Factors Associated with Failure of Bakri Balloon Tamponade for the Management of Postpartum Haemorrhage. Case Series Study and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Series of BBT for PPH
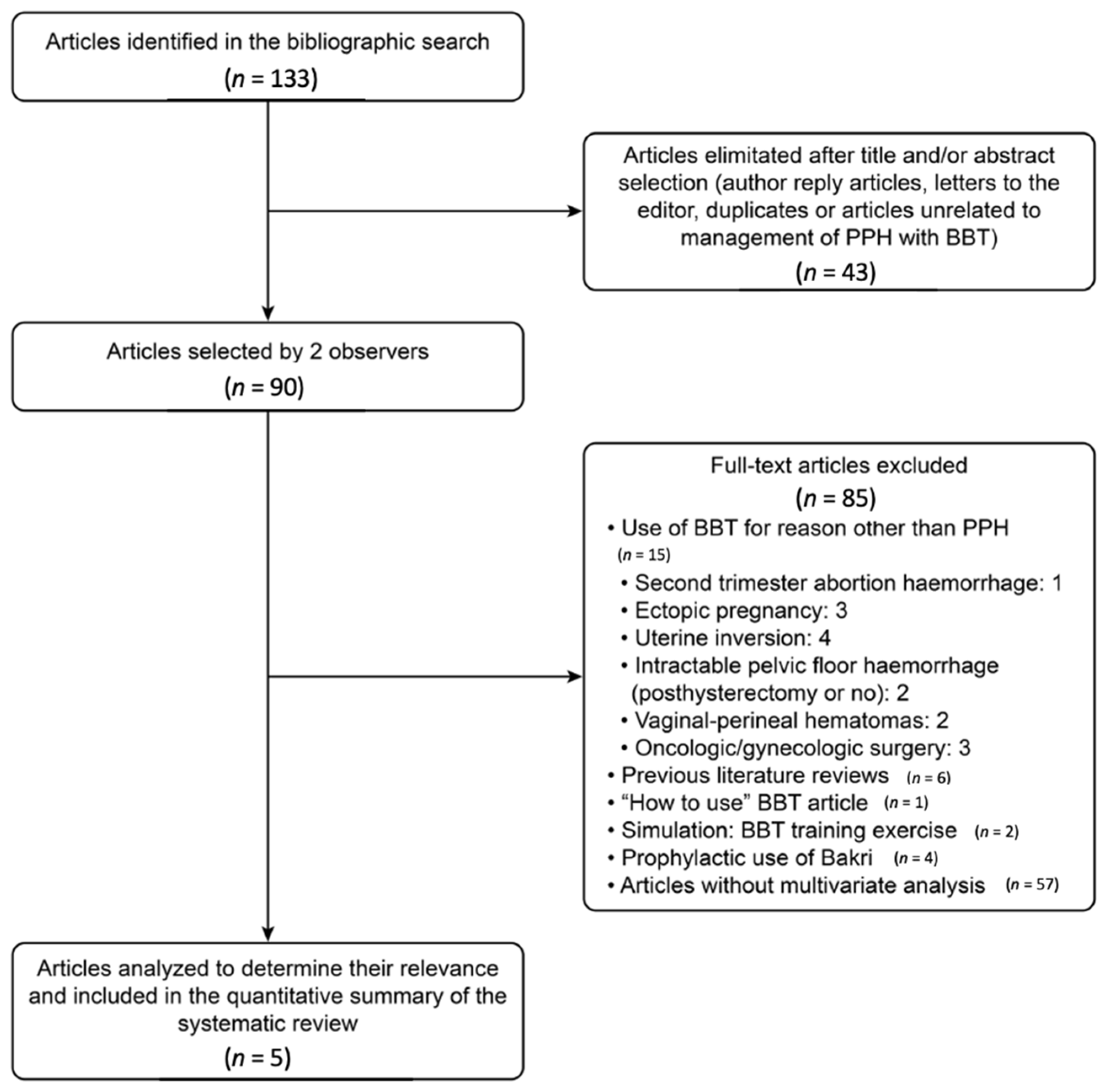
2.2. Systematic Review
2.3. Eligibility Criteria and Outcome Measures
2.4. Information Sources and Search Strategies
2.5. Study Selection, Data Extraction, Statistical Analysis, and Risk of Bias
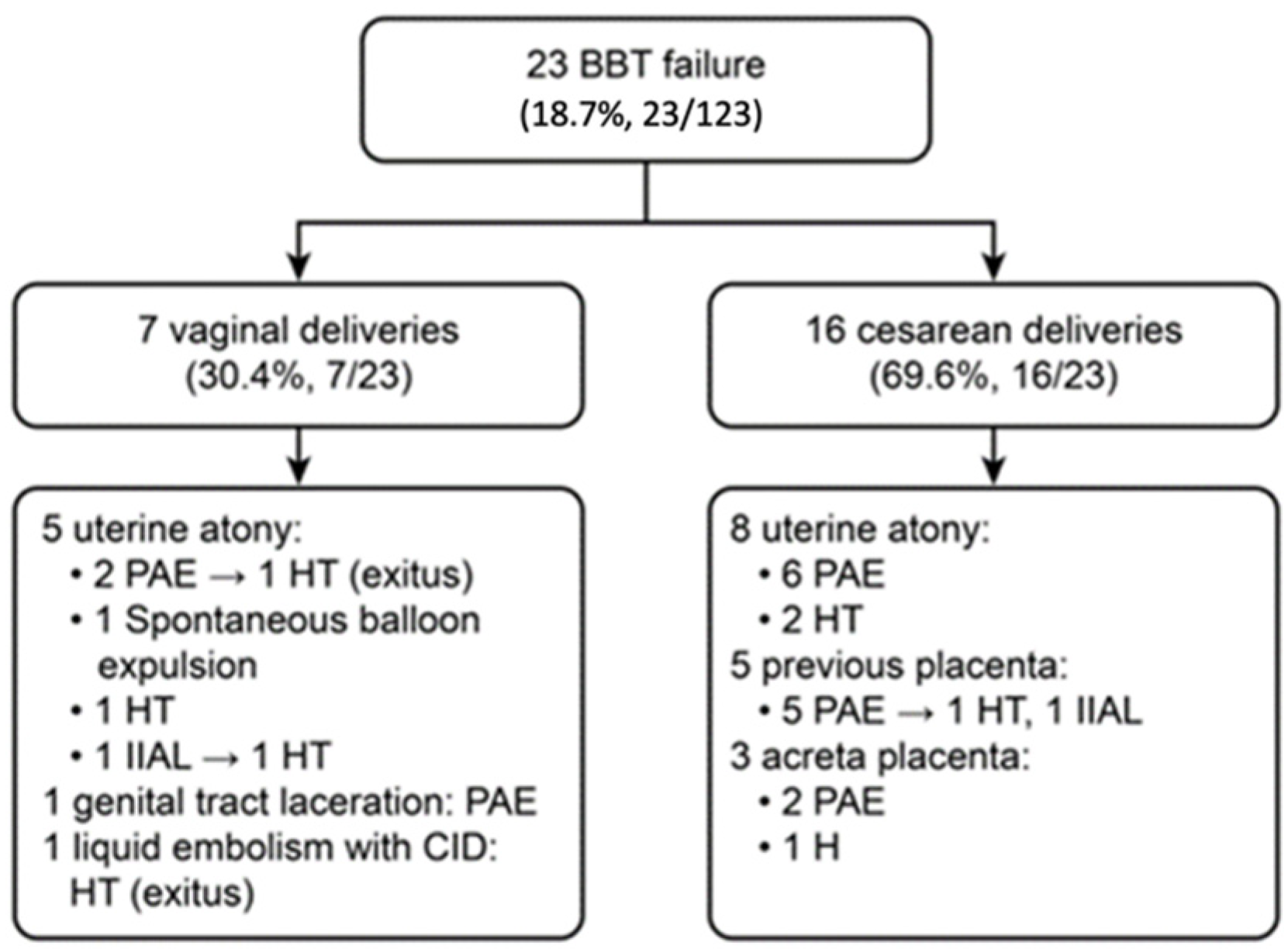
3. Results
3.1. Case Series of BBT for PPH
3.2. Systematic Review
4. Discussion
4.1. Main Findings
4.1.1. Case Series of BBT for PPH
4.1.2. Systematic Review
4.1.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Hutcheon, J.A.; Lee, L.; Kramer, M.S.; Liston, R.M.; Joseph, K.S. Epidemiological investigation of a temporal in-crease in atonic postpartum haemorrhage: A population-based retrospective cohort study. BJOG 2013, 120, 853–862. [Google Scholar] [CrossRef]
- Dupont, C.; Rudigoz, R.C.; Cortet, M.; Touzet, S.; Colin, C.; Rabilloud, M.; Lansac, J.; Harvey, T.; Tessier, V.; Chauleur, C.; et al. Frecuency, causes and risk factors of postpartum haemorrhage: A population-based study in 106 French maternity units. J. Gynecol. Obstet. Biol. Reprod. 2014, 43, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.; Liu, S.; Bartholomew, S.; Hutcheon, J.A.; Kramer, M.S.; Liston, R.M.; Joseph, K. Temporal Trends in Postpartum Hemorrhage and Severe Postpartum Hemorrhage in Canada From 2003 to 2010. J. Obstet. Gynaecol. Can. 2014, 36, 21–33. [Google Scholar] [CrossRef]
- Devine, P.C. Obstetric Hemorrhage. Semin. Perinatol. 2009, 33, 76–81. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recommendations on Prevention and Treatment of Postpartum Haemorrhage; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Dahlke, J.D.; Mendez-Figueroa, H.; Maggio, L.; Hauspurg, A.K.; Sperling, J.D.; Chauhan, S.P.; Rouse, D.J. Prevention and management of postpartum hemorrhage: A comparison of 4 national guidelines. Am. J. Obstet. Gynecol. 2015, 213, 76.e1–76.e10. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: Postpartum hemorrhage. Obstet. Gynecol. 2006, 108, 1039–1047. [Google Scholar]
- No, G.G. Prevention and Management of Postpartum Haemorrhage: Green-top Guideline No. 52. BJOG 2017, 124, e106–e149. [Google Scholar]
