Implications of Lifestyle and Occupational Factors on the Risk of Breast Cancer in Shiftwork Nurses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Sample
2.2. Shift Work and Night Work Definition
2.3. Instrument
2.4. Variables
- Sociodemographic data (age, sex and marital relationship).
- Self-perception of health. An ad hoc evaluation tool consisting of 5 direct questions was designed to assess nurses’ perception of their own health. The valuation scale was a Likert type with ten response possibilities ranging from 1 “very low” to 10 “very high”. The questions used were: “How do you value your overall health?”, “How do you value the quality of your rest?”, “How do you value the effect shift work has on your health?”, “How do you value your level of work stress?” and “How do you value your satisfaction with your current job?”.
- General data on disease and cancer (current disease, oncological disease, number of mammograms, use of oral contraceptives and presence of first-degree familial cancer).
- Lifestyle habits (Body Mass Index-BMI-was calculated with the weight and height indicated by the participants. Working exertion, measured by light, moderate, hard or very hard. Free-time physical activity, measured by the time spent in hours).
- Family burdens (care for children under 14, and care for dependents or elderly family members at home).
- Sleeping aids (“Do you take any medication to sleep?”, “which?”).
- Exposure to tobacco (consumption habits (did you ever smoked?), exposure to tobacco in the workplace and at home).
- Labour information (type of current working schedule, working experience (throughout life), number of years working regularly 3 nights per month or more, number of worked nights accumulated throughout life, and sick leaves throughout life and in the last year).
2.5. Data Collection Procedure
2.6. Statistical Analysis
- Root Node: represents a single input variable and a split point on that variable. It does not have any parent node and gives two children nodes based on the question.
- Internal Node: it will have a parent node and gives two children nodes.
- Terminal or Leaf Node: contains an output variable which is used to make a prediction. This node will have a parent node but will not have any children nodes.
2.7. Ethical Considerations
3. Results
3.1. Two-Dimensional Analysis for Healthy Participants and Those Who Have or Ever Had Breast Cancer
3.2. Self-Perception of Health Descriptive Analysis
3.3. Breast Cancer Prediction
3.3.1. Considering Labour Variables and Sleep Medication
