Echocardiographic Screening of Anomalous Origin of Coronary Arteries in Athletes with a Focus on High Take-Off
Abstract
:1. Introduction
2. Methods
Search Strategy
3. Results
3.1. Literature Search and Demographic Characteristics
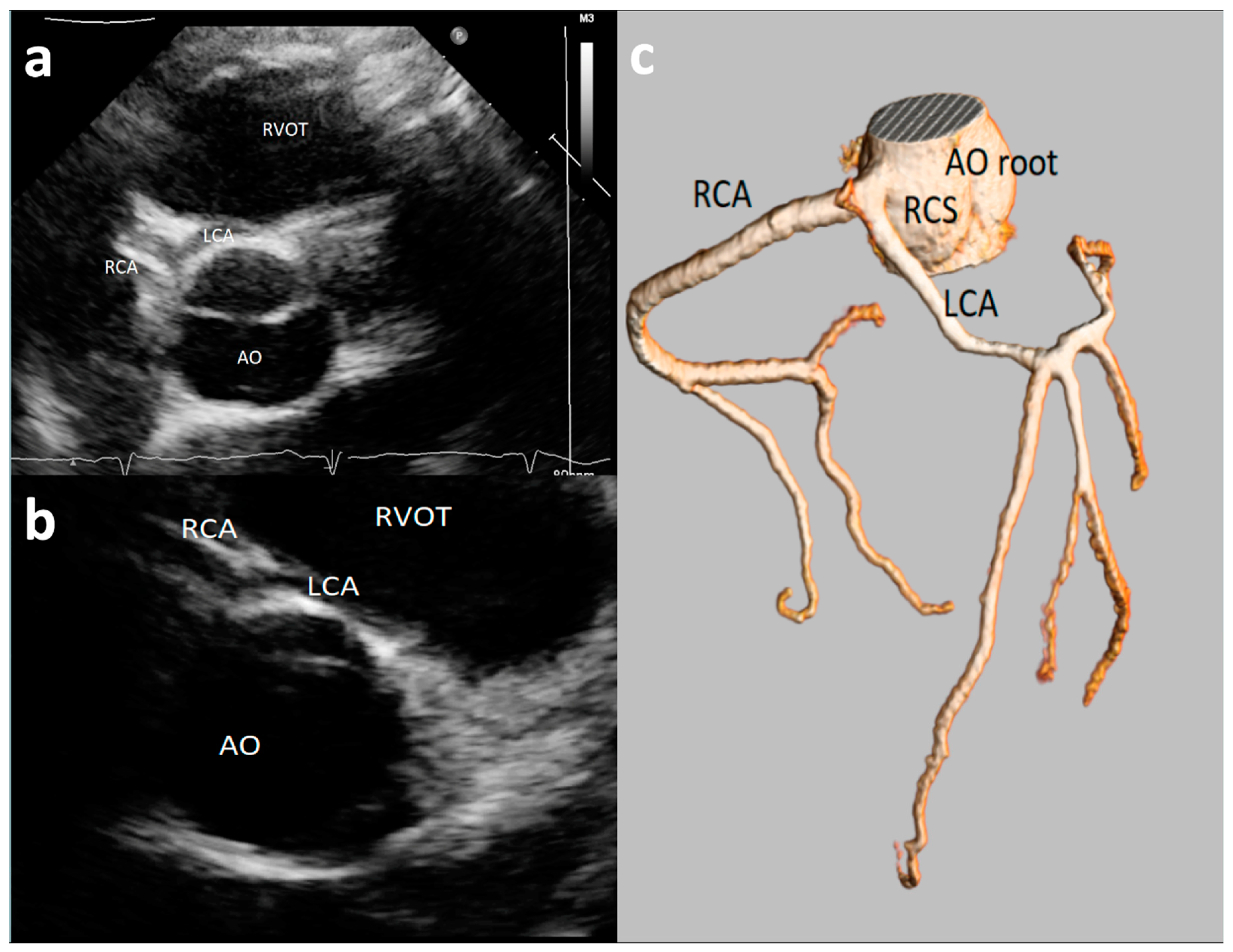
3.2. Feasibility of Visualizing Coronary Arteries’ Origins
3.3. Echocardiographic Views Employed to Visualize Origin of Coronary Artery
3.4. Detection Rate of Major and Minor AAOCAs by Echocardiography
3.5. Symptoms, ECG, Stress Testing, and Clinical Management
3.6. Coronary Artery High Take-Off
4. Discussion
5. Limitations
6. Conclusions
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author | Sample Size and Age | Anomaly/Course | Reason for Referral | ECG | Stress Test | Management |
|---|---|---|---|---|---|---|
| Zeppilli [20], 1998, Italy | 3,650 (30 ± 12 years) Athletes | RCA from LSV (n°2):1 screening, 1 fatigue during effort: LCA from RSV (n°1): LBB at stress test | All normal | RCA from LSV positive and 1 negative myocardial perfusion scintigraphy. Positive had left-axis deviation at ECG stress test. 1 LCA from RSV: positive scintigraphy | No (old study) All disqualified from competition | |
| Gerling [13], 2018, Germany | 1,045 (12-15 years) Athletes | RCA high take-off (n°14), interarterial course (n°2) | Screening all Asymptomatic | All negative | All negative stress test | No sport restriction |
| Labombarda, [21], 2013, France | 350 (84% adult,16% children) Athletes | LCA from RSV; interarterial (n°1) RCA from RSV; interarterial (n°8) | LCA from RSV, Cardiac arrest (n°1) SC (n°3), 2 acute coronary syndrome, 1 asympt. RCA from LSV (n°8) 4 asympt, 1 stroke, 1chest pain, 2 dyspnea | LCA from RSV (n°1): Normal SC (n°3):1 T wave inversion, 2 normal RCA from LSV (n°8): 3 normal, 1 LVH, T wave inversion (V5, V6), 1 Q wave (V2, V3),1 ST changes (V5-v6),1 AF | LCA from RSV A (n°1): not done (urgency) SC (n°3): 1 positive (myocardial perfusion scintigraphy), 1 normal, 1 not done. RCA from LSV (n°8): 1 positive (myocardial perfusion scintigraphy, 3 negatives (scintigraphy), 4 not done | Surgery: 1 LCA from RSV, 1 SC,3 ARCA Conservative management: all the rest. No indication on sport |
| Lytrivi [23], 2008, USA | 14546 General pediatric population | LCA from RSV (n°6) 3 interarterial, 1 retro-aortic, 1 LAD anterior, Cx posterior RCA from LSV (n°24) 1 intramural, 1 anterior, 22 interarterial | LCA from RSV (n°6): 1 chest pain, 1 murmur, 2VSD, 2 pre-liver tx RCA from LSV (n°24): 2 chest pain, 2 tachycardia, 3 suspected coronary anomalies on echo, 2 VSD, 2 PDA. 1 AS, 1 ASD, 1 Down syndrome, 3 murmur, 1 abnormal ECG, 1 metabolic syndrome | LCA from RSV (n°6): 3 normal, 1 LVH, 2 RVH RCA from LSV (n°24): 17 normal. 2 RVH, 2 BVH, 1 left-axis deviation, 1 superior axis | Surgery LCA from RSV (n°3): 1 unroofing, 2 surgery for VSD RCA (n°8): from LSV, 4 unroofing, 4 surgery for VDS/ASD Conservative management: all the rest. No indication on sport |
References
