign. Pediatrics 2016, 137, e20150389. [Google Scholar] [CrossRef] [PubMed]
- Baloh, J.; Zhu, X.; Ward, M.M. Implementing team huddles in small rural hospitals: How does the Kotter model of change apply? J. Nurs. Manag. 2018, 26, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Lewin, K. Frontiers in Group Dynamics: Concept, Method and Reality in Social Science; Social Equilibria and Social Change. Hum. Relat. 1947, 1, 5–41. [Google Scholar] [CrossRef]
- Šuc, J.; Prokosch, H.U.; Ganslandt, T. Applicability of lewińs change management model in a hospital setting. Methods Inf. Med. 2009, 48, 419–428. [Google Scholar] [PubMed]
- Abd El-Shafy, I.; Zapke, J.; Sargeant, D.; Prince, J.M.; Christopherson, N.A.M. Decreased Pediatric Trauma Length of Stay and Improved Disposition with Implementation of Lewin’s Change Model. J. Trauma Nurs. 2019, 26, 84–88. [Google Scholar] [CrossRef]
- Shatpattananunt, B.; Petpichetchian, W.; Kitrungrote, L. Development of the Change Implementation Strategies Model Regarding Evidence-Based Chronic Wound Pain Management. Pac. Rim Int. J. Nurs. Res. 2015, 19, 359–372. [Google Scholar]
- Tetef, S. Successful Implementation of New Technology Using an Interdepartmental Collaborative Approach Recognizing an Opportunity for Growth in New Technology. J. PeriAnesthesia Nurs. 2017, 32, 225–230. [Google Scholar] [CrossRef]
- Welbourn, D.; Warwick, R.; Carnall, C.; Fathers, D. Leadership of Whole Systems; The King’s Fund: London, UK, 2012. [Google Scholar]
- Newell, K.; Corrigan, C.; Punshon, J.; Leary, A. Severe asthma: Emergency care patient driven solutions. Int. J. Health Care Qual. Assur. 2017, 30, 628–637. [Google Scholar] [CrossRef]
- Checkland, P. Achieving ‘desirable and feasible’ change: An application of soft systems methodology. J. Oper. Res. Soc. 1985, 36, 821–831. [Google Scholar]
- Emes, M.; Smith, S.; Ward, S.; Smith, A. Improving the patient discharge process: Implementing actions derived from a soft systems methodology study. Health Syst. 2019, 8, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.E. Why good management ideas fail: The neglected power of organizational culture. Strategy Leadersh. 2000, 28, 24–29. [Google Scholar] [CrossRef]
- Checkland, P.; Poulter, J. Systems Approaches to Managing Change: A Practical Guide; Springer: London, UK, 2010. [Google Scholar]
- Hřebíček, J. Modelování soft (měkkých) systémů. In 5. Letní Škola Matematické Biologie „Analýza Biologických a Klinických dat v Mezioborovém Pojetí; Masarykova univerzita v Brně: Brno, Czechia, 2009. [Google Scholar]
- Mukotekwa, C.; Carson, E. Improving the discharge planning process: A systems study. J. Res. Nurs. 2007, 12, 667–686. [Google Scholar] [CrossRef]
- Emes, M.; Smith, S.; Ward, S.; Smith, A.; Ming, T. Care and Flow: Using Soft Systems Methodology to understand tensions in the patient discharge process. Health Syst. 2017, 6, 260–278. [Google Scholar] [CrossRef]
- Deiters, W.; Burmann, A.; Meister, S. Strategies for digitalizing the hospital of the future. Urologe 2018, 57, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Bruthans, J. The past and current state of the Czech outpatient electronic prescription (eRecept). Int. J. Med. Inform. 2019, 123, 49–53. [Google Scholar] [CrossRef]
- Brice, S.; Almond, H. Health professional digital capabilities frameworks: A scoping review. J. Multidiscip. Healthc. 2020, 2, 1375–1390. [Google Scholar] [CrossRef]
- Shaw, B.; Chisholm, O. Creeping Through the Backdoor: Disruption in Medicine and Health. Front. Pharmacol. 2020, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- OECD. Health in the 21st Century; OECD: Paris, France, 2019. [Google Scholar]
- PROSCI. Communication Checklist for Achieving Change Management. Available online: https://www.prosci.com/resources/articles/change-managementcommunication-checklist (accessed on 18 August 2021).
- Ústav Zdravotnických Informací a Statistiky ČR. Interaktivní Prohlížeč dat-DRG Restart. Available online: https://drg.uzis.cz/index.php?pg=referencni-sit--mapovani-akutni-luzkove-121%20pece--interaktivni-prohlizecdat&studie=i&analyza=i.1&zz_type=&var1=pocet_luzek&var2=osa_b (accessed on 18 August 2021).
- German Hospital Directory. Available online: https://www.german-hospital-directory.com/app/search/maps (accessed on 18 August 2021).
- Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz. Available online: https://kliniksuche.at/suche/bundesland (accessed on 18 August 2021).
- Ministerstvo Zdravotníctva Slovenskej Republiky. Available online: https://www.health.gov.sk/?subjekty-hospodarskej-mobilizacie (accessed on 18 August 2021).
