Potential Benefits of N-Acetylcysteine in Preventing Pregabalin-Induced Seeking-Like Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
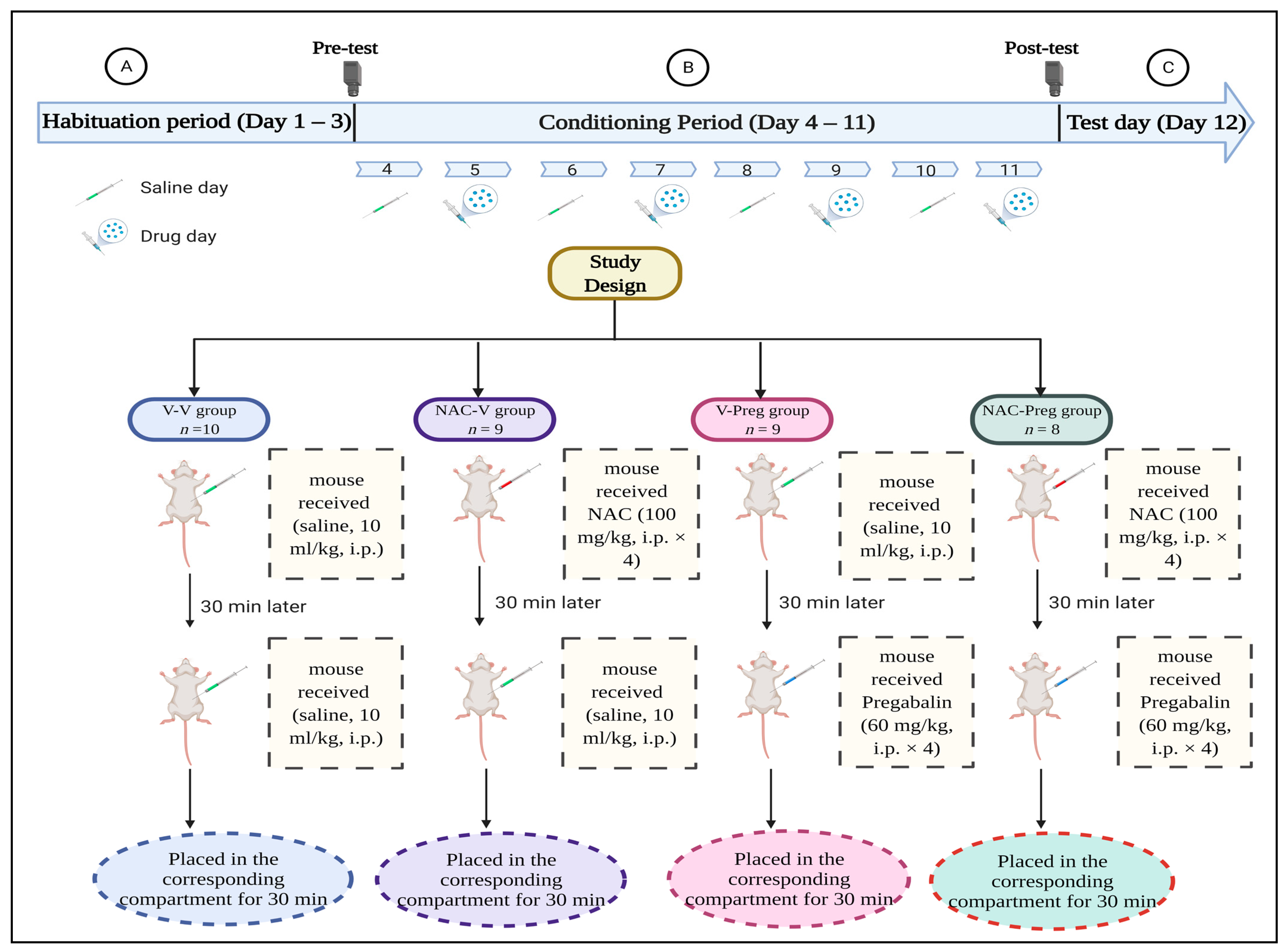
2.3. Experimental Design
2.3.1. CPP Model Apparatus
2.3.2. Habituation Period
2.3.3. Conditioning Period
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Taylor, C.P.; Weber, M.; Piechan, J.; Prior, F.; Bian, F.; Cui, M.; Hoffman, D.; Donevan, S. Pregabalin is a potent and selective ligand for α2δ-1 and α2δ-2 calcium channel subunits. Eur. J. Pharmacol. 2011, 667, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Pexton, T.; Moeller-Bertram, T.; Schilling, J.M.; Wallace, M.S. Targeting voltage-gated calcium channels for the treatment of neuropathic pain: A review of drug development. Expert Opin. Investig. Drugs 2011, 20, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Boschen, M.J. A meta-analysis of the efficacy of pregabalin in the treatment of generalized anxiety disorder. Can. J. Psychiatry 2011, 56, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bağımlılığı, P.; Oğuz, G.; Güven, F.M.; Batmaz, S. Pregabalin dependence: A case report. J. Depend. 2015, 16, 160–163. [Google Scholar]
- Gahr, M.; Freudenmann, R.W.; Hiemke, C.; Kölle, M.A.; Schönfeldt-Lecuona, C. Pregabalin abuse and dependence in Germany: Results from a database query. Eur. J. Clin. Pharmacol. 2013, 69, 1335–1342. [Google Scholar] [CrossRef]
- Halaby, A.; Kassm, S.A.; Naja, W.J. Pregabalin dependence: A case report. Curr. Drug Saf. 2015, 10, 184–186. [Google Scholar] [CrossRef]
- Saudigazette. Saudi Youth and the Abuse of Prescription Drugs. Available online: http://saudigazette.com.sa/article/155386/Saudi-youth-and-the-abuse-of-prescription-drugs (accessed on 13 February 2020).