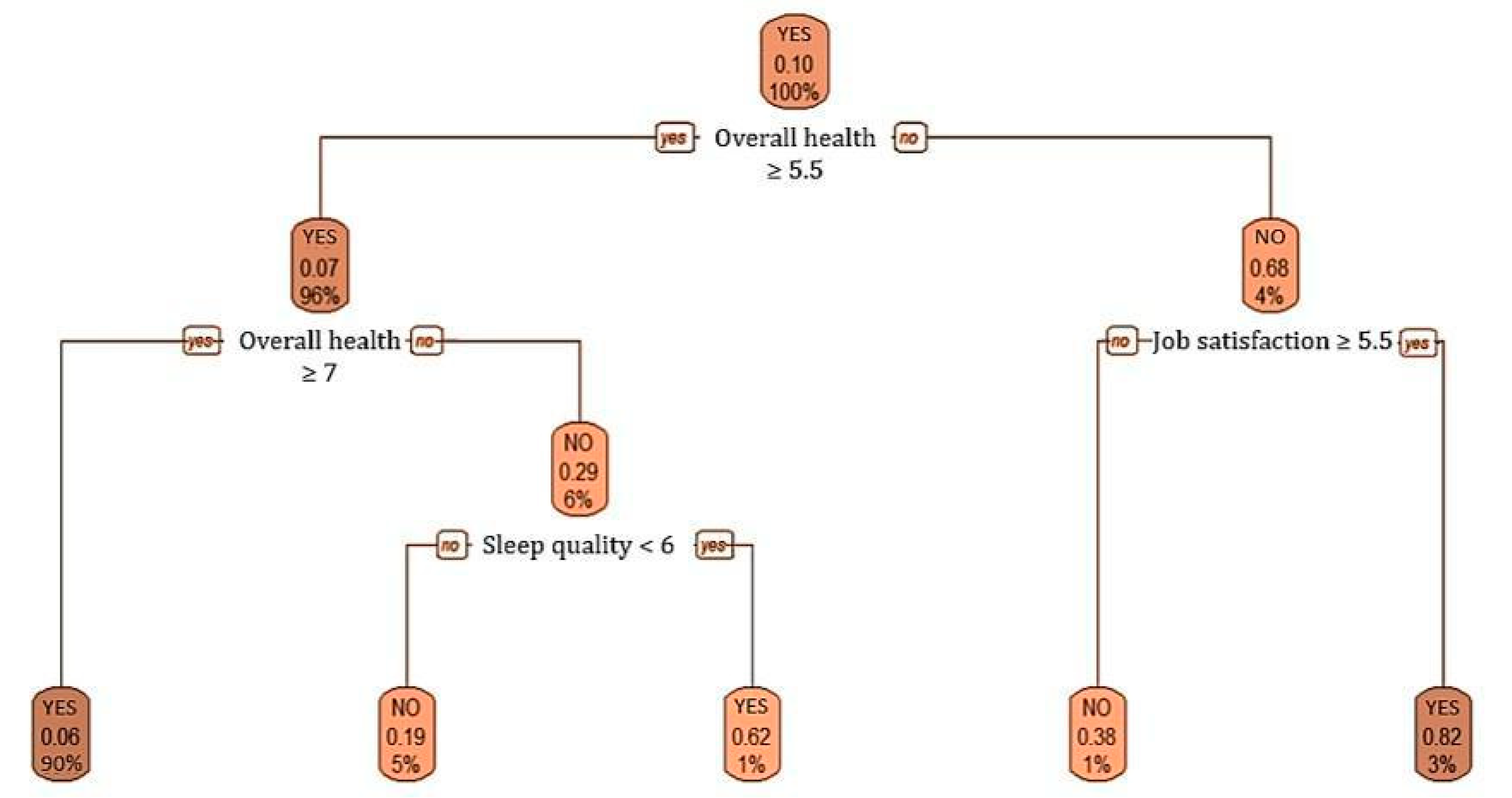
3.3.2. Self-Perception of Health Variable
4. Discussion
4.1. Implications for the Practice and Applicability
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reszka, E.; Peplonska, B.; Wieczorek, E.; Sobala, W.; Bukowska, A.; Gromadzinska, J.; Lie, J.-A.; Kjuus, H.; Wasowicz, W. Rotating night shift work and polymorphism of genes important for the regulation of circadian rhythm. Scand. J. Work. Environ. Health 2012, 39, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Peplonska, B.; Wieczorek, E.; Sobala, W.; Bukowska, A.; Gromadzinska, J.; Lie, J.-A.; Kjuus, H.; Wasowicz, W. Circadian gene expression in peripheral blood leukocytes of rotating night shift nurses. Scand. J. Work. Environ. Health 2013, 39, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, J.-A.S.; Kjuus, H.; Zienolddiny, S.; Haugen, A.; Stevens, R.G.; Kjaerheim, K. Night Work and Breast Cancer Risk Among Norwegian Nurses: Assessment by Different Exposure Metrics. Am. J. Epidemiol. 2011, 173, 1272–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, J.-A.S.; Kjuus, H.; Zienolddiny, S.; Haugen, A.; Kjaerheim, K. Breast Cancer Among Nurses: Is the Intensity of Night Work Related to Hormone Receptor Status? Am. J. Epidemiol. 2013, 178, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Bracci, M.; Manzella, N.; Copertaro, A.; Staffolani, S.; Strafella, E.; Barbaresi, M.; Copertaro, B.; Rapisarda, V.; Valentino, M.; Santarelli, L. Rotating-shift nurses after a day off: Peripheral clock gene expression, urinary melatonin, and serum 17-β-estradiol levels. Scand. J. Work. Environ. Health 2014, 40, 295–304. [Google Scholar] [CrossRef]
- Zienolddiny, S.; Haugen, A.; Lie, J.-A.S.; Kjuus, H.; Anmarkrud, K.H.; Kjærheim, K. Analysis of polymorphisms in the circadian-related genes and breast cancer risk in Norwegian nurses working night shifts. Breast Cancer Res. 2013, 15, R53. [Google Scholar] [CrossRef] [Green Version]
- Erdem, J.S.; Notø, H.Ø.; Skare, Ø.; Lie, J.S.; Petersen-Øverleir, M.; Reszka, E.; Pepłońska, B.; Zienolddiny, S. Mechanisms of breast cancer risk in shift workers: Association of telomere shortening with the duration and intensity of night work. Cancer Med. 2017, 6, 1988–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carugno, M.; Maggioni, C.; Crespi, E.; Bonzini, M.; Cuocina, S.; Dioni, L.; Tarantini, L.; Consonni, D.; Ferrari, L.; Pesatori, A.C. Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses. Int. J. Environ. Res. Public Health 2019, 16, 2292. [Google Scholar] [CrossRef] [Green Version]