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Ding, H.; Fatehi, F.; Maiorana, A.; Bashi, N.; Hu, W.; Edwards, I. Digital health for COPD care: The current state of play. J. Thorac. Dis. 2019, 11 (Suppl. 17), S2210–S2220. [Google Scholar] [CrossRef]
- Fatehi, F.; Wootton, R. Telemedicine, telehealth or e-health? A bibliometric analysis of the trends in the use of these terms. J. Telemed. Telecare 2012, 18, 460–464. [Google Scholar] [CrossRef]
- Meinert, E.; Van Velthoven, M.; Brindley, D.; Alturkistani, A.; Foley, K.; Rees, S.; Wells, G.; De Pennington, N. The internet of things in health care in Oxford: Protocol for proof-of-concept projects. J. Med. Internet Res. 2018, 7, e12077. [Google Scholar] [CrossRef]
- Olze, H.; Uecker, F.C.; Häubler, S.M.; Knopke, S.; Szczepek, A.J.; Gräbel, S. Hearing Implants in the Era of Digitization. Laryngo- Rhino- Otol. 2019, 98, S1–S24. [Google Scholar]
- Ferrante, G.; Licari, A.; Marseglia, G.L.; La Grutta, S. Digital health interventions in children with asthma. Clin. Exp. Allergy 2021, 51, 212–220. [Google Scholar] [CrossRef]
- Rose, K.J.; Petrut, C.; L’heveder, R.; De Sabata, S. IDF Europe’s position on mobile applications in diabetes. Diabetes Res. Clin. Pract. 2019, 149, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kernebeck, S.; Busse, T.S.; Böttcher, M.D.; Weitz, J.; Ehlers, J.; Bork, U. Impact of mobile health and medical applications on clinical practice in gastroenterology. World J. Gastroenterol. 2020, 26, 4182–4197. [Google Scholar] [CrossRef] [PubMed]
- Wechkunanukul, K.; Parajuli, D.R.; Hamiduzzaman, M. Utilising digital health to improve medication-related quality of care for hypertensive patients: An integrative literature review. World J. Clin. Cases 2020, 8, 2266–2279. [Google Scholar] [CrossRef]
- Griauzde, D.; Kullgren, J.T.; Liestenfeltz, B.; Ansari, T.; Johnson, E.H.; Fedewa, A.; Saslow, L.R.; Richardson, C.; Heisler, M. A mobile phone-based program to promote healthy behaviors among adults with prediabetes who declined participation in free diabetes prevention programs: Mixed-methods pilot randomized controlled trial. JMIR Mhealth Uhealth 2019, 7, e11267. [Google Scholar] [CrossRef]
- Tripoliti, E.E.; Karanasiou, G.S.; Kalatzis, F.G.; Naka, K.K.; Fotiadis, D.I. The Evolution of mHealth Solutions for Heart Failure Management. In Heart Failure: From Research to Clinical Practice; Springer: New York, NY, USA, 2018; pp. 353–371. [Google Scholar]
- Wongvibulsin, S.; Martin, S.S.; Steinhubl, S.R.; Muse, E.D. Connected Health Technology for Cardiovascular Disease Prevention and Management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 1–15. [Google Scholar] [CrossRef]
- Gopal, G.; Suter-Crazzolara, C.; Toldo, L.; Eberhardt, W. Digital transformation in healthcare-Architectures of present and future information technologies. Clin. Chem. Lab. Med. 2018, 57, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A.; Carini, C. Are innovation and new technologies in precision medicine paving a new era in patients centric care? J. Transl. Med. 2019, 17, 114. [Google Scholar] [CrossRef]
- Kataria, S.; Ravindran, V. Digital health: A new dimension in rheumatology patient care. Rheumatol. Int. 2018, 38, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Gessa, A.; Jiménez, A.; Sancha, P. Open innovation in digital healthcare: Users’ discrimination between certified and non-certified mhealth applications. J. Open Innov. Technol. Mark. Complex. 2020, 6, 1–20. [Google Scholar]
- Lo, C.; Yu, J.; Görges, M.; Matava, C. Anesthesia in the modern world of apps and technology: Implications and impact on wellness. Pediatric Anesth. 2021, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kouroubali, A.; Katehakis, D.G. The new European interoperability framework as a facilitator of digital transformation for citizen empowerment. J. Biomed. Inform. 2019, 94, 103166. [Google Scholar] [CrossRef]
- Scott, I.A.; Sullivan, C.; Staib, A. Going digital: A checklist in preparing for hospital-wide electronic medical record implementation and digital transformation. Aust. Health Rev. 2019, 43, 302–313. [Google Scholar] [CrossRef]
- Meinert, E.; Alturkistani, A.; Brindley, D.; Knight, P.; Wells, G.; De Pennington, N. The technological imperative for value-based health care. Br. J. Hosp. Med. 2018, 79, 328–332. [Google Scholar] [CrossRef]
- Faggini, M.; Cosimato, S.; Nota, F.D.; Nota, G. Pursuing Sustainability for Healthcare through Digital Platforms. Sustainability 2019, 11, 165. [Google Scholar] [CrossRef]
- Hermes, S.; Riasanow, T.; Clemons, E.K.; Böhm, M.; Krcmar, H. The digital transformation of the healthcare industry: Exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus. Res. 2020, 13, 1033–1069. [Google Scholar] [CrossRef]
- Park, C.-W.; Seo, S.W.; Kang, N.; Ko, B.S.; Choi, B.W.; Park, C.M.; Chang, D.K.; Kim, H.; Kim, H.; Lee, H.; et al. Artificial Intelligence in Health Care: Current Applications and Issues. J. Korean Med. Sci. 2020, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gehring, H.; Rackebrandt, K.; Imhoff, M. E-Health und die Realität–was sehen wir heute schon in der Klinik? Bundesgesundheitsblatt-Gesundh. -Gesundh. 2018, 61, 252–262. [Google Scholar] [CrossRef]
- Despotou, G.; Laleci Erturkmen, G.B.; Yuksel, M.; Sarigul, B.; Lindman, P.; Jaulent, M.C.; Bouaud, J.; Traore, L.; Lim Choi Keung, S.N.; De Manuel, E.; et al. Localisation, personalisation and delivery of best practice guidelines on an integrated care and cure cloud architecture: The C3-cloud approach to managing multimorbidity. Stud. Health Technol. Inform. 2020, 270, 623–627. [Google Scholar]
- Kobusinge, G. Managing as Designing: Transforming Digital Healthcare Interoperability. Proceedings of the 26th Americas Conference on Information Systems, AMCIS. 2020. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85097714752&partnerID=40&md5=f4e265b215fc68c6b6aec74633053412 (accessed on 29 August 2021).