- Maron, B.J.; Haas, T.S.; Ahluwalia, A.; Murphy, C.J.; Garberich, R.F. Demographics and epidemiology of sudden deaths in young competitive athletes: From the United States National Registry. Am. J. Med. 2016, 129, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.F.; Siebert, D.M.; Kucera, K.L.; Thomas, L.C.; Maleszewski, J.J.; Lopez-Anderson, M.; Suchsland, M.Z.; Harmon, K.G.; Drezner, J.A. Etiology of sudden cardiac arrest and death in us competitive athletes: A 2-year prospective surveillance study. Clin. J. Sport Med. 2018, 30, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of sudden death in sports: Insights from a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Alkhulaifi, A.M.; Chooriyil, N.; Alkuwari, M.; Ghareep, A.N.; Carr, C. Coronary artery anomalies: Unusually high incidence of anomalies with a malignant course in an Asian population. SAGE Open Med. 2017, 5. [Google Scholar] [CrossRef]
- Sperandii, F.; Guerra, E.; Tranchita, E.; Minganti, C.; Lanzillo, C.; Nigro, A.; Quaranta, F.; Parisi, A.; Di Roma, M.; Maresca, L.; et al. Clinical significance of ST depression at exercise stress testing in competitive athletes: Usefulness of coronary CT during screening. J. Sports Med. Phys. Fit. 2018, 58, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Link, M.S. Sudden cardiac death in the young: Epidemiology and overview. Congenit. Heart Dis. 2017, 12, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Girzadas, M.; Varga, P.; Dajani, K. A single-center experience of detecting coronary anomalies on 64-slice computed tomography. J. Cardiovasc. Med. 2009, 10, 842–847. [Google Scholar] [CrossRef]
- Ugalde, H.; Ramírez, A.; Ugalde, D.; Farías, E.; Silva, A.M. Coronary artery origin anomalies. Analysis of 10,000 coronary angiographies. Rev. Med. Chile 2010, 138, 7–14. [Google Scholar]
- Basso, C.; Maron, B.J.; Corrado, D.; Thiene, G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J. Am. Coll. Cardiol. 2000, 35, 1493–1501. [Google Scholar] [CrossRef]
- Molossi, S.; Martínez-Bravo, L.E.; Mery, C.M. Anomalous aortic origin of a coronary artery. Methodist Debakey Cardiovasc. J. 2019, 15, 111–121. [Google Scholar]
- Angelini, P. Imaging approaches for coronary artery anomalies: Purpose and techniques. Curr. Cardiol. Rep. 2019, 21, 101. [Google Scholar] [CrossRef]
- Rigatelli, G.; Zuin, M.; Galasso, P.; Carraro, M.; D’Elia, K.; Daniela, L.; Roncon, L.; Truyen, T.T.; Nguyen, T. Mechanisms of myocardial ischemia inducing sudden cardiac death in athletes with anomalous coronary origin from the opposite sinus: Insights from a computational fluid dynamic study. Cardiovasc. Revascularization Med. 2019, 20, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Gerling, S.; Loose, O.; Zant, R.; Michel, H.; Melter, M.; Gündisch, C.; Krutsch, V.; Krutsch, W. Echocardiographic diagnosis of congenital coronary artery abnormalities in a continuous series of adolescent football players. Eur. J. Prev. Cardiol. 2019, 26, 988–994. [Google Scholar] [CrossRef]
- Lorber, R.; Srivastava, S.; Wilder, T.J.; McIntyre, S.; DeCampli, W.M.; Williams, W.G.; Frommelt, P.C.; Parness, I.A.; Blackstone, E.H.; Jacobs, M.L.; et al. Anomalous aortic origin of coronary arteries in the young: Echocardiographic evaluation with surgical correlation. JACC Cardiovasc. Imaging 2015, 8, 1239–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyt, W.J.; Dean, P.N.; Schneider, D.S.; Conaway, M.R.; Kramer, C.M.; Battle, R.W. Coronary artery evaluation by screening echocardiogram in intercollegiate athletes. Med. Sci. Sports Exerc. 2017, 49, 863–869. [Google Scholar] [CrossRef]