- Bassiony, M. Substance use disorders in Saudi Arabia. J. Subst. Use 2013, 18, 450–466. [Google Scholar] [CrossRef]
- Carrus, D.; Schifano, F. Pregabalin misuse–related issues; intake of large dosages, drug-smoking allegations, and possible association with myositis: Two case reports. J. Clin. Psychopharmacol. 2012, 32, 839–840. [Google Scholar] [CrossRef]
- Schifano, F. Misuse and abuse of pregabalin and gabapentin: Cause for concern? CNS Drugs 2014, 28, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinotti, G.; Lupi, M.; Sarchione, F.; Santacroce, R.; Salone, A.; De Berardis, D.; Serroni, N.; Cavuto, M.; Signorelli, M.; Aguglia, E. The potential of pregabalin in neurology, psychiatry and addiction: A qualitative overview. Curr. Pharm. Des. 2013, 19, 6367–6374. [Google Scholar] [CrossRef]
- Gahr, M.; Freudenmann, R.W.; Kölle, M.A.; Schönfeldt-Lecuona, C. Pregabalin and addiction: Lessons from published cases. J. Subst. Use 2014, 19, 448–449. [Google Scholar] [CrossRef]
- Althobaiti, Y.S.; Almalki, A.; Alsaab, H.; Alsanie, W.; Gaber, A.; Alhadidi, Q.; Hardy, A.M.G.; Nasr, A.; Alzahrani, O.; Stary, C.M. Pregabalin: Potential for addiction and a possible glutamatergic mechanism. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Reissner, K.J.; Gipson, C.D.; Tran, P.K.; Knackstedt, L.A.; Scofield, M.D.; Kalivas, P.W. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict. Biol. 2015, 20, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Althobaiti, Y.S.; Alshehri, F.S.; Almalki, A.H.; Sari, Y. Effects of ceftriaxone on glial glutamate transporters in Wistar rats administered sequential ethanol and methamphetamine. Front. Neurosci. 2016, 10, 427. [Google Scholar] [CrossRef]
- Das, S.C.; Yamamoto, B.K.; Hristov, A.M.; Sari, Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 2015, 97, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Knackstedt, L.A.; Melendez, R.I.; Kalivas, P.W. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry 2010, 67, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Dravolina, O.A.; Zakharova, E.S.; Shekunova, E.V.; Zvartau, E.E.; Danysz, W.; Bespalov, A.Y. mGlu1 receptor blockade attenuates cue-and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology 2007, 52, 263–269. [Google Scholar] [CrossRef]
- Tessari, M.; Pilla, M.; Andreoli, M.; Hutcheson, D.M.; Heidbreder, C.A. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine-and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur. J. Pharmacol. 2004, 499, 121–133. [Google Scholar] [CrossRef]
- Lee, B.; Platt, D.M.; Rowlett, J.K.; Adewale, A.S.; Spealman, R.D. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: Comparison with dizocilpine. J. Pharmacol. Exp. Ther. 2005, 312, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2009, 35, 217–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornish, J.L.; Kalivas, P.W. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J. Neurosci. 2000, 20, RC89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.K.; Bari, A.; Jey, A.; Anderson, S.; Spealman, R.; Rowlett, J.; Pierce, R. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J. Neurosci. 2002, 22, 2916–2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, K.; Davidge, S.B.; Lapish, C.C.; Kalivas, P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004, 24, 1551–1560. [Google Scholar] [CrossRef]
- Bäckström, P.; Hyytiä, P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 2007, 192, 571–580. [Google Scholar] [CrossRef]
- McClure, E.A.; Gipson, C.D.; Malcolm, R.J.; Kalivas, P.W.; Gray, K.M. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs 2014, 28, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussawi, K.; Pacchioni, A.; Moran, M.; Olive, M.F.; Gass, J.T.; Lavin, A.; Kalivas, P.W. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009, 12, 182–189. [Google Scholar] [CrossRef] [Green Version]
- LaRowe, S.D.; Mardikian, P.; Malcolm, R.; Myrick, H.; Kalivas, P.; McFarland, K.; Saladin, M.; McRae, A.; Brady, K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am. J. Addict. 2006, 15, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Knackstedt, L.A.; LaRowe, S.; Mardikian, P.; Malcolm, R.; Upadhyaya, H.; Hedden, S.; Markou, A.; Kalivas, P.W. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry 2009, 65, 841–845. [Google Scholar] [CrossRef] [Green Version]
