How Fear of COVID-19 Can Affect Treatment Choices for Anaplastic Large Cell Lymphomas ALK+ Therapy: A Case Report
Abstract
1. Introduction
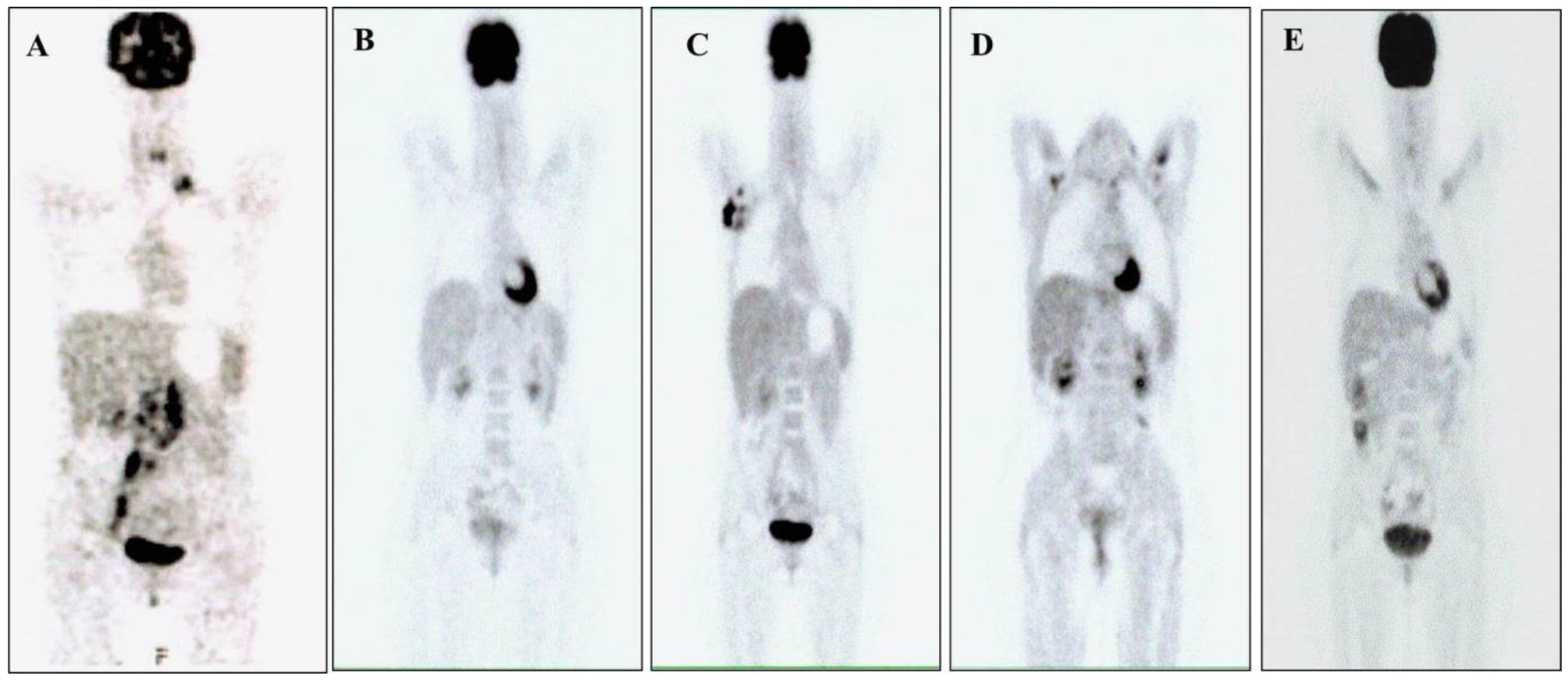
2. Case Report
3. Statement of Ethics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sica, A.; Vitiello, P.; Ronchi, A.; Casale, B.; Calogero, A.; Sagnelli, E.; Nachtigal, G.C.; Troiani, T.; Franco, R.; Argenziano, G.; et al. Primary Cutaneous Anaplastic Large Cell Lymphoma (pcALCL) in the elderly and the importance of sport activity training. Int. J. Environ. Res. Public Health 2020, 17, 839. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; D’Andrilli, A.; Carlucci, A.; Vicidomini, G.; Loizzi, D.; Ardò, N.P.; Marasco, R.D.; Ventura, L.; Ampollini, L.; Carbognani, P.; et al. Prognostic factors of lung cancer in lymphoma survivors (the LuCiLyS study). Transl. Lung Cancer Res. 2020, 9, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Vitiello, P.; Sorriento, A.; Ronchi, A.; Calogero, A.; Sagnelli, C.; Troiani, T.; Fasano, M.; Dodaro, C.A.; Franco, R.; et al. Lymphomatoid papulosis. Minerva Med. 2020, 111, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Caccavale, S.; Vitiello, P.; Franco, R.; Panarese, I.; Ronchi, A.; Sica, A.; Toncic, R.J.; Alfano, R.; Argenziano, G. Dermoscopic characterization of folliculotropic mycosis fungoides selectively localized on trunk and limbs. Int. J. Dermatol. 2019, 58, e187–e189. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Casale, B.; Spada, A.; Di Dato, M.T.; Sagnelli, C.; Calogero, A.; Buonavolontà, P.; Salzano, A.; Martinelli, E.; Saracco, E.; et al. Differential diagnosis: Retroperitoneal fibrosis and oncological diseases. Open Med. 2019, 15, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Cascone, R.; Sica, A.; Sagnelli, C.; Carlucci, A.; Calogero, A.; Santini, M.; Fiorelli, A. Endoscopic treatment and pulmonary rehabilitation for management of lung abscess in elderly lymphoma patients. Int. J. Environ. Res. Public Health 2020, 17, 997. [Google Scholar] [CrossRef]
- Sica, A.; Casale, D.; Rossi, G.; Casale, B.; Ciccozzi, M.; Fasano, M.; Ciotti, M.; Sagnelli, E.; Papa, A.; Sagnelli, C. The impact of the SARS-CoV-2 infection, with special reference to the hematological setting. J. Med. Virol. 2021, 93, 223–233. [Google Scholar] [CrossRef]
- Passerini, C.G.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase–positive lymphoma patients. J. Natl. Cancer Inst. 2014, 106, djt378. [Google Scholar] [CrossRef]
- Calogero, A.; Sagnelli, C.; Carlomagno, N.; Tammaro, V.; Candida, M.; Vernillo, A.; Peluso, G.; Minieri, G.; Sica, A.; Ciccozzi, M.; et al. Familial polyposis coli: The management of desmoid tumor bleeding. Open Med. 2019, 14, 572–576. [Google Scholar] [CrossRef]