- Villa, A.D.; Sammut, E.; Nair, A.; Rajani, R.; Bonamini, R.; Chiribiri, A. Coronary artery anomalies overview: The normal and the abnormal. World J. Radiol. 2016, 8, 537–555. [Google Scholar] [CrossRef] [Green Version]
- Kastellanos, S.; Aznaouridis, K.; Vlachopoulos, C.; Tsiamis, E.; Oikonomou, E.; Tousoulis, D. Overview of coronary artery variants, aberrations and anomalies. World J. Cardiol. 2018, 10, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Attili, A.; Hensley, A.K.; Jones, F.D.; Grabham, J.; Disessa, T.G. Echocardiography and coronary CT angiography imaging of variations in coronary anatomy and coronary abnormalities in athletic children: Detection of coronary abnormalities that create a risk for sudden death. Echocardiography 2012, 30, 225–233. [Google Scholar] [CrossRef]
- Pelliccia, A.; Spataro, A.; Maron, B.J. Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. Am. J. Cardiol. 1993, 72, 978–979. [Google Scholar] [CrossRef]
- Zeppilli, P.; Russo, A.D.; Santini, C.; Palmieri, V.; Natale, L.; Giordano, A.; Frustaci, A. In vivo detection of coronary artery anomalies in asymptomatic athletes by echocardiographic screening. Chest 1998, 114, 89–93. [Google Scholar] [CrossRef]
- Labombarda, F.; Coutance, G.; Pellissier, A.; Mery-Alexandre, C.; Roule, V.; Maragnes, P.; Milliez, P.; Saloux, E. Major congenital coronary artery anomalies in a paediatric and adult population: A prospective echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thankavel, P.P.; Lemler, M.S.; Ramaciotti, C. Utility and importance of new echocardiographic screening methods in diagnosis of anomalous coronary origins in the pediatric population: Assessment of quality improvement. Pediatr. Cardiol. 2014, 36, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lytrivi, I.D.; Wong, A.H.; Ko, H.H.; Chandra, S.; Nielsen, J.C.; Srivastava, S.; Lai, W.W.; Parness, I.A. Echocardiographic diagnosis of clinically silent congenital coronary artery anomalies. Int. J. Cardiol. 2008, 126, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Wyman, R.A.; Chiu, R.Y.; Rahko, P.S. The 5-minute screening echocardiogram for athletes. J. Am. Soc. Echocardiogr. 2008, 21, 786–788. [Google Scholar] [CrossRef]
- Modaff, D.S.; Hegde, S.M.; Wyman, R.A.; Rahko, P.S.; Wyman, R.A. Usefulness of focused screening echocardiography for collegiate athletes. Am. J. Cardiol. 2019, 123, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Frommelt, P.; Lopez, L.; Dimas, V.V.; Eidem, B.; Han, B.K.; Ko, H.H.; Lorber, R.; Nii, M.; Printz, B.; Srivastava, S.; et al. Recommendations for multimodality assessment of congenital coronary anomalies: A guide from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 259–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, E.S.; Basilico, F.; Corrado, G. Early screening for cardiovascular abnormalities with preparticipation echocardiography: Utility of focused physician-operated echocardiography in preparticipation screening of athletes. J. Ultrasound Med. 2014, 33, 307–313. [Google Scholar] [CrossRef]