- Kolasa, K.; Goettsch, W.; Petrova, G.; Berler, A. ‘Without data, you’re just another person with an opinion’. Expert Rev. Pharm. Outcomes Res. 2020, 20, 147–154. [Google Scholar] [CrossRef]
- Laleci Erturkmen, G.B.; Yuksel, M.; Sarigul, B.; Arvanitis, T.N.; Lindman, P.; Chen, R.; Zhao, L.; Sadou, E.; Bouaud, J.; Traore, L.; et al. A Collaborative Platform for Management of Chronic Diseases via Guideline-Driven Individualized Care Plans. Comput. Struct. Biotechnol. J. 2019, 17, 869–885. [Google Scholar] [CrossRef]
- Wang, S.Y.; Pershing, S.; Lee, A.Y. Big data requirements for artificial intelligence. Curr. Opin. Ophthalmol. 2020, 31, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Odisho, A.Y.; Lui, H.; Yerramsetty, R.; Bautista, F.; Gleason, N.; Martin, E.; Young, J.J.; Blum, M.; Neinstein, A.B. Design and development of Referrals Automation, a SMART on FHIR solution to improve patient access to specialty care. JAMIA Open 2020, 3, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/wearable devices opportunity. NPJ Digit. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Cai, S.; Han, Z.; Liu, X.; Wang, F.; Cao, Y.; Wang, Z.; Li, H.; Chen, Y.; et al. Flexible Hybrid Electronics for Digital Healthcare. Adv. Mater. 2020, 32, 1902062. [Google Scholar] [CrossRef]
- Binder, A.F.; Handley, N.R.; Wilde, L.; Palmisiano, N.; Lopez, A.M. Treating Hematologic Malignancies During a Pandemic: Utilizing Telehealth and Digital Technology to Optimize Care. Front. Oncol. 2020, 10, 1183. [Google Scholar] [CrossRef]
- Noel, K.; Ellison, B. Inclusive innovation in telehealth. Digit. Med. 2020, 3, 1–3. [Google Scholar]
- Guy, M.; Koizia, L.; Kooner, A.; Cafferkey, J.; Ross, C.; Purkayastha, S.; Sivananthan, A.; Tanna, A.; Pratt, P.; Kinross, J. Use of the HoloLens2 mixed reality headset for protecting health care workers during the COVID-19 pandemic: Prospective, observational evaluation. J. Med. Internet Res. 2020, 22, e21486. [Google Scholar]
- Umoren, R.A.; Gray, M.M.; Handley, S.; Johnson, N.; Kunimura, C.; Mietzsch, U.; Billimoria, Z.; Lo, M.D. In-Hospital Telehealth Supports Care for Neonatal Patients in Strict Isolation. Am. J. Perinatol. 2020, 37, 857–860. [Google Scholar] [CrossRef]
- Raphael, B.P.; Schumann, C.; Garrity-Gentille, S.; Mcclelland, J.; Rosa, C.; Tascione, C.; Gallotto, M.; Takvorian-Bené, M.; Carey, A.N.; Mccarthy, P.; et al. Virtual Telemedicine Visits in Pediatric Home Parenteral Nutrition Patients: A Quality Improvement Initiative. Telemed. e-Health 2019, 25, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Tahir, M.; Sheikh, M.A.; Ahmed, K.I.; Mughees, A.; Numani, A. Technologies trend towards 5g network for smart health-care using iot: A review. Sensors 2020, 20, 4047. [Google Scholar] [CrossRef]
- Shayevitz, C.; Breitinger, S.; Lerario, M.P.; Mroczkowski, M.; Osuji, M.; Fleischut, P.; Khan, M.; Murray, J.; Wilner, P.; Sombrotto, L. Implementation of a Centralized Telepsychiatry Consult Service in a Multi-Hospital Metropolitan Health Care System: Challenges and Opportunities. Psychosomatics 2020, 62, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.R.; Hall, C.L.; Andrén, P.; Bethan Davies, E.; Kilgariff, J.; Kouzoupi, N.; Murphy, T.; Hollis, C. Therapist-supported online interventions for children and young people with tic disorders: Lessons learned from a randomized controlled trial and considerations for future practice. JMIR Ment. Health 2020, 7, e19600. [Google Scholar] [CrossRef]
- Mamyrbekova, S.; Nurgaliyeva, Z.; Saktapov, A.; Zholdasbekova, A.; Kudaibergenova, A. Medicine of the Future: Digital Technologies in Healthcare. E3S Web Conf. 2020, 159, 04036. [Google Scholar] [CrossRef]
- Dohan, M.S.; Califf, C.B.; Ghosh, K.; Tan, J. Digital transformation in healthcare: New value for a new movement. Health Policy Technol. 2020, 9, 177–178. [Google Scholar] [CrossRef]
- El Hayek, S.; Nofal, M.; Abdelrahman, D.; Adra, A.; Al Harthi, M.; Al Shamli, S.; Alnuaimi, N.; Bensid, L.; Cheaito, M.A.; Emberish, A.M.; et al. Telepsychiatry in the Arab World: A Viewpoint Before and During COVID-19. Neuropsychiatr. Dis. Treat. 2020, 16, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Horsham, C.; Koh, U.; Gillespie, N.; Vagenas, D.; Loescher, L.J.; Curiel-Lewandrowski, C.; Hofmann-Wellenhof, R.; Peter Soyer, H. Evaluating healthcare practitioners’ views on store-and-forward teledermoscopy services for the diagnosis of skin cancer. Digit. Health 2019, 5, 2055207619828225. [Google Scholar] [CrossRef]