- Grönvall, M.; Tikkanen, M.; Tallberg, E.; Paavonen, J.; Stefanovic, V. Use of Bakri balloon tamponade in the treatment of postpartum hemorrhage: A series of 50 cases from a tertiary teaching hospital. Acta Obstet. Gynecol. Scand. 2013, 92, 433–438. [Google Scholar] [CrossRef]
- Ruiz Labarta, F.J.; Pintado Recarte, M.P.; Alvarez Luque, A.; Joigneau Prieto, L.; Perez Martín, L.; Gonzalez Leyte, M.; Palacio Abizanda, F.; Morillas Ramirez, F.; Perez Corral, A.; Ortiz Quintana, L.; et al. Outcomes of pelvic arterial embolization in the management of postpartum haemorrhage: A case series study and systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 12–21. [Google Scholar] [CrossRef]
- Rath, W.; Hackethal, A.; Bohlmann, M.K. Second-line treatment of postpartum haemorrhage (PPH). Arch. Gynecol. Obstet. 2012, 286, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Doumouchtsis, S.K.; Papageorghiou, A.T.; Arulkumaran, S. Systematic Review of Conservative Management of Postpartum Hemorrhage: What to Do When Medical Treatment Fails. Obstet. Gynecol. Surv. 2007, 62, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Diemert, A.; Ortmeyer, G.; Hollwitz, B.; Lotz, M.; Somville, T.; Glosemeyer, P.; Diehl, W.; Hecher, K. The combination of intrauterine balloon tamponade and the B-Lynch procedure for the treatment of severe postpartum hemorrhage. Am. J. Obstet. Gynecol. 2012, 206, 65.e1–65.e4. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.M.; Otiv, S.R.; Majumder, R.; Nikam, Y.A.; Shrivastava, M. Internal iliac artery ligation for arresting postpartum haemorrhage. BJOG 2007, 114, 356–361. [Google Scholar] [CrossRef]
- Knight, M. Ukoss Peripartum hysterectomy in the UK: Management and outcomes of the associated haemorrhage. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 1380–1387. [Google Scholar] [CrossRef]
- Georgiou, C. Balloon tamponade in the management of postpartum haemorrhage: A review. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 748–757. [Google Scholar] [CrossRef]
- Wright, C.E.; Abuhamad, A.Z.; Chauhan, S.P. Bakri Balloon in the Management of Postpartum Hemorrhage: A Review. Am. J. Perinatol. 2014, 31, 957–964. [Google Scholar] [CrossRef]
