Experiences of Using a Continuous Glucose Monitoring System in Children—A Descriptive Study with Parents in the Republic of Georgia
Abstract
:1. Background
2. Materials and Method
2.1. Design and Informants
2.2. Data Collection
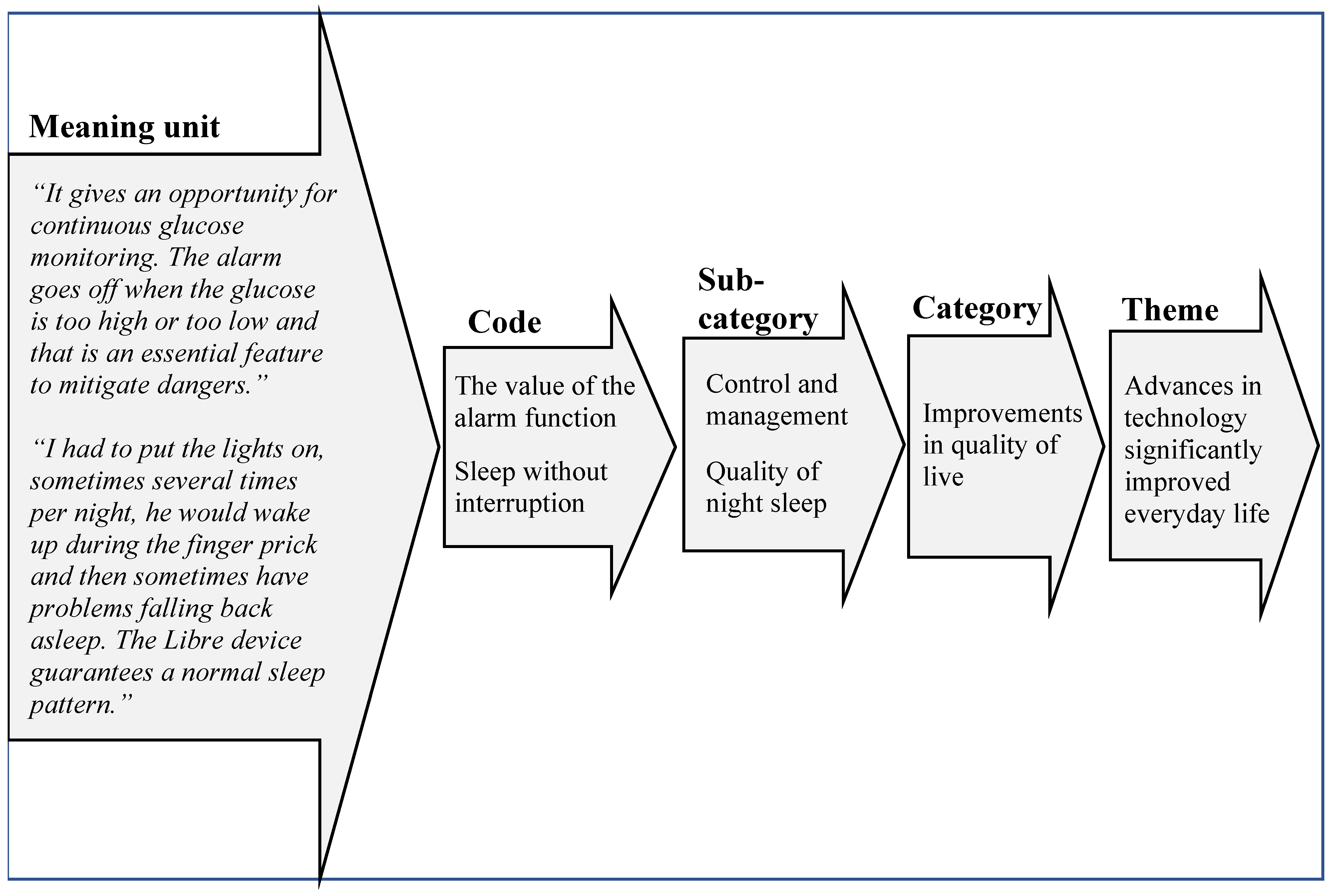
2.3. Analysis
3. Results
3.1. Descriptive Findings
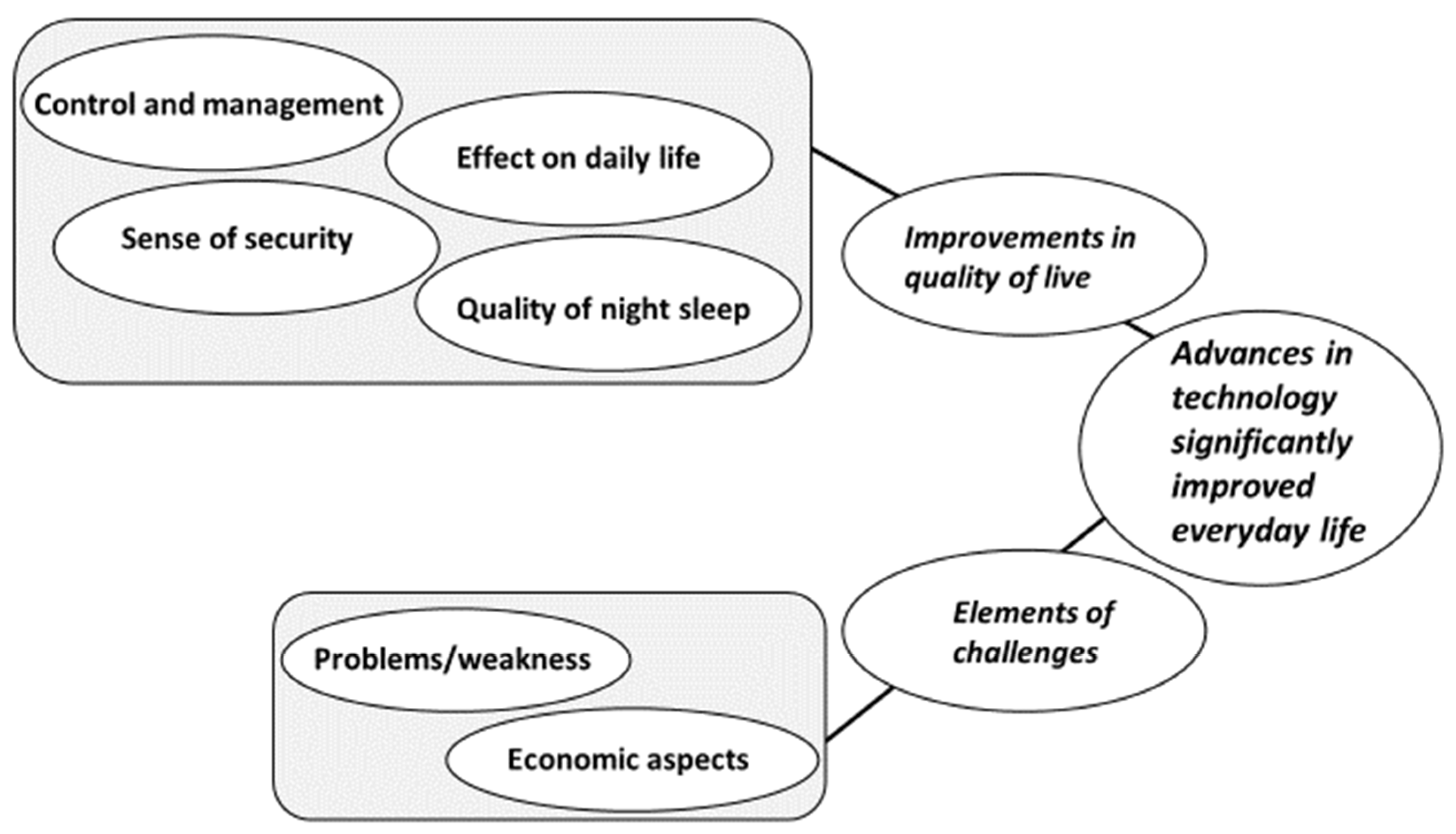
3.2. Qualitative Findings
3.2.1. Improvements in Quality of Life
Control and Management
“It’s a huge comfort for us and our child is so relieved. Her fingertips even become softer. I am now able to understand the condition better, things that I didn’t understand before, and realise they are becoming much clearer.”(R13)
“Very important. I was afraid to let her go to school without it. I was thinking I had to quit work, so I could be close to her school all the time before we got to use the Libre. She feels kind of cool with a fancy device.”(R10)
Effect on Daily Life
“Of course. Before the Libre she would not consider what she was eating and she would not restrict sweets. Now, when she can see what happens with her blood glucose when she eats something unsuitable. I think it helps her with her food choices and overall understanding of her condition”.(R9)
“We have info about the trends and if it tends to go up or down. It’s super useful especially when my son plays rugby”.(R6)
“Due to the pandemic there is no school, so I can only imagine it will be a great help”.(R4)
“Most people don’t know what it is, my kid loves to explain to them”.(R7)
Sense of Security
“It’s absolutely a must-have item. We can only let her attend day-care with this device”.(R12)
“When our child had an acute viral infection, he had high blood glucose levels and the Libre device was essential for us to determine the insulin dosage”.(R1)
“The first time I had some help from someone who had experience, after that, I was able to do it easily”.(R13)
Quality of Night Sleep