- Stephenson, D.; Alexander, R.; Aggarwal, V.; Badawy, R.; Bain, L.; Bhatnagar, R.; Bloem, B.R.; Boroojerdi, B.; Burton, J.; Cedarbaum, J.M.; et al. Precompetitive Consensus Building to Facilitate the Use of Digital Health Technologies to Support Parkinson Disease Drug Development through Regulatory Science. Digit. Biomark. 2020, 4, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. A Showcase of Medical, Therapeutic and Pastime Uses of Virtual Reality (VR) and How (VR) Is Impacting the Dementia Sector. Adv. Exp. Med. Biol. 2019, 1156, 135–141. [Google Scholar]
- Bhavnani, S.P. Digital Health: Opportunities and Challenges to Develop the Next-Generation Technology-Enabled Models of Cardiovascular Care. Methodist DeBakey Cardiovasc. J. 2020, 16, 296–303. [Google Scholar] [CrossRef]
- Gruska, M.; Aigner, G.; Altenberger, J.; Burkart-Küttner, D.; Fiedler, L.; Gwechenberger, M.; Lercher, P.; Martinek, M.; Nürnberg, M.; Pölzl, G.; et al. Recommendations on the utilization of telemedicine in cardiology. Wien. Klin. Wochenschr. 2020, 132, 782–800. [Google Scholar] [CrossRef]
- Horgan, D.; Romao, M.; Morré, S.A.; Kalra, D. Artificial Intelligence: Power for Civilisation–and for Better Healthcare. Public Health Genom. 2019, 22, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Basset, M.; Chang, V.; Nabeeh, N.A. An intelligent framework using disruptive technologies for COVID-19 analysis. Technol. Forecast. Soc. Chang. 2021, 163, 120431. [Google Scholar] [CrossRef]
- Improta, G.; Luca, V.; Illario, M.; Triassi, M. Digital Innovation in Healthcare: A Device with A Method for Monitoring, Managing and Preventing the Risk of Chronic Polypathological Patients. Transl. Med. UniSa 2020, 21, 61–64. [Google Scholar]
- Pesapane, F.; Codari, M.; Sardanelli, F. Artificial intelligence in medical imaging: Threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur. Radiol. Exp. 2018, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Burnside, M.; Crocket, H.; Mayo, M.; Pickering, J.; Tappe, A.; De Bock, M. Do-It-Yourself Automated Insulin Delivery: A Leading Example of the Democratization of Medicine. J. Diabetes Sci. Technol. 2020, 14, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Coiera, E. The Price of Artificial Intelligence. Yearb. Med. Inform. 2019, 28, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Casey, D.; Joshi, I.; Zhu, J.; Cheng, F. Emergence of New Disease: How Can Artificial Intelligence Help? Trends Mol. Med. 2020, 26, 627–629. [Google Scholar] [CrossRef]
- Chehade, M.J.; Yadav, L.; Kopansky-Giles, D.; Merolli, M.; Palmer, E.; Jayatilaka, A.; Slater, H. Innovations to improve access to musculoskeletal care. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101559. [Google Scholar] [CrossRef]
- Lai, L.; Wittbold, K.A.; Dadabhoy, F.Z.; Sato, R.; Landman, A.B.; Schwamm, L.H.; He, S.; Patel, R.; Wei, N.; Zuccotti, G.; et al. Digital triage: Novel strategies for population health management in response to the COVID-19 pandemic. Healthcare 2020, 8, 100493. [Google Scholar] [CrossRef]
- Arora, A. Conceptualising artificial intelligence as a digital healthcare innovation: An introductory review. Med. Devices Evid. Res. 2020, 13, 223–230. [Google Scholar] [CrossRef]
- Bhavnani, S.P.; Sitapati, A.M. Virtual Care 2.0—a Vision for the Future of Data-Driven Technology-Enabled Healthcare. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Alami, H.; Lehoux, P.; Denis, J.L.; Motulsky, A.; Petitgand, C.; Savoldelli, M.; Rouquet, R.; Gagnon, M.P.; Roy, D.; Fortin, J.P. Organizational readiness for artificial intelligence in health care: Insights for decision-making and practice. J. Health Organ. Manag. 2020, 35, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Decary, M. Artificial intelligence in healthcare: An essential guide for health leaders. Healthc. Manag. Forum 2020, 33, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Suehling, M.; Flohr, T.; Comaniciu, D. Artificial Intelligence in Diagnostic Imaging: Status Quo, Challenges, and Future Opportunities. J. Thorac. Imaging 2020, 35 (Suppl. 1), S11–S16. [Google Scholar] [CrossRef]
- Siering, L.; Ludden, G.D.S.; Mader, A.; Van Rees, H. A Theoretical Framework and Conceptual Design for Engaging Children in Therapy at Home—The Design of a Wearable Breathing Trainer. J. Pers. Med. 2019, 9, 27. [Google Scholar] [CrossRef]