- Bakri, Y.; Amri, A.; Jabbar, F.A. Tamponade-balloon for obstetrical bleeding. Int. J. Gynecol. Obstet. 2001, 74, 139–142. [Google Scholar] [CrossRef]
- Revert, M.; Cottenet, J.; Raynal, P.; Cibot, E.; Quantin, C.; Rozenberg, P. Intrauterine balloon tamponade for management of severe postpartum haemorrhage in a perinatal network: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.A.; Abdelaziz, A. Comparison between two management protocols for postpartum hemorrhage during cesarean section in placenta previa: Balloon protocol versus non-balloon protocol. J. Obstet. Gynaecol. Res. 2016, 43, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Park, Y.W.; Kim, Y.H.; Jung, I.; Kwon, J.-Y. Efficacy of Intrauterine Bakri Balloon Tamponade in Cesarean Section for Placenta Previa Patients. PLoS ONE 2015, 10, e0134282. [Google Scholar] [CrossRef]
- Kong, C.W.; To, W.W. Prognostic factors for the use of intrauterine balloon tamponade in the management of severe postpartum hemorrhage. Int. J. Gynecol. Obstet. 2018, 142, 48–53. [Google Scholar] [CrossRef]
- Grange, J.; Chatellier, M.; Cheve, M.-T.; Paumier, A.; Launay-Bourillon, C.; Legendre, G.; Olivier, M.; Ducarme, G. Predictors of failed intrauterine balloon tamponade for persistent postpartum hemorrhage after vaginal delivery. PLoS ONE 2018, 13, e0206663. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.; Dildy, G.A.; Clark, S.L.; Belfort, M.A. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am. J. Obstet. Gynecol. 2008, 199, 519.e1–519.e7. [Google Scholar] [CrossRef]
- Al-Zirqi, I.; Vangen, S.; Forsen, L.; Stray-Pedersen, B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.L. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, S.; Qiu, X.; Zhu, C.; Li, Z.; Wang, Z.; Hou, H.; Gao, Y.; Wang, X.; He, P.; et al. Early usage of Bakri postpartum balloon in the management of postpartum hemorrhage: A large prospective, observational multicenter clinical study in South China. J. Perinat. Med. 2018, 28, 649–656. [Google Scholar] [CrossRef]
- Burke, T.F.; Danso-Bamfo, S.; Guha, M.; Oguttu, M.; Tarimo, V.; Nelson, B.D. Shock progression and survival after use of a con-dom uterine balloon tamponade package in women with uncontrolled postpartum hemorrhage. Int. J. Gynaecol. Obstet. 2017, 139, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Conde-Agudelo, A.; Borovac-Pinheiro, A.; Suarez-Rebling, D.; Eckardt, M.; Theron, G.; Burke, T.F. Uterine balloon tamponade for the treatment of postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 222, 293.e1–293.e52. [Google Scholar] [CrossRef]