- Gleason, C.N.; Kerkhof, D.L.; Cilia, E.A.; Lanyi, M.A.; Finnoff, J.; Sugimoto, D.; Corrado, G.D. Early Screening for cardiovascular abnormalities with preparticipation echocardiography: Feasibility study. Clin. J. Sport Med. 2017, 27, 423–429. [Google Scholar] [CrossRef]
- Moulson, N.; Jaff, Z.; Wiltshire, V.; Taylor, T.; O’Connor, H.M.; Hopman, W.M.; Johri, A.M. Feasibility and reliability of nonexpert POCUS for cardiovascular preparticipation screening of varsity athletes: The sharp protocol. Can. J. Cardiol. 2019, 35, 35–41. [Google Scholar] [CrossRef]
- Anderson, J.B.; Grenier, M.; Edwards, N.M.; Madsen, N.L.; Czosek, R.J.; Spar, D.S.; Barnes, A.; Pratt, J.; King, E.; Knilans, T.K. Usefulness of combined history, physical examination, electrocardiogram, and limited echocardiogram in screening adolescent athletes for risk for sudden cardiac death. Am. J. Cardiol. 2014, 114, 1763–1767. [Google Scholar] [CrossRef]
- Maron, B.J.; Bodison, S.A.; Wesley, Y.E.; Tucker, E.; Green, K.J. Results of screening a large group of intercollegiate competitive athletes for cardiovascular disease. J. Am. Coll. Cardiol. 1987, 10, 1214–1221. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.F.; Maron, B.J.; Diggs, J.A.; Spencer, J.E.; Mehrotra, P.P.; Curry, C.L. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominantly black population of collegiate athletes. Am. J. Cardiol. 1989, 64, 1029–1033. [Google Scholar] [CrossRef]
- Loukas, M.; Andall, R.G.; Khan, A.Z.; Patel, K.; Muresian, H.; Spicer, D.E.; Tubbs, R.S. The clinical anatomy of high take-off coronary arteries. Clin. Anat. 2016, 29, 408–419. [Google Scholar] [CrossRef]
- Clemente, A.; del Borrello, M.; Greco, P.; Mannella, P.; Di Gregorio, F.; Romano, S.; Morra, A. Anomalous origin of the coronary arteries in children: Diagnostic role of three-dimensional coronary MR angiography. Clin. Imaging 2010, 34, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Hobbs, R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheter. Cardiovasc. Diagn. 1990, 21, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.W.; Griffith, G.C. Anomalies of the coronary arteries and their clinical significance. Circulation 1956, 14, 800–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, K.; Jeng, C.; Liu, Y.P.; Wang, T.; Lin, T.; Chen, S.; Chen, C.; Mo, Y. Diagnosis of anomalous coronary arteries in 64-MDCT. Chin. J. Radiol. Taipei 2007, 32, 111–119. [Google Scholar]
- E Hobbs, R.; Millit, H.D.; Raghavan, P.V.; Moodie, D.S.; Sheldon, W.C. Congenital coronary artery anomalies: Clinical and therapeutic implications. Cardiovasc. Clin. 1981, 12, 43–58. [Google Scholar]
- Sivri, N.; Aktoz, M.; Yalta, K.; Ozcelik, F.; Altun, A. A retrospective study of angiographic ally determined anomalous coronary arteries in 12,844 subjects in Thrace region of Turkey. Hippokratia 2012, 16, 246–249. [Google Scholar]
- Yildiz, A.; Okcun, B.; Peker, T.; Arslan, C.; Olcay, A.; Vatan, M.B. Prevalence of coronary artery anomalies in 12,457 adult Patients who underwent coronary angiography. Clin. Cardiol. 2010, 33, E60–E64. [Google Scholar] [CrossRef]
- Yuksel, S.; Meric, M.; Soylu, K.; Gulel, O.; Zengin, H.; Demircan, S.; Yilmaz, O.; Sahin, M. The primary anomalies of coronary artery origin and course: A coronary angiographic analysis of 16,573 patients. Exp. Clin. Cardiol. 2013, 18, 121–123. [Google Scholar]