- Badawy, R.; Hameed, F.; Bataille, L.; Little, M.A.; Claes, K.; Saria, S.; Cedarbaum, J.M.; Stephenson, D.; Neville, J.; Maetzler, W.; et al. Metadata Concepts for Advancing the Use of Digital Health Technologies in Clinical Research. Digit. Biomark. 2019, 3, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.P.; Wieringa, F.P.; Han, M.; Chaudhuri, S.; Van Der Sande, F.M.; Usvyat, L.A.; Kotanko, P. Wearable health devices and personal area networks: Can they improve outcomes in haemodialysis patients? Nephrol. Dial. Transplant. 2020, 35 (Suppl. 2), II43–II50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Corsini, E.M.; Jin, S.; Barbosa, G.R.; Kell, T.; Antonoff, M.H.; Antonoff, M.B. Advanced Data Analytics for Clinical Research Part I: What are the Tools? Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2020, 15, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Uslu, B.Ç.; Okay, E.; Dursun, E. Analysis of factors affecting IoT-based smart hospital design. J. Cloud Comput. 2020, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, J.; Glasziou, P.; Westbrook, J. The three numbers you need to know about healthcare: The 60–30–10 Challenge. BMC Med. 2020, 18, 102. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Papoutsi, C. Studying complexity in health services research: Desperately seeking an overdue paradigm shift. BMC Med. 2018, 16, 95. [Google Scholar] [CrossRef]
- Kraus, S.; Schiavone, F.; Pluzhnikova, A.; Invernizzi, A.C. Digital transformation in healthcare: Analyzing the current state-of-research. J. Bus. Res. 2021, 123, 557–567. [Google Scholar] [CrossRef]
- National Academies of Sciences Engineering and Medicine. Crossing the Global Quality Chasm: Improving Health Care Worldwide; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Nielsen, P.; Nielsen, R.N.; Bamberger, S.G.; Stamhus, J.; Fonager, K.; Larsen, A.; Vinding, A.L.; Ryom, P.; Omland, O. Capabilities for innovation: The nordic model and employee participation. Nord. J. Work. Life Stud. 2012, 2, 85–115. [Google Scholar] [CrossRef][Green Version]
- Garmann-Johnsen, N.F.; Helmersen, M.; Eikebrokk, T.R. Digital Transformation in Healthcare: Enabling Employee Co-Creation through web 2.0. In Proceedings of the 26th Americas Conference on Information Systems 2018, Digital Disruption, AMCIS, New Orleans, LA, USA, 16 August 2018; Available online: https://www.researchgate.net/publication/328997843_Digital_Transformation_in_Healthcare_Enabling_Employee_Co-Creation_through_Web_20_Completed_Research (accessed on 31 August 2021).
- Hartvigsen, G.; Pedersen, S. Lessons Learned from 25 Years with Telemedicine in Northern Norway, 1st ed.; University Hospital of North Norway: Tromsø, Norway, 2015. [Google Scholar]
- Nonaka, I. Toward Middle-Up-Down Management: Accelerating Information Creation. MIT Sloan Manag. Rev. 1988, 20, 9–18. [Google Scholar]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond adoption: A new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef]
- Weiner, B.J.; Amick, H.; Lee, S.Y.D. Conceptualization and measurement of organizational readiness for change. A review of the literature in health services research and other fields. Med. Care Res. Rev. 2008, 65, 379–436. [Google Scholar] [CrossRef]
- Blease, C.; Kaptchuk, T.J.; Bernstein, M.H.; Mandl, K.D.; Halamka, J.D.; Desroches, C.M. Artificial intelligence and the future of primary care: Exploratory qualitative study of UK general practitioners’ views. J. Med. Internet Res. 2019, 21, e12802. [Google Scholar] [CrossRef] [PubMed]
- Sdělení Komise Evropskému Parlamentu, Radě, Evropskému Hospodářskému a Sociálnímu Výboru a Výboru Regionů. Available online: https://eur-lex.europa.eu/legal-content/CS/TXT/?uri=CELEX%3A52016AE3545 (accessed on 30 August 2021).
- Muffly, M.; Scheinker, D.; Muffly, T.; Singleton, M.; Agarwal, R.; Honkanen, A. Practice Characteristics of Board-certified Pediatric Anesthesiologists in the US: A Nationwide Survey. Cureus 2019, 11, e5745. [Google Scholar] [CrossRef] [PubMed]
- Faddis, A. The digital transformation of healthcare technology management. Biomed. Instrum. Technol. 2018, 52, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Elshaug, A.G.; Rosenthal, M.B.; Lavis, J.N.; Brownlee, S.; Schmidt, H.; Nagpal, S.; Littlejohns, P.; Srivastava, D.; Tunis, S.; Saini, V. Levers for addressing medical underuse and overuse: Achieving high-value health care. Lancet 2017, 390, 191–202. [Google Scholar] [CrossRef]
- Nolte, E.; World Health Organization. How Do We Ensure That Innovation in Health Service Delivery and Organization Is Implemented, Sustained and Spread? Available online: https://apps.who.int/iris/bitstream/handle/10665/331980/Policy-brief-3-1997-8073-2018-eng.pdf?sequence=5&isAllowed=y (accessed on 30 August 2021).