- Kaya, B.; Tuten, A.; Daglar, K.; Misirlioglu, M.; Polat, M.; Yildirim, Y.; Unal, O.; Kilic, G.S.; Guralp, O. Balloon tamponade for the management of postpartum uterine hemorrhage. J. Perinat. Med. 2014, 42, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.; Reisner, D.P.; Benedetti, T.J.; Dunsmoor-Su, R.F. Bakri balloon effectiveness for postpartum hemorrhage: A “real world experience”. J. Matern. Neonatal Med. 2013, 26, 1720–1723. [Google Scholar] [CrossRef]
- Danisman, N.; Kahyaoglu, S.; Celen, S.; Akselim, B.; Tuncer, E.G.; Timur, H.; Kaymak, O.; Kahyaoglu, I. The outcomes of surgical treatment modalities to decrease “near miss” maternal morbidity caused by peripartum hemorrhage. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1092–1097. [Google Scholar] [PubMed]
- Cekmez, Y.; Ozkaya, E.; Öcal, F.D.; Kücüközkan, T. Experience with different techniques for the management of postpartum haemorrhage due to uterine atony: Compression sutures, artery ligation and Bakri balloon. Ir. J. Med. Sci. 2015, 184, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Ramler, P.I.; Henriquez, D.D.C.A.; Akker, T.V.D.; Caram-Deelder, C.; Groenwold, R.H.H.; Bloemenkamp, K.W.M.; Van Roosmalen, J.; Van Lith, J.M.M.; Van Der Bom, J.G. Comparison of outcome between intrauterine balloon tamponade and uterine artery embolization in the management of persistent postpartum hemorrhage: A propensity score-matched cohort study. Acta Obstet. Gynecol. Scand. 2019, 98, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 123) | BBT Success Group (n = 100) | BBT Failure Group (n = 23) | p Value |
|---|---|---|---|---|
| Maternal and perinatal characteristics | ||||
| Maternal age (years) | 33.7 ± 5.7 (21–45) | 34.3 ± 5.3 (21–45) | 31.1 ± 6.7 (21–43) | 0.043 |
| Primiparous | 81 (65.9%) | 63 (63%) | 18 (78.3%) | 0.164 |
| History of caesarean section | 15 (12.2%) | 11 (11%) | 4 (17.4%) | 0.398 |
| Twin pregnancy | 27 (22%) | 24 (24%) | 3 (13%) | 0.252 |
| Gestational age at delivery (weeks) | 37.4 ± 3.3 (24–42) | 37.3 ± 3.4 (24–41) | 37.7 ± 2.6 (31–42) | 0.898 |