- Tsuyama, N.; Sakamoto, K.; Sakata, S.; Dobashi, A.; Takeuchi, K. Anaplastic large cell lymphoma: Pathology, genetics, and clinical aspects. J. Clin. Exp. Hematop. 2017, 57, 120–142. [Google Scholar] [CrossRef]
- Hallberg, B.; Palmer, R.H. The role of the ALK receptor in cancer biology. Ann. Oncol. 2016, 27, iii4–iii15. [Google Scholar] [CrossRef] [PubMed]
- Childress, M.A.; Himmelberg, S.M.; Chen, H.; Deng, W.; Davies, M.A.; Lovly, C.M. ALK fusion partners impact response to ALK inhibition: Differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol. Cancer Res. 2018, 16, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Fukano, R.; Mori, T.; Sekimizu, M.; Choi, I.; Kada, A.; Saito, A.M.; Asada, R.; Takeuchi, K.; Terauchi, T.; Tateishi, U.; et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: An open-label phase II trial. Cancer Sci. 2020, 111, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P.; Voss, S.D.; Lim, M.S.; Rolland, D.; Minard, C.G.; Fox, E.; Adamson, P.; Wilner, K.; Blaney, S.M.; Weigel, B.J. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A children’s oncology group study. J. Clin. Oncol. 2017, 35, 3215–3221. [Google Scholar] [CrossRef]
- Stucklin, A.S.G.; Ryall, S.; Fukuoka, K.; Zapotocky, M.; Lassaletta, A.; Li, C.; Bridge, T.; Kim, B.; Arnoldo, A.; Kowalski, P.E.; et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Risum, S.; Knigge, U.; Langer, S.W. Hitherto unseen survival in an ALK-positive-patient with advanced stage adult ganglioneuroblastoma treated with personalized medicine. Clin. Case Rep. 2017, 5, 2085–2087. [Google Scholar] [CrossRef]
- Sica, A.; Casale, B.; Di Dato, M.T.; Calogero, A.; Spada, A.; Sagnelli, C.; Santagata, M.; Buonavolontà, P.; Fiorelli, A.; Salzano, A.; et al. Cancer- and non-cancer related chronic pain: From the physiopathological basics to management. Open Med. 2019, 14, 761–766. [Google Scholar] [CrossRef]
- Sica, A.; Casale, B.; Sagnelli, C.; Di Dato, M.T.; Buonavolontà, P.; Salzano, A.M.; Sagnelli, E.; Famiglietti, V.; Saracco, E.; Tammaro, D.; et al. All-in-one spinal cord stimulation in lymphoproliferative diseases. Front. Neurol. 2020, 11, 550554. [Google Scholar] [CrossRef]
- Merli, M.; Frigeni, M.; Alric, L.; Visco, C.; Besson, C.; Mannelli, L.; Di Rocco, A.; Ferrari, A.; Farina, L.; Pirisi, M.; et al. Direct-Acting Antivirals in Hepatitis C Virus-Associated Diffuse Large B-cell Lymphomas. Oncologist 2019, 24, e720–e729. [Google Scholar] [CrossRef]
- Gravina, A.G.; Federico, A.; Sica, A.; D’Armiento, F.P.; Ferrara, M.G.; Falcone, U.; Dallio, M.; Cozzolino, D.; Guastafierro, S.; Loguercio, C.; et al. Secondary Extramedullary Plasmacytoma of the Duodenum: An Unusual Endoscopic Presentation. Gastroenterol. Res. 2013, 6, 110–111. [Google Scholar] [CrossRef][Green Version]