- Muriago, M.; Sheppard, M.N.; Ho, S.Y.; Anderson, R.H. Location of the coronary arterial orifices in the normal heart. Clin. Anat. 1997, 10, 297–302. [Google Scholar] [CrossRef]
- Priemer, D.S.; Danon, S.; Guzman, M.A. Unexpected cardiac death due to a slit-like left coronary ostium with associated high take-off of the right coronary artery in a previously healthy child. Forensic Sci. Med. Pathol. 2014, 11, 124–126. [Google Scholar] [CrossRef]
- Palmieri, V.; Gervasi, S.; Bianco, M.; Cogliani, R.; Poscolieri, B.; Cuccaro, F.; Marano, R.; Mazzari, M.; Basso, C.; Zeppilli, P. Anomalous origin of coronary arteries from the “wrong” sinus in athletes: Diagnosis and management strategies. Int. J. Cardiol. 2018, 252, 13–20. [Google Scholar] [CrossRef]
- Angelini, P.; Cheong, B.Y.; Lenge De Rosen, V.V.; Lopez, A.; Uribe, C.; Masso, A.H.; Ali, S.W.; Davis, B.R.; Muthupillai, R.; Willerson, J.T. High-risk cardiovascular conditions in sports-related sudden death: Prevalence in 5,169 schoolchildren screened via cardiac magnetic resonance. TX Heart Inst. J. 2018, 45, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Malagò, R.; Pezzato, A.; Barbiani, C.; Alfonsi, U.; Nicolì, L.; Caliari, G.; Mucelli, R.P. Coronary artery anatomy and variants. Pediatr. Radiol. 2011, 41, 1505–1515. [Google Scholar] [CrossRef]
- Mery, C.M.; Lopez, K.N.; Molossi, S.; Sexson-Tejtel, S.K.; Krishnamurthy, R.; McKenzie, E.D.; Fraser, C.D.; Cantor, S.B. Decision analysis to define the optimal management of athletes with anomalous aortic origin of a coronary artery. J. Thorac. Cardiovasc. Surg. 2016, 152, 1366–1375. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.D.; Myerburg, R.J.; Levine, B.D.; Udelson, J.E.; Kovacs, R.J. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 8: Coronary artery disease: A scientific statement from the american heart association and american college of cardiology. Circulation 2015, 132, e310–e314. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Mery, C.M.; Day, P.E.; Tejtel, S.K.S.; McKenzie, E.D.; Fraser, C.D.; Qureshi, A.M.; Molossi, S. Current practices are variable in the evaluation and management of patients with anomalous aortic origin of a coronary artery: Results of a survey. Congenit. Heart Dis. 2017, 12, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Padalino, M.A.; Franchetti, N.; Sarris, G.E.; Hazekamp, M.; Carrel, T.; Frigiola, A.; Horer, J.; Roussin, R.; Cleuziou, J.; Meyns, B.; et al. Anomalous aortic origin of coronary arteries: Early results on clinical management from an international multicenter study. Int. J. Cardiol. 2019, 291, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirico, F.; Fernando, F.; Di Paolo, F.; Adami, P.E.; Signorello, M.G.; Sannino, G.; Bianco, A.; Cerrone, A.; Baioccato, V.; Filippi, N.; et al. Exercise stress test in apparently healthy individuals—Where to place the finish line? The ferrari corporate wellness programme experience. Eur. J. Prev. Cardiol. 2019, 26, 731–738. [Google Scholar] [CrossRef]