- Hansen, S.; Baroody, A.J. Electronic health records and the logics of care: Complementarity and conflict in the U.S. Healthcare system. Inf. Syst. Res. 2020, 31, 57–75. [Google Scholar] [CrossRef]
- Malhotra, N.K.; Kim, S.S.; Agarwal, J. Internet users’ information privacy concerns (IUIPC): The construct, the scale, and a causal model. Inf. Syst. Res. 2004, 15, 336–355. [Google Scholar] [CrossRef]
- Naidoo, R. Building a critical mass of users for digital healthcare promotion programs: A teaching case. J. Cases Inf. Technol. 2020, 22, 44–59. [Google Scholar] [CrossRef]
- Henke, N.; Ehrbeck, T.; Kibasi, T. Unlocking Productivity through Healthcare Delivery Innovations: Lessons from Entrepreneurs around the World; McKinsey & Company: Chicago, IL, USA, 2010. [Google Scholar]
- Britnell, M. Human: Solving the Global Workforce Crisis in Healthcare; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Greenhalgh, T.; Robert, G.; Macfarlane, F.; Bate, P.; Kyriakidou, O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Q. 2004, 82, 581–629. [Google Scholar] [CrossRef]
- Frick, N.R.J.; Möllmann, H.L.; Mirbabaie, M.; Stieglitz, S. Driving Digital Transformation During a Pandemic: Case Study of Virtual Collaboration in a German Hospital. JMIR Med. Inform. 2021, 9, e25183. [Google Scholar] [CrossRef]
- Allard, J.; Dzwonczyk, R.; Yablok, D.; Block, F.E.; Mcdonald, J.S. Effect of automatic record keeping on vigilance and record keeping time. Br. J. Anaesth. 1995, 74, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Stol, I.S.; Ehrenfeld, J.M.; Epstein, R.H. Technology Diffusion of Anesthesia Information Management Systems into Academic Anesthesia Departments in the United States. Anesth. Analg. 2014, 118, 644–650. [Google Scholar] [CrossRef]
- Bruthans, J. Anesthesia Information Management Systems in the Czech Republic from the Perspective of Early Adopters. J. Med. Syst. 2020, 44, 70. [Google Scholar] [CrossRef] [PubMed]





| Field | Lewin | Kotter | SSM |
|---|---|---|---|
| External expert | maybe | maybe | no |
| Pre-defined problem | yes | yes | no |
| Feedback | yes | yes | yes |
| Sociological approach | positivism | combination | interpretivism |
| Support tools | field theory, action research, group dynamics | “see-feel-change” | CATWOE, PQR, “root definitions” |
| Dismantling existing situation | in step 1 | in step 1 | during the process |
| Cyclical process (constant problem solving) | yes | yes | yes |
| Management/leading coalition | yes | yes | no |
| Recommended step sequence | yes | yes | steps overlap in reality |
| Building awareness why the change is necessary | yes | yes | not necessary, awareness arises spontaneously |
| Adaptability to changing environment | no | partly | yes |
| Suitable application | smaller changes | bigger changes | complex problems |
| CHM approach | top-down | top-down, partly bottom-up | combination of bottom-up and top-down |
| Question No. | Key Research Question |
|---|---|
| 1. | Do the majority of healthcare managers use CHM tools? |
| 2. | What specific CHM method do healthcare managers use the most? |
| 3. | What hospital actors are most involved in change implementation and management? |
| 4. | What changes (of what type) are currently most frequently being implemented in hospitals? |
| 5. | What is the target of the change implementation and what information do mangers provide to those involved in the change? |
| 6. | What do managers think about the implemented change? How do they judge the success/failure of a change? |
| 7. | What opportunities and threats can be identified in the context of digital innovations? |
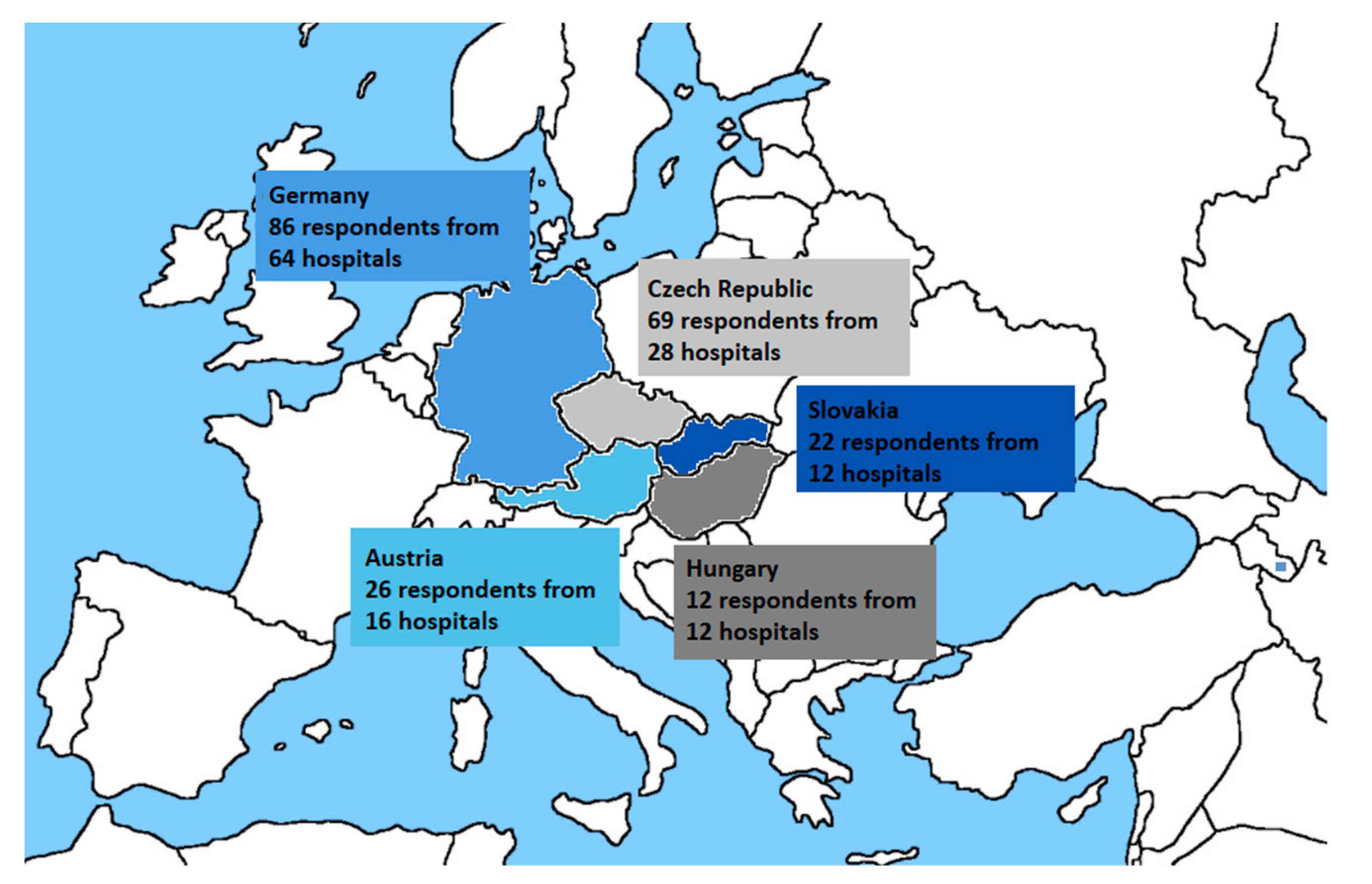
| Country | Population 1 | Beds Per 100,000 Inhabitants (Rounded off) 2 | Number of Hospitals with 500+ Beds 3 | Number of Participating Hospitals |
|---|---|---|---|---|
| Czech Republic | 10,693,861 | 662 | 36 | 28 |
| Germany | 83,135,181 | 800 | 244 | 64 |
| Austria | 8,904,262 | 727 | 26 | 16 |
| Poland 3 | 37,941,122 | 654 | n/a | n/a |
| Hungary | 9,771,975 | 701 | 44 | 12 |
| Slovakia | 5,457,679 | 570 | 18 | 12 |
| In total | 368 | 132 |
| Web of Science | PubMed | Scopus | |
|---|---|---|---|