- Guastafierro, S.; Sica, A.; Parascandola, R.R.; Ferrara, M.G.; Di Martino, A.; Pezone, L.; Falcone, U. Clinical significance of serum triple monoclonal components: A report of 6 cases and a review of the literature. Leuk. Res. 2014, 38, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; DeFrancesco, I.; Visco, C.; Besson, C.; Di Rocco, A.; Arcari, A.; Sica, A.; Cencini, E.; Tisi, M.C.; Frigeni, M.; et al. Direct-acting antivirals in relapsed or refractory hepatitis C virus-associated diffuse large B-cell lymphoma. Leuk. Lymphoma 2020, 61, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Tonziello, G.; Pisaturo, M.; Sica, A.; Ferrara, M.G.; Sagnelli, C.; Pasquale, G.; Sagnelli, E.; Guastafierro, S.; Coppola, N. Transient reactivation of occult hepatitis B virus infection despite lamivudine prophylaxis in a patient treated for non-Hodgkin lymphoma. Infection 2012, 41, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Guastafierro, S.; Ferrara, M.G.; Sica, A.; Parascandola, R.R.; Santangelo, S.; Falcone, U. Serum double monoclonal components and hematological malignancies: Only a casual association? Review of 34 cases. Leuk. Res. 2012, 36, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Pisaturo, M.; Guastafierro, S.; Filippini, P.; Tonziello, G.; Sica, A.; Di Martino, F.; Sagnelli, C.; Ferrara, M.G.; Martini, S.; Cozzolino, D.; et al. Absence of occult HCV infection in patients experiencing an immunodepression condition. Infez. Med. 2013, 21, 296–301. [Google Scholar]
- Coppola, N.; Pisaturo, M.; Guastafierro, S.; Tonziello, G.; Sica, A.; Sagnelli, C.; Ferrara, M.G.; Sagnelli, E. Absence of occult hepatitis C virus infection in patients under immunosupressive therapy for oncohematological diseases. Hepatology 2011, 54, 1487–1489. [Google Scholar] [CrossRef]
- Coppola, N.; Pisaturo, M.; Guastafierro, S.; Tonziello, G.; Sica, A.; Iodice, V.; Sagnelli, C.; Ferrara, M.G.; Sagnelli, E. Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig. Liver Dis. 2012, 44, 49–54. [Google Scholar] [CrossRef]
- Bagaglio, S.; Uberti-Foppa, C.; Sagnelli, C.; Lai, A.; Hasson, H.; Salpietro, S.; Messina, E.; Morsica, G.; Zaffina, C.; Sica, A.; et al. HIV-1 recombinant forms in immigrants regularly residing in Milan, northern Italy. Infection 2020, 48, 553–558. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Grillo, C.M.; Scandurra, G.; Torino, F.; Libra, M. Cancer management during COVID-19 pandemic: Is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Al-Quteimat, O.M.; Amer, A.M. The impact of the COVID-19 pandemic on cancer patients. Am. J. Clin. Oncol. 2020, 43, 452–455. [Google Scholar] [CrossRef]