| hematocrit on admission (%) | 35.2 ± 4.1 (22.1–43.9) | 35.6 ± 4.2 (22.1–43.9) | 33.9 ± 2.9 (26.9–37.5) | 0.019 |
| Induction of labour | 58 (47.2%) | 50 (50%) | 8 (34.8%) | 0.187 |
| Caesarean delivery | 52 (42.3%) | 36 (36%) | 16 (69.6%) | 0.003 |
| Neonatal birth weight (g) | 2883.2 ± 758.4 (600–4320) | 2851.2 ± 794.5 (600–4320) | 3022.2 ± 569.7 (1820–4020) | 0.431 |
| PPH characteristics and maternal haemodynamic state data | ||||
| Primary PPH | 115 (93.5%) | 93 (93%) | 22 (95.7%) | 0.642 |
| Aetiology of PPH | ||||
| Uterine atony | 86 (69.9%) | 73 (73%) | 13 (56.5%) | |
| Retention of placental fragments | 14 (11.4%) | 14 (14%) | 0 | |
| Placenta previa | 6 (4.9%) | 1 (1%) | 5 (21.7%) | |
| Placenta accreta | 8 (6.5%) | 5 (5%) | 3 (13%) | |
| Vaginal/cervical tears | 3 (2.4%) | 2 (2%) | 1 (4.3%) | |
| DIC | 2 (1.6%) | 1 (1%) | 1 (4.3%) | 0.001 |
| Curettage before BBT insertion | 80 (65%) | 71 (71%) | 9 (39.1%) | 0.004 |
| Estimated blood loss before BBT | ||||
| 1000 mL | 23 (18.7%) | 21 (21%) | 2 (8.7%) | |
| 1000–2500 mL | 87 (70.7%) | 75 (75%) | 12 (52.2%) | |
| 2500–5000 ml | 13 (10.6%) | 4 (4%) | 9 (39.9%) | 0.001 |
| First post-bleed hematocrit (%) | 25.6 ± 5.2 (11.8–38.7) | 25.6 ± 5.3 (14–38.7) | 25.2 ± 4.6 (11.8–31.6) | 0.984 |
| Need for transfusion | 85 (69.1%) | 64 (64%) | 21 (91.3%) | 0.011 |
| RBCUs per patient | 5.2 ± 5.4 (0–40) | 3.4 ± 2.1 (0–11) | 11.1 ± 7.9 (1–40) | 0.001 |
| ≥7 RBCUs transfused | 21 (17.1%) | 5 (5%) | 16 (69.6%) | 0.001 |
| FFPUs per patient | 3.7 ± 4.9 (0–30) | 1.9 ± 1.4 (0–4) | 5.8 ± 6.4 (0–30) | 0.001 |
| Number of concentrated platelets transfused per patient | 1.7 ± 2.6 (0–14) | 0.6 ± 0.6 (0–2) | 2.6 ± 3.4 (0–14) | 0.002 |
| Fibrinógeno (gr) per patient | 2.9 ± 2.7 (0–20) | 2.1 ± 0.9 (0–6) | 4.8 ± 4.4 (1–20) | 0.001 |
| Admission to PARU | 101 (82.1%) | 98 (98%) | 3 (13%) | 0.001 |
| Admission to ICU | 21 (17.1%) | 2 (2%) | 19 (82.6%) | 0.001 |
| Hospital stay (days) | 5.2 ± 5.3 (1–49) | 4.4 ± 3.3 (2–25) | 9.1 ± 9.3 (1–49) | 0.001 |
| BBT main parameters | ||||
| Vaginal BBT placement | 117 (95.1%) | 97 (97%) | 20 (87%) | 0.044 |
| Filling volumen (mL) | 255.6 ± 100.7 (60–540) | 250.3 ± 92 (90–500) | 286.5 ± 141.3 (60–540) | 0.435 |
| Balloon output (mL) | 173.4 ± 264.5 (0–1800) | 109.5 ± 89.9 (0–700) | 573.1 ± 534.3 (25–1800) | 0.001 |