“We sleep much, much better. We wake up if the alarm goes off to take appropriate measures”.(R1)
3.2.2. Elements of Challenges
Problems/Weakness
“My son is an active rugby player and even though we try to attach it with extra measures it often gets removed during exercise or games”.(R6)
“I was quite worried that she may have some allergic reaction to the tape or sensor, but she has had zero discomforts so far. She has a history with allergies”.(R3)
“I had a case when four sensors were damaged, and the manufacturer replaced all four of them”.(R8)
Economic Aspects
“For Georgians, it is quite expensive and not many families can afford it. I am absolutely sure that, at least at first, and especially for smaller children, it is essential to use the Libre. It is also becoming difficult to purchase the Libre; our friend buys it for us in Germany and sends it by mail or if someone is travelling to Georgia they bring it for us. We also try to have a supply at home”.(R1)
4. Discussion
Methodical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Included Questions in Used Questionnaire Survey
| 1. What does the Libre device mean to your child and your family? |
| 2. What feature of the Libre device is most useful for you and your child? |
| 3. How do you perceive the device importance for your child at school/kindergarten? |
| 4. Have your child’s medical parameters (mostly HbA1c) improved or changed in any way? |
| 5. Do you feel safer when your child uses the Libre device (hyper-/hypo-glycaemia)? |
| 6. How much do you think the use of Libre device helps you/your child with decision making for proper insulin dosing? Eating? Physical activity? |
| 7. What is your experience according to the glucose control when stress situations and acute illnesses occur (using the Libre device)? |
| 8. How do you think the use of the Libre device effects your child’s coping to the disease? |
| 9. How do you think the device has affected/facilitate your child’s hobbies and favorite activities? |