| Search terms | (((“trend”[Title] OR “evolution”[Title] OR “digital”)[Title]) AND ((“hospital”[Title] OR “healthcare”)[Title])) AND ((“transformation”[Topic] OR “innovation”)[Topic]) | (((“trend”[Title] OR “evolution”[Title] OR “digital”)[Title]) AND ((“hospital”[Title] OR “healthcare”)[Title])) AND ((“transformation”[Title/Abstract] OR “innovation”)[Title/Abstract]) | TITLE (“trend”OR “evolution” OR “digital”) AND Title (“hospital” OR “healthcare”) AND TitleABS (“transformation” OR “innovation”) |
| Time period | 2018–2020 | 2018–2020 (6 December 2020) | 2018–2021 (7 Mar 2021) |
| Languages | English, German | English, German | English, German |
| Document type | Papers, conferences, reviews, editorial material, early access | Papers, conferences, reviews, case studies, clinical trials, systematic literature review, randomized controlled trials | Papers, conferences, reviews |
| Question | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Does the study focus on the use of digital innovations in healthcare? | implementation or use of digital innovation as the main topic; digital innovation in any healthcare intervention with elements of e-Health, virtual reality, smart phones/portable devices, or telemedicine as part of its implementation; aimed at understanding why the innovation is being incorporated into healthcare activities | the main topic focuses on the creation of measures, checklists or other metrics that do not represent a healthcare digitisation intervention; merely a technical description of an innovation; studies focusing on safety, education, or ethical issues related to digital innovations |
| Does the study deal with the framework for a digital innovation implementation? | studies focusing on theoretical mathematical models or statistical models and simulations of | |
| Is the study rooted in the hospital environment? | hospital or clinic environment | other environments (e.g., pharmaceutical companies, medical device manufacturers) |
| Country | Number of Potential Respondents Contacted | Number of Answers | Return Rate |
|---|---|---|---|
| Czech Republic | 279 | 69 | 26% |
| Germany | 1389 | 86 | 7% |
| Austria | 293 | 26 | 10% |
| Slovakia | 78 | 22 | 30% |
| Hungary | 298 | 12 | 4% |
| Total | 2337 | 215 | 11% |
| Top Management | Whole Team (Leadership Coalition) | Middle Management | Quality Managers | Project Managers | HR Dept. | Outsourcing | Other | |
|---|---|---|---|---|---|---|---|---|
| Czech Republic | 70% | 32% | 19% | 33% | 9% | 6% | 1% | 6% |
| Germany | 46% | 71% | 40% | 27% | 31% | 6% | 2% | 1% |
| Austria | 35% | 77% | 31% | 15% | 12% | 4% | 4% | 4% |
| Slovakia | 65% | 17% | 0% | 26% | 4% | 0% | 0% | 9% |
| Hungary | 67% | 33% | 17% | 8% | 8% | 0% | 0% | 0% |
| % (average) | 48% | 51% | 27% | 26% | 17% | 5% | 2% | 4% |
| Absolute number | 104 | 110 | 57 | 56 | 37 | 10 | 4 | 8 |
| Technology | Opportunity | Threat |
|---|---|---|
| mHealth | Wide user basis of mobile phone users [49,50] Rapid growth in the number of applications supporting self-management [51,52,53] Applicable to a wide scope of diagnoses [47,53] Increased patient engagement during treatment [47,52,53,54,55] | Ethical and legal aspects [53,56,57,58] Limited evidence of outcomes and benefits (insufficient randomised controlled trials) [47,52,56,59,60] Low interoperability and integration with existing work procedures [56] Uncertainty concerning data reliability [47,56] Declining patient self-discipline over time [52] Absence of personal contact with physician [55] Non-certified applications, large number of applications [61,62] Level of physician acceptance of mobile health applications [62] |
| Electronic Health Record (EHR), Electronic Medical Record (EMR), Personal Health Record (PHR) | Access to information for all stakeholders [63,64,65,66,67] Benefits if combined with AI [58,65,68] Higher accuracy, legibility, reliability, and better information search functions [64,65,69,70] Risk management—reminders, warnings (allergies, patient history) [64,67,70] Less burden on treating medical staff [36,64] Reduction of cost related to poor documentation [64,65,69] | Violation of the interoperability condition [53,63,70,71] Problem with aligning operating standards with the current information exchange protocols for Big Data [72] Regulatory restraints [72,73,74] The risk of possible re-identification [74] Financial sustainability [75] |
| Digital biomarkers | Wide user base [76] Wide range of information [76] Better diagnostic and decision-making on interventions thanks to continual data collection [58,59] Developing flexible electronic materials for integrating chip technology [77,78] | Bad choice of monitored attributes [59] Problems with technology validation [59] |