| Duration of placement (hours) | 18.7 ± 8.1 (0–36) | 20.4 ± 5.8 (2–36) | 11.5 ± 12.1 (0–36) | 0.003 |
| Variables Significantly Associated with Failed BBT | OR | 95% CI | p Value |
|---|---|---|---|
| Maternal age | 1.26 | 1.07–1.47 | 0.01 |
| Caesarean delivery | 6.90 | 1.23–38.65 | 0.03 |
| Curettage before BBT insertion | 9.02 | 1.69–48.22 | 0.01 |
| ≥7 RBCUs transfused | 68.39 | 12.60–371.33 | <0.001 |
| Report (Author and Year) | Country | Type of Study | n | Vaginal/Caesarean Delivery n (%) | Indications for BBT n (%) | Clinical Success n (%) | Hysterectomy n (%) | Death n (%) | BBT-Related Complications |
|---|---|---|---|---|---|---|---|---|---|
| Revert M et al. 2016 [20] | France | Prospective cohort study; 10 maternity units | 226 | 171 (75.5%)/55 (24.3%) | Uterine atony: 183 (81%); Placenta previa: 33 (14.6%); Others: 10 (4.4%) | 188 (83.2%) | 11 (4.9%) | 0 | 1 endometritis |
| Maher MA et al. 2017 [21] | Saudi Arabia | Prospective cohort study; 2 hospitals | 72 | Cesarean: 72 (100%) | Placenta low-lying: 42 (58.5%); Placenta incomplete centralis: 20 (27.7%); Placenta complete centralis: 10 (13.8%) | 63 (87.5%) | 1 (1.4%) | 0 | 0 |
| Kong CW et al. 2018 [23] | Hong Kong | Retrospective | 81 | 24 (29.6%)/57 (70.4%) | Uterine atony: 53 (65.4%); Placenta previa/accreta: 25 (30.9%); Uterine/vaginal/cervical tears: 3 (3.7%) | 59 (72.8%) | 11 (13.6%) | 1 (1.2%) | 0 |
| Cho HY et al. 2015 [22] | Korea | Retrospective | 64 | Cesarean: 64 (100%) | Placenta previa totalis: 50 (78%); Placenta previa partialis. marginalis: 11 (17%); Low-lying placenta: 3 (5%) | 48 (75%) | 5 (8%) | 0 | 0 |
| Grange J et al. 2018 [24] | France | Retrospective case series study; 5 maternity units | 108 | Vaginal: 108 (100%) | Uterine atony: 39 (36.1%); Placenta previa: 6 (5.5%); Placenta accreta: 3 (2.8%); Retained placenta: 23 (21.3%) | 80 (74.1%) | 5 (4.6%) | 0 | 2 thromboembolic events |
| Total | 551 | 303 (55%)/248 (45%) | Uterine atony: 275 (50%); Placenta previa: 155 (28.1%) | 438 (79.5%) | 33 (6%) | 1 (0.2%) |
| Report (Author and Year) | Variables Significantly Associated with Failed BBT | OR | 95% CI | p Value |
| Revert M et al. 2016 [20] | Caesarean delivery | 3.5 | 1.6–7.6 | <0.05 |
| Estimated blood loss before BBT | 3.2 | 1.5–6.8 | <0.05 | |