| 10. What are your thoughts about the otherwise society’s perception (in general), when your child are using the Libre device? |
| 11. In what way affect the Libre device personal hygiene and self-care? |
| 12. If you have had device malfunction episodes, could you please describe them? |
| 13. Have you found the alarms for high/low useful? |
| 14. What features do you think are lacking? |
| 15. When I say costs for the Libre device, what comes to your mind? |
| 16. As a parent, estimate your total satisfaction with the Libre device using a Likert scale 1–10 (1 = not at all satisfied-10 = totally satisfied). |
References
- International Diabetes Federation. IDF Diabetes Atlas. 2019. Available online: https://www.diabetesatlas.org/en (accessed on 30 August 2021).
- WHO. Diabetes—Fact-Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 30 August 2021).
- Patterson, C.; Guariguata, L.; Dahlquist, G.; Soltesz, G.; Ogle, G.; Silink, M. Diabetes in young—A global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res. Clin. Pract. 2014, 103, 161–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Managing Diabetes in Preschool Children. ISPAD Guidelines Pediatric Diabetes (2017). ISPAD Clinical Practice Consensus Guidelines 2018—International Society for Pediatric and Adolescent Diabetes. Available online: https://www.ispad.org/page/ISPADGuidelines2018 (accessed on 30 August 2021).
- Mastrototaro, J. The MiniMed Continuous Glucose Monitoring System (CGMS). J. Pediatr. Endocrinol. Metab. 1999, 12, 751–758. [Google Scholar] [PubMed]
- Leelarathna, L.; Wilmot, E.G. Flash forward: A review of flash glucose monitoring. Diabet. Med. 2018, 35, 472–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidonde, J.; Fagerlund, B.C.; Frønsdal, K.B.; Højslev Lund, U.; Robberstad, B. FreeStyle Libre Flash Glucose Self-Monitoring System: A Single-Technology Assessment. Available online: https://pubmed.ncbi.nlm.nih.gov/29553668/ (accessed on 30 August 2021).