- LaRowe, S.D.; Kalivas, P.W.; Nicholas, J.S.; Randall, P.K.; Mardikian, P.N.; Malcolm, R.J. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am. J. Addict. 2013, 22, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Ducret, E.; Puaud, M.; Lacoste, J.; Belin-Rauscent, A.; Fouyssac, M.; Dugast, E.; Murray, J.E.; Everitt, B.J.; Houeto, J.L.; Belin, D. N-acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biol. Psychiatry 2016, 80, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.A.; McFarland, K.; Lake, R.W.; Shen, H.; Tang, X.C.; Toda, S.; Kalivas, P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003, 6, 743–749. [Google Scholar] [CrossRef]
- Zhou, W.; Kalivas, P.W. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue-and heroin-induced drug-seeking. Biol. Psychiatry 2008, 63, 338–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amen, S.L.; Piacentine, L.B.; Ahmad, M.E.; Li, S.J.; Mantsch, J.R.; Risinger, R.C.; Baker, D.A. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 2011, 36, 871. [Google Scholar] [CrossRef]
- Xi, Z.X.; Kiyatkin, M.; Li, X.; Peng, X.Q.; Wiggins, A.; Spiller, K.; Li, J.; Gardner, E.L. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology 2010, 58, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berk, M.; Malhi, G.S.; Gray, L.J.; Dean, O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013, 34, 167–177. [Google Scholar] [CrossRef]
- Brown, R.M.; Kupchik, Y.M.; Kalivas, P.W. The story of glutamate in drug addiction and of N-acetylcysteine as a potential pharmacotherapy. Jama Psychiatry 2013, 70, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M.; Schmidt, W.J. N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neurosci. Lett. 1995, 193, 37–40. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Dong, H.; Su, R.; Gong, Z.; Yan, L. Inhibition of vesicular glutamate transporters contributes to attenuate methamphetamine-induced conditioned place preference in rats. Behav. Brain Res. 2014, 267, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Gremel, C.M.; Groblewski, P.A. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006, 1, 1662. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Ferree, N.K.; Howard, M.A. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology 2003, 170, 409–422. [Google Scholar] [CrossRef]
- Tzschentke, T.M. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998, 56, 613–672. [Google Scholar] [CrossRef]
- Aldemir, E.; Altintoprak, A.E.; Coskunol, H. Pregabalin dependence: A case report. Turk. Psikiyatri Derg. 2015, 26, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, M.; Mutschler, J.; Hermann, D.; Klein, O.; Dressing, H.; Kiefer, F.; Mann, K. Pregabalin abuse, dependence, and withdrawal: A case report. Am. J. Psychiatry 2010, 167, 869. [Google Scholar] [CrossRef]
- Filipetto, F.A.; Zipp, C.P.; Coren, J.S. Potential for pregabalin abuse or diversion after past drug-seeking behavior. J. Am. Osteopath. Assoc. 2010, 110, 605–607. [Google Scholar]
- Andrews, N.; Loomis, S.; Blake, R.; Ferrigan, L.; Singh, L.; McKnight, A.T. Effect of gabapentin-like compounds on development and maintenance of morphine-induced conditioned place preference. Psychopharmacology 2001, 157, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ruttenl, K.; Vry, J.; Robens, A.; Tzschentke, T.M.; Kam, E.L. Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. Eur. J. Pain 2011, 15, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Seiva, F.R.; Amauchi, J.F.; Rocha, K.K.; Ebaid, G.X.; Souza, G.; Fernandes, A.A.; Cataneo, A.C.; Novelli, E.L. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol 2009, 43, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, Y.S.; Almalki, A.H. Effects of environmental enrichment on reinstatement of methamphetamine-induced conditioned place preference. Behav. Brain Res. 2020, 379, 112372. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, Y.S.; Alghorabi, A.; Alshehri, F.S.; Baothman, B.; Almalki, A.H.; Alsaab, H.O.; Alsanie, W.; Gaber, A.; Almalki, H.; Alghamdi, A.S. Gabapentin-induced drug-seeking-like behavior: A potential role for the dopaminergic system. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Nakagawa, T.; Fujio, M.; Ozawa, T.; Minami, M.; Satoh, M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav. Brain Res. 2005, 156, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Fujio, M.; Nakagawa, T.; Sekiya, Y.; Ozawa, T.; Suzuki, Y.; Minami, M.; Satoh, M.; Kaneko, S. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine-and morphine-induced conditioned place preference in rats. Eur. J. Neurosci. 2005, 22, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, Y.S.; Alshehri, F.S.; Hakami, A.Y.; Hammad, A.M.; Sari, Y. Effects of Clavulanic Acid Treatment on Reinstatement to Methamphetamine, Glial Glutamate Transporters, and mGluR 2/3 Expression in P Rats Exposed to Ethanol. J. Mol. Neurosci. 2019, 67, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Acquas, E.; Carboni, E.; Leone, P.; Di Chiara, G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: Anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology 1989, 99, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Amit, Z.; Splawinsky, J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol 1984, 1, 193–195. [Google Scholar] [CrossRef]
- Freynhagen, R.; Strojek, K.; Griesing, T.; Whalen, E.; Balkenohl, M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible-and fixed-dose regimens. Pain 2005, 115, 254–263. [Google Scholar] [CrossRef]
- Fink, K.; Dooley, D.J.; Meder, W.P.; Suman-Chauhan, N.; Duffy, S.; Clusmann, H.; Göthert, M.J.N. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 2002, 42, 229–236. [Google Scholar] [CrossRef]
- Errante, L.D.; Petroff, O.A. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure 2003, 12, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Sills, G.J. The mechanisms of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 2006, 6, 108–113. [Google Scholar] [CrossRef]
- Narita, N.; Kumar, N.; Cherkas, P.S.; Chiang, C.Y.; Dostrovsky, J.O.; Coderre, T.J.; Sessle, B.J.J.N. Systemic pregabalin attenuates sensorimotor responses and medullary glutamate release in inflammatory tooth pain model. Neuroscience 2012, 218, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Cherkas, P.S.; Varathan, V.; Miyamoto, M.; Chiang, C.Y.; Dostrovsky, J.O.; Sessle, B.J.; Coderre, T.J. Systemic pregabalin attenuates facial hypersensitivity and noxious stimulus-evoked release of glutamate in medullary dorsal horn in a rodent model of trigeminal neuropathic pain. Neurochem. Int. 2013, 62, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Laferriere, A.; Yu, J.S.; Leavitt, A.; Coderre, T.J. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J. Neurochem. 2010, 113, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.E.; Napadow, V.; Huggins, J.P.; Pauer, L.; Kim, J.; Hampson, J.; Sundgren, P.C.; Foerster, B.; Petrou, M.; Schmidt-Wilcke, T.; et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013, 119, 1453–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.H.; Lee, P.B.; Kim, J.H.; Do, S.H.; Kim, C.S. Effects of pregabalin on the activity of glutamate transporter type 3. Br. J. Anaesth. 2012, 109, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.M.; Swanson, R.A.J.G. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Gila 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Gipson, C.D.; Reissner, K.J.; Kupchik, Y.M.; Smith, A.C.; Stankeviciute, N.; Hensley-Simon, M.E.; Kalivas, P.W. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc. Natl. Acad. Sci. USA 2013, 110, 9124–9129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalivas, P.W.; McFarland, K.; Bowers, S.; Szumlinski, K.; Xi, Z.X.; Baker, D. Glutamate transmission and addiction to cocaine. Ann. N. Y. Acad. Sci. 2003, 1003, 169–175. [Google Scholar] [CrossRef]