| Telemedicine | Lower risk of disease transmission [79,80,81] Suitable for “social distancing” [82] Reduction in hospitalization cost [83,84] Comparable or better care than that of in-person consultations [79,83,85] Elimination of the feeling of isolation during hospitalization [79] Alleviation of resource scarcity (staff, geographical location) [84,86,87,88] Shorter waiting times [60,86] Applicable to numerous diagnoses (e.g., in psychiatry, dermatology, etc.) [60,89,90,91,92] | Limited applicability based on diagnosis [79,85] Unreliable Internet connection [79,85] Lack of training in the use of digital devices [60,79,93] Violation of interoperability between healthcare providers and healthcare systems [94] Discrimination of certain patient groups (e.g., people with particular handicaps) [80] Limited evidence of outcomes and benefits (insufficient randomised controlled trials) [60,80] |
| Artificial intelligence (AI) | Prediction of illness development [94,95,96,97,98] Improvements in treatment optimization and effectiveness [94,97,99,100] Evidence-based recommendations [60,98,101] Delegation of simple and repeating tasks to AI [96] Lower number of hospitalizations [95] Cost cutting [77,95,97] Less pressure on scarce HR in healthcare [102,103] Automatic recall and rescheduling of patients [98] Bigger potential of other digital innovations [68,104] Ability to process huge amounts of data [101] AI-biosensors (miniaturization, scalability, low power consumption, high sensitivity, multifunction, safety, non-toxicity, and degradation) [77] | Incompatible with older infrastructure [105] Lack of understanding of AI functionality [68,106] Inefficient use of AI in day-to-day workflows [107,108] Potential conflict between human ability to act autonomously and the complicated, allegedly infallible machine logic (known as automation bias [69,100] Legal and ethical issues [68,95,100,101,104] Physicians’ concern about AI (security, privacy, and confidentiality) [68,101] Missing multidisciplinary AI teams [98] |
| Wearable technologies | Wide user base [76,77,93] Better diagnostics and decision-making about interventions thanks to continual data collection [76,77,91,106,109] Source of objective data (measured in real-life conditions) [76,91,110] Reduction of “unnecessary” out-patient visits [94] 4P medicine (predictive, precise, preventative and personalized) [76,77,111] | Data smog [76,91] Standardization and validation issues with sensor placement [91] Energy consumption (limited battery capacity) [49,84] Different levels of digital literacy and/or aproach to technologies among patients [47,91] Declining patient self-discipline over time [91] Limited availability due to high production costs of some technologies [77] |
| Internet of Things (IoT) | Higher operational efficiency [49,112] Integration of data from various sources [49,112] Disease prevention and monitoring [49,113] Use of AI in analyses [49,65,113] | Loss of safe and stable communication with devices [84] Higher demands on network infrastructure [49] Unauthorised manipulation [49,112] There are currently no clear instructions for healthcare staff how to use IoT (e.g., in recommendations to patients concerning their use) [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hospodková, P.; Berežná, J.; Barták, M.; Rogalewicz, V.; Severová, L.; Svoboda, R. Change Management and Digital Innovations in Hospitals of Five European Countries. Healthcare 2021, 9, 1508. https://doi.org/10.3390/healthcare9111508
Hospodková P, Berežná J, Barták M, Rogalewicz V, Severová L, Svoboda R. Change Management and Digital Innovations in Hospitals of Five European Countries. Healthcare. 2021; 9(11):1508. https://doi.org/10.3390/healthcare9111508
Chicago/Turabian StyleHospodková, Petra, Jana Berežná, Miroslav Barták, Vladimír Rogalewicz, Lucie Severová, and Roman Svoboda. 2021. "Change Management and Digital Innovations in Hospitals of Five European Countries" Healthcare 9, no. 11: 1508. https://doi.org/10.3390/healthcare9111508
APA StyleHospodková, P., Berežná, J., Barták, M., Rogalewicz, V., Severová, L., & Svoboda, R. (2021). Change Management and Digital Innovations in Hospitals of Five European Countries. Healthcare, 9(11), 1508. https://doi.org/10.3390/healthcare9111508