- van Hare, G.F.; Ackerman, M.J.; Evangelista, J.A.K.; Kovacs, R.J.; Myerburg, R.J.; Shafer, K.M.; Warnes, C.A.; Washington, R.L. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 4: Congenital heart disease: A scientific statement from the American Heart Association and American College of Cardiology. Circulation 2015, 132, e281–e291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biffi, A.; Delise, P.; Zeppilli, P.; Giada, F.; Pelliccia, A.; Penco, M.; Casasco, M.; Colonna, P.; D’Andrea, A.; D’Andrea, L.; et al. Italian cardiological guidelines for sports eligibility in athletes with heart disease: Part 2. J. Cardiovasc. Med. 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Stoebe, S.; Lange, K.; Pfeiffer, D.; Hagendorff, A. Feasibility of proximal right coronary artery imaging by 2D and 3D echocardiography in comparison to coronary angiography. Echo Res. Pract. 2015, 2, 7379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen-Urstad, M. Sudden death and physical activity in athletes and nonathletes. Scand. J. Med. Sci. Sports 2007, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]



| Study, Authors | Study Design | Echo Protocol | Coronary Artery Echo View | Echo Machine | High Take-off Definition |
|---|---|---|---|---|---|
| Hyot [15] 2017, USA | Retrospective Athletes Different sports, different levels, pre-participation screening | NR | NR | NR | - |
| Zeppilli [20] 1998, Italy | Prospective Athletes Different sports, different levels, preparticipation screening | NR | PSA | Sonoline CD (Siemens, Medical Solutions USA, Inc, Mountain View, CA, USA) | - |
| Pellicia [19] 1993, Italy | Prospective Athletes Elite, different sport, preparticipation screening | NR | PSA | NR | - |
| Gerling [13] 2018, Germany | Prospective Athletes Adolescent elite football players-participation screening | NR | PSA or PLA | Aplio 500 CV (Toshiba Medical System, Otawara, Japan), Vivid I, (General Electric, Healthcare, USA) | High take-off above to the SJ |
| Lombardara [21] 2013, France | Prospective General population | Standard full examination | Modified PSA Color doppler with decreased velocity | IE33 Philips Healthcare (DA best, The Netherlands) | - |
| Thanraval [22] 2014, USA | Retrospective General pediatric population | NR | 2010 2D and color doppler PSA at the level of coronary sinuses 2011 Added PSA superiorly into the Asc Ao +/-color Doppler with decreased velocity | Siemens Sequoia C512 ultrasound equipment (Siemens, Medical Solutions USA, Inc, Mountain View, CA, USA) or Philips iE33 (Philips Medical Systems, Bothell, WA, USA) | - |
| Lytrivi [23], 2008, USA | Retrospective General pediatric population | NR | PSA, left or right PLA and/or parasagittal planes | Aplio 500 CV (Toshiba Medical System, Otawara, Japan) | High take-off distal to the SJ |
| Wyman [24] 2007, USA | Prospective Athletes Different sports, different levels, preparticipation screening | 5 minutes echo | PSA | Siemens Acuson Sequoia, General Electric Vivid 7, USA. Philips Sonos 5500 (Philips Medical Systems, Bothell, WA, USA) | - |
| Maron [30] 1987, USA | Prospective Athletes Adolescents, different sports, different levels, preparticipation screening | Standard full examination | LMCA origin PSA | Advance Technology Laboratory (ATL) Mark 500 (Advanced Technology Laboratory-Seattle) | - |