- Sari, Y.; Sreemantula, S.N.; Lee, M.R.; Choi, D.S. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J. Mol. Neurosci. 2013, 51, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Fischer-Smith, K.D.; Houston, A.C.; Rebec, G.V. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience 2012, 210, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Moussawi, K.; Kalivas, P.W. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur. J. Pharmacol. 2010, 639, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Melendez, R.I.; Vuthiganon, J.; Kalivas, P.W. Regulation of extracellular glutamate in the prefrontal cortex: Focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2005, 314, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbe, D.; Bockaert, J.; Manzoni, O.J. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur. J. Neurosci. 2002, 16, 2231–2235. [Google Scholar] [CrossRef] [Green Version]
- Madayag, A.; Lobner, D.; Kau, K.S.; Mantsch, J.R.; Abdulhameed, O.; Hearing, M.; Grier, M.D.; Baker, D.A. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J. Neurosci. 2007, 27, 13968–13976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar]
- Krzyżanowska, W.; Pomierny, B.; Bystrowska, B.; Pomierny-Chamioło, L.; Filip, M.; Budziszewska, B.; Pera, J. Ceftriaxone-and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS ONE 2017, 12, e0186243. [Google Scholar] [CrossRef] [Green Version]
- Kalivas, P.; Volkow, N. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol. Psychiatry 2011, 16, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhouib, I.E.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Robaczewska, J.; Kedziora-Kornatowska, K.; Kozakiewicz, M.; Zary-Sikorska, E.; Pawluk, H.; Pawliszak, W.; Kedziora, J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J. Physiol. Pharmacol. 2016, 67, 331–337. [Google Scholar]
- Sözbir, E.; Nazıroğlu, M. Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab. Brain Dis. 2016, 31, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.K.; Tandan, S.K.; Dudhgaonkar, S.P.; Jadhav, S.H.; Kataria, M.; Prakash, V.R.; Kumar, D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-l-cysteine in rats. Eur. J. Pain 2006, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Horst, A.; Kolberg, C.; Moraes, M.S.; Riffel, A.P.K.; Finamor, I.A.; Belló-Klein, A.; Pavanato, M.A.; Partata, W.A. Effect of N-acetylcysteine on the spinal-cord glutathione system and nitric-oxide metabolites in rats with neuropathic pain. Neurosci. Lett. 2014, 569, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tata, D.A.; Yamamoto, B.K. Interactions between methamphetamine and environmental stress: Role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction 2007, 102, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ozaras, R.; Tahan, V.; Aydin, S.; Uzun, H.; Kaya, S.; Senturk, H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J. Gastroenterol. 2003, 9, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Bandiera, S.; Souza, D.G.; Bellaver, B.; Caletti, G.; Quincozes-Santos, A.; Elisabetsky, E.; Gomez, R. N-acetylcysteine prevents alcohol related neuroinflammation in rats. Neurochem. Res. 2017, 42, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Bevins, R.; Cunningham, C. Tasks and techniques. In A Sampling of the Methodologies for the Investigation of Animal Learning, Behavior and Cognition; Anderson, M.J., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2006. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almalki, A.H.; Alsaab, H.O.; Alsanie, W.F.; Gaber, A.; Alkhalifa, T.; Almalki, A.; Alzahrani, O.; Hardy, A.M.G.; Alhadidi, Q.; Shah, Z.A.; et al. Potential Benefits of N-Acetylcysteine in Preventing Pregabalin-Induced Seeking-Like Behavior. Healthcare 2021, 9, 376. https://doi.org/10.3390/healthcare9040376
Almalki AH, Alsaab HO, Alsanie WF, Gaber A, Alkhalifa T, Almalki A, Alzahrani O, Hardy AMG, Alhadidi Q, Shah ZA, et al. Potential Benefits of N-Acetylcysteine in Preventing Pregabalin-Induced Seeking-Like Behavior. Healthcare. 2021; 9(4):376. https://doi.org/10.3390/healthcare9040376
Chicago/Turabian StyleAlmalki, Atiah H., Hashem O. Alsaab, Walaa F. Alsanie, Ahmed Gaber, Turki Alkhalifa, Ahmad Almalki, Omar Alzahrani, Ana Maria Gregio Hardy, Qasim Alhadidi, Zahoor A. Shah, and et al. 2021. "Potential Benefits of N-Acetylcysteine in Preventing Pregabalin-Induced Seeking-Like Behavior" Healthcare 9, no. 4: 376. https://doi.org/10.3390/healthcare9040376