- Richardson, E.; Berdzuli, N.; World Health Organization. Georgia: Health system review. Health Syst. Transit. 2017, 19, 1–90. [Google Scholar] [PubMed]
- American Academy of Pediatrics. Patient- and family-centered care and the pediatrician’s role. Pediatrics 2012, 129, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. SRQR, Standards for Reporting Qualitative Research: A Synthesis of Recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Etikan, I.; Babtope, O. A basic approach in sampling methodology and sample size calculation. Med. Life Clin. 2019, 1, 1006. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesén, E.; Björholt, I.; Ingelgård AOlson, F.J. Exploration and preferential ranking of patient benefits of medical devices: A new and generic instrument for health economic assessments. Int. J. Technol. Assess. Health Care. 2017, 33, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Graneheim, U.H.; Lundman, B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Educ. Today 2004, 24, 105–112. [Google Scholar] [CrossRef]
- Perez, L.; Romo, L.K.; Bell, T. Communicatively exploring uncertainty of parents of children with type 1 diabetes. Health Commun. 2019, 34, 949–957. [Google Scholar] [CrossRef]
- Nurmi, M.A.; Stieber-Roger, K. Parenting children living with type 1 diabetes: A qualitative study. Diabetes Educ. 2012, 384, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Streisand, R.; Monaghan, M. Young Children with type 1 diabetes: Challenges, research, and future directions. Curr. Diabetes Rep. 2014, 14, 520. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr. Diabetes 2018, 19, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarnblad, S.; Berg, L.; Detlofsson, I.; Jonsson, A.; Forsander, G. Diabetes management in Swedish schools: A national survey of attitudes of parents, children, and diabetes teams. Pediatr. Diabetes 2014, 15, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.; Lloyd, C.E.; Gosden, C.; Macdougall, C.; Brown, N.; Matyka, K. The concerns of school staff in caring for children with diabetes in primary school. Pediatr. Diabetes 2012, 13, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Burgmann, J.; Biester, T.; Grothaus, J.; Kordonouri, O.; Ott, H. Pediatric diabetes and skin disease (PeDiSkin): A cross-sectional study in 369 children, adolescents and young adults with type 1 diabetes. Pediatric Diabetes 2020, 21, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.T.; Zachariae, C.; Christensen, K.B.; Svensson, J.; Berg, A.K. Five-month follow-up shows no improvement in dermatological complications in children with type 1 diabetes using continuous glucose monitoring systems and insulin pumps. J. Diabetes Sci. Technol. 2021, 15, 317–323. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Webbsite: Skin Complications | ADA. Available online: Diabetes.org (accessed on 5 August 2021).
- Farsani, S.F.; Souverein, P.C.; van der Vorst, M.M.; Knibbe, C.A.; de Boer AMantel-Teeuwisse, A.K. Chronic comorbidities in children with type 1 diabetes: A population-based cohort study. Arch. Dis. Child. 2015, 100, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bastida, J.; López-Siguero, J.P.; Oliva-Moreno, J.; Perez-Nieves Villoro, R.; Dilla, T.; Vázquez, L.A. Social economic costs of type 1 diabetes mellitus in pediatric patients in Spain: CHRYSTAL observational study. Diabetes Res. Clin. Pract. 2017, 127, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parents N = 20 | Children N = 20 | Length of Diabetes Disease & Libre Experience (Month) | ||
|---|---|---|---|---|
| (n/%) | (n/%) | Length of diabetes disease | ||
| Men | 3 (15) | Boys | 9 (45) | (median (IQR)) 17 (6–62.25) |
| Women | 17 (85) | Girls | 11 (55) | (median-year & month) 1 year 5 month |
| min-max 4–96 | ||||
| Age (year) | Age (year, (n/%)) | Libre experience | ||
| mean ± SD | 38 ± 4 | 0–3 | 2 (10) | (median (IQR)) 7 (3.25–30) |
| min–max | 30–45 | 4–7 | 7 (35) | min–max 1–72 |
| 8–12 | 7 (35) | |||
| 12–16 | 4 (20) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheladze, N.; Kristensson, L.; Johansson, A.; Crang-Svalenius, E.; Ivarsson, B. Experiences of Using a Continuous Glucose Monitoring System in Children—A Descriptive Study with Parents in the Republic of Georgia. Healthcare 2021, 9, 1556. https://doi.org/10.3390/healthcare9111556
Kheladze N, Kristensson L, Johansson A, Crang-Svalenius E, Ivarsson B. Experiences of Using a Continuous Glucose Monitoring System in Children—A Descriptive Study with Parents in the Republic of Georgia. Healthcare. 2021; 9(11):1556. https://doi.org/10.3390/healthcare9111556
Chicago/Turabian StyleKheladze, Nino, Lars Kristensson, Anders Johansson, Elizabeth Crang-Svalenius, and Bodil Ivarsson. 2021. "Experiences of Using a Continuous Glucose Monitoring System in Children—A Descriptive Study with Parents in the Republic of Georgia" Healthcare 9, no. 11: 1556. https://doi.org/10.3390/healthcare9111556
APA StyleKheladze, N., Kristensson, L., Johansson, A., Crang-Svalenius, E., & Ivarsson, B. (2021). Experiences of Using a Continuous Glucose Monitoring System in Children—A Descriptive Study with Parents in the Republic of Georgia. Healthcare, 9(11), 1556. https://doi.org/10.3390/healthcare9111556