| Coagulopathy | 5.6 | 2.5–13.0 | <0.05 | |
| Cho HY et al. 2015 [22] | Anterior placenta | 12.75 | 1.04–155.94 | 0.04 |
| History of cesarean section | 8.9 | 2.27–34.83 | <0.01 | |
| Grange J et al. 2018 [24] | Pre-pregnancy obesity | 4.4 | 1.06–18.31 | <0.05 |
| Report (Author and Year) | Variables Significantly Associated with Successful BBT | OR | 95% CI | p Value |
| Maher MA et al. 2017 [21] | Placenta accreta | 0.01 | 0.00–0.97 | 0.049 |
| Operation duration (min) | 1.14 | 1.02–1.28 | 0.023 | |
| Kong CW et al. 2018 [23] | Blood loss at the time of insertion of BBT | 0.99 | 0.99–0.99 | 0.041 |
| Volume of blood drained from the uterine cavity within first 30 min | 0.97 | 0.95–0.99 | 0.034 | |
| Placenta accreta | 0.01 | 0.01–0.98 | 0.048 | |
| Coagulopathy | 0.02 | 0.01–0.96 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Labarta, F.J.; Pintado Recarte, M.P.; Joigneau Prieto, L.; Bravo Arribas, C.; Bujan, J.; Ortega, M.A.; De León-Luis, J.A. Factors Associated with Failure of Bakri Balloon Tamponade for the Management of Postpartum Haemorrhage. Case Series Study and Systematic Review. Healthcare 2021, 9, 295. https://doi.org/10.3390/healthcare9030295
Ruiz Labarta FJ, Pintado Recarte MP, Joigneau Prieto L, Bravo Arribas C, Bujan J, Ortega MA, De León-Luis JA. Factors Associated with Failure of Bakri Balloon Tamponade for the Management of Postpartum Haemorrhage. Case Series Study and Systematic Review. Healthcare. 2021; 9(3):295. https://doi.org/10.3390/healthcare9030295
Chicago/Turabian StyleRuiz Labarta, Francisco Javier, María Pilar Pintado Recarte, Laura Joigneau Prieto, Coral Bravo Arribas, Julia Bujan, Miguel A. Ortega, and Juan A. De León-Luis. 2021. "Factors Associated with Failure of Bakri Balloon Tamponade for the Management of Postpartum Haemorrhage. Case Series Study and Systematic Review" Healthcare 9, no. 3: 295. https://doi.org/10.3390/healthcare9030295
APA StyleRuiz Labarta, F. J., Pintado Recarte, M. P., Joigneau Prieto, L., Bravo Arribas, C., Bujan, J., Ortega, M. A., & De León-Luis, J. A. (2021). Factors Associated with Failure of Bakri Balloon Tamponade for the Management of Postpartum Haemorrhage. Case Series Study and Systematic Review. Healthcare, 9(3), 295. https://doi.org/10.3390/healthcare9030295