| First Author, Year of Publication | Sample Size and Age | LCA | RCA | All | Major Anomalies | Minor Anomalies |
|---|---|---|---|---|---|---|
| Hyot [15], 2017, USA | 146 (18–23 years) | 98% 81% | 98% 82% | NR | 0% | NR |
| Zeppilli [20], 1998, Italy | 3650 (30 ± 12 years) | NR | NR | Ostium and proximal tract: 90% | 0.09%: RCA from left sinus, LCA from right sinus | 1.6%: separate origin of LCA and CFx from left sinus of Valsalva and two distinct ostia in the right sinus for RCA and the conus branch |
| Pellicia [19], 1993, Italy | 1273 (13–49 years) | 98.7% | 80% | 93% | 0% | 2.19%: Separate ostia for LCA and CFx (n°6), short LCA (<5 mm) with real bifurcation (n°22) |
| Gerling [13], 2018, Germany | 1045 (12–15 years) | NR | NR | NR | 0.19% RCA with high take-off and partial intra-arterial course (n°2) | 1.5% RCA high take-off with acute angle (n°1), small fistulas (n°2), ectasias of LCA (n°2), and RCA high take-off (n°11) Incidence of High take-off 1.14% |
| Labombarda, [21], 2013, France | 350 (84% adult,16% children) | NR | NR | Ostium and first tract 98.5% children | 0.39% RCA from left sinus (n°8), single coronary osmium (n°3), LCA from right sinus (n°1), fistula (n°1) | NR |
| Thanraval [22], 2014, USA | 2010 (n 5669 0–21 years) 2012 N 6.428 (0–21 years) | NR | NR | NR | 0.02% Intramural LCA for right sinus 0.22% RCA from left sinus (n°13), LCA from right sinus (n°2) | NR |
| Lytrivi [23], 2008, USA | 14,546 | NR | NR | NR | 0.76% Major and Minor RCA from LSV (n°24), Cx from RCA (n°9), Single RCA (n°8), LCA from RSV (n°6), LAD off RSV (n°3), Dual LAD (n°1), Single LCA (n°1), RCA high take-off (n°53) (0.36%, LCA high take-off (n°4), high take-off of both (n°2), fistulas (n°57) Incidence of RCA high take-off 0.36% All 0.41% | NR |
| Maron [30], 1987, USA | 90 17–30 years | 100% | NR | NR | 0% | |
| Wyman [24], 2008, USA | 395 17–23 years | 99% | 96 | NR | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantinotti, M.; Giordano, R.; Assanta, N.; Koestenberger, M.; Franchi, E.; Marchese, P.; Clemente, A.; Kutty, S.; D’Ascenzi, F. Echocardiographic Screening of Anomalous Origin of Coronary Arteries in Athletes with a Focus on High Take-Off. Healthcare 2021, 9, 231. https://doi.org/10.3390/healthcare9020231
Cantinotti M, Giordano R, Assanta N, Koestenberger M, Franchi E, Marchese P, Clemente A, Kutty S, D’Ascenzi F. Echocardiographic Screening of Anomalous Origin of Coronary Arteries in Athletes with a Focus on High Take-Off. Healthcare. 2021; 9(2):231. https://doi.org/10.3390/healthcare9020231
Chicago/Turabian StyleCantinotti, Massimiliano, Raffaele Giordano, Nadia Assanta, Martin Koestenberger, Eliana Franchi, Pietro Marchese, Alberto Clemente, Shelby Kutty, and Flavio D’Ascenzi. 2021. "Echocardiographic Screening of Anomalous Origin of Coronary Arteries in Athletes with a Focus on High Take-Off" Healthcare 9, no. 2: 231. https://doi.org/10.3390/healthcare9020231
APA StyleCantinotti, M., Giordano, R., Assanta, N., Koestenberger, M., Franchi, E., Marchese, P., Clemente, A., Kutty, S., & D’Ascenzi, F. (2021). Echocardiographic Screening of Anomalous Origin of Coronary Arteries in Athletes with a Focus on High Take-Off. Healthcare, 9(2), 231. https://doi.org/10.3390/healthcare9020231