- Sica, A.; Vitiello, P.; Papa, A.; Calogero, A.; Sagnelli, C.; Casale, D.; Mottola, M.; Svanera, G.; Dodaro, C.A.; Martinelli, E.; et al. Use of rituximab in NHL malt type pregnant in I° trimester for two times. Open Med. 2019, 14, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Falcone, U.; Parascandola, R.R.; Ferrara, M.G.; Sica, A.; Di Martino, A.; Guastafierro, S. Bilateral lacrimal gland involvement with mantle cell lymphoma. Am. J. Med Sci. 2015, 349, 268. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.; Sica, A.; et al. A preliminary study for quantitative assessment with HFUS (High- Frequency Ultrasound) of nodular skin melanoma breslow thickness in adults before surgery: Interdisciplinary team experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Vitiello, P.; Caccavale, S.; Sagnelli, C.; Calogero, A.; Dodaro, C.A.; Pastore, F.; Ciardiello, F.; Argenziano, G.; Reginelli, A.; et al. Primary cutaneous DLBCL non-GCB type: Challenges of a rare case. Open Med. 2020, 15, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Sagnelli, C.; Papa, A.; Ciccozzi, M.; Sagnelli, E.; Calogero, A.; Martinelli, E.; Casale, B. An anecdotal case report of chronic lymphatic leukemia with del(11q) treated with ibrutinib: Artificial nourishment and physical activity program. Int. J. Environ. Res. Public Health 2020, 17, 1929. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, P.; Sica, A.; Ronchi, A.; Caccavale, S.; Franco, R.; Argenziano, G. Primary cutaneous b-cell lymphomas: An update. Front. Oncol. 2020, 10, 651. [Google Scholar] [CrossRef]
- Ronchi, A.; Marino, F.Z.; Vitiello, P.; Caccavale, S.; Argenziano, G.; Crisci, S.; Franco, R.; Sica, A. A case of primary cutaneous B-cell lymphoma with immature features in an old man. Diffuse Large B-cell Lymhpoma with immature features or B-cell Lymhpoblastic Lymphoma? J. Cutan. Pathol. 2020. [Google Scholar] [CrossRef]
- Visco, C.; Di Rocco, A.; Evangelista, A.; Quaglia, F.M.; Tisi, M.C.; Morello, L.; Zilioli, V.R.; Rusconi, C.; Hohaus, S.; Sciarra, R.; et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: Results from the MANTLE-FIRST study. Leukemia 2020, 1–9. [Google Scholar] [CrossRef]
- Reginelli, A.; Urraro, F.; Sangiovanni, A.; Russo, G.M.; Russo, C.; Grassi, R.; Agostini, A.; Belfiore, M.P.; Cellina, M.; Floridi, C.; et al. Extranodal lymphomas: A pictorial review for CT and MRI classification. Acta Bio-med. 2020, 91, 34–42. [Google Scholar] [CrossRef]
- Dumont, D.; Dô, P.; Lerouge, D.; Planchard, G.; Riffet, M.; Dubos-Arvis, C.; Danhier, S.; Gervais, R. Off-label use of crizotinib as a neoadjuvant treatment for a young patient when conventional chemotherapy gave no benefits in stage IIIA non-small cell lung cancer. Am. J. Case Rep. 2017, 18, 890–893. [Google Scholar] [CrossRef][Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guo, L.; Zhang, H.; Shao, W. Crizotinib as a personalized alternative for targeted anaplastic lymphoma kinase rearrangement in previously treated patients with non-small-cell lung cancer. Drug Des. Dev. Ther. 2015, 9, 5491–5497. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Gao, F.; Wang, H.-J.; Ma, F. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: A meta-analysis of clinical trials. BMC Cancer 2014, 14, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viscardi, G.; Zanaletti, N.; Ferrara, M.G.; Sica, A.; Falcone, U.; Guastafierro, S.; Bracale, U.; Ribero, D.; Fasano, M.; Napolitano, S.; et al. Atypical haemolytic-uraemic syndrome in patient with metastatic colorectal cancer treated with fluorouracil and oxaliplatin: A case report and a review of literature. ESMO Open 2019, 4, e000551. [Google Scholar] [CrossRef] [PubMed]
- Capece, M.; Creta, M.; Calogero, A.; La Rocca, R.; Napolitano, L.; Barone, B.; Sica, A.; Fusco, F.; Santangelo, M.L.; Dodaro, C.; et al. Does physical activity regulate prostate carcinogenesis and prostate cancer outcomes? A narrative review. Int. J. Environ. Res. Public Health 2020, 17, 1441. [Google Scholar] [CrossRef]
| Period | Therapy |
|---|---|
| From October 2016 to February 2017 | CEOP: 6 cycles |
| From April 2017 to August 2017 | MTX-ARAC-THIOthepa: 4 cycles plus 6 rachicentesis with infusion of MTX, ARAC and steroids |
| From October 2017 to November 2017 | ASCT after HDT with Thio-Bu |
| From July 2019 to June 2020 | Brentuximab: 16 cycles |
| From July 2020 to present | Crizotinib: 250 mg, twice daily |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sica, A.; Sagnelli, C.; Casale, B.; Svanera, G.; Creta, M.; Calogero, A.; Franco, R.; Sagnelli, E.; Ronchi, A. How Fear of COVID-19 Can Affect Treatment Choices for Anaplastic Large Cell Lymphomas ALK+ Therapy: A Case Report. Healthcare 2021, 9, 135. https://doi.org/10.3390/healthcare9020135
Sica A, Sagnelli C, Casale B, Svanera G, Creta M, Calogero A, Franco R, Sagnelli E, Ronchi A. How Fear of COVID-19 Can Affect Treatment Choices for Anaplastic Large Cell Lymphomas ALK+ Therapy: A Case Report. Healthcare. 2021; 9(2):135. https://doi.org/10.3390/healthcare9020135
Chicago/Turabian StyleSica, Antonello, Caterina Sagnelli, Beniamino Casale, Gino Svanera, Massimiliano Creta, Armando Calogero, Renato Franco, Evangelista Sagnelli, and Andrea Ronchi. 2021. "How Fear of COVID-19 Can Affect Treatment Choices for Anaplastic Large Cell Lymphomas ALK+ Therapy: A Case Report" Healthcare 9, no. 2: 135. https://doi.org/10.3390/healthcare9020135
APA StyleSica, A., Sagnelli, C., Casale, B., Svanera, G., Creta, M., Calogero, A., Franco, R., Sagnelli, E., & Ronchi, A. (2021). How Fear of COVID-19 Can Affect Treatment Choices for Anaplastic Large Cell Lymphomas ALK+ Therapy: A Case Report. Healthcare, 9(2), 135. https://doi.org/10.3390/healthcare9020135