- Bracci, M.; Ciarapica, V.; Zabaleta, M.E.; Tartaglione, M.F.; Pirozzi, S.; Giuliani, L.; Piva, F.; Valentino, M.; Ledda, C.; Rapisarda, V.; et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers 2019, 11, 1146. [Google Scholar] [CrossRef] [Green Version]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peplonska, B.; Bukowska, A.; Lie, J.A.; Gromadzinska, J.; Zienolddiny, S. Night shift work and other determinants of estradiol, testosterone, and dehydroepiandrosterone sulfate among middle-aged nurses and midwives. Scand. J. Work. Environ. Health 2016, 42, 435–446. [Google Scholar] [CrossRef]
- Cordina-Duverger, E.; Menegaux, F.; Popa, A.; Rabstein, S.; Harth, V.; Pesch, B.; Brüning, T.; Fritschi, L.; Glass, D.; Heyworth, J.S.; et al. Night shift work and breast cancer: A pooled analysis of population-based case–control studies with complete work history. Eur. J. Epidemiol. 2018, 33, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Kroenke, C.H.; Laden, F.; Hankinson, S.E. Night work and risk of breast cancer. Epidemiology 2006, 17, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Inostrosa, M.P.A.; Robles, J.M.D.L.T.; Del Rio, M.V.C.; Lazo, C.E. Trabajo nocturno y cáncer de mama en personal sanitario. Rev. Soc. Esp. Med. Trap. 2018, 27, 141–149. [Google Scholar]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragones, N.; Pérez-Gómez, B.; Ardanaz, E.; Altzibar, J.M.; Sanchez, V.M.; Gomez-Acebo, I.; Llorca, J.; et al. Breast cancer risk and night shift work in a case–control study in a Spanish population. Eur. J. Epidemiol. 2015, 31, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Tamimi, R.M.; Baer, H.J.; Marotti, J.; Galan, M.; Galaburda, L.; Fu, Y.; Deitz, A.C.; Connolly, J.L.; Schnitt, S.J.; Colditz, G.; et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008, 10, R67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotti, J.D.; Collins, L.C.; Hu, R.; Tamimi, R.M. Estrogen receptor-β expression in invasive breast cancer in relation to molecular phenotype: Results from the Nurses’ Health Study. Mod. Pathol. 2009, 23, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Rosa, D.; Terzoni, S.; Dellafiore, F.; Destrebecq, A. Systematic review of shift work and nurses’ health. Occup. Med. 2019, 69, 237–243. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Painting, Firefighting and Shiftwork; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Night Shift Work; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Ward, E.M.; Germolec, D.; Kogevinas, M.; McCormick, D.; Vermeulen, R.; Anisimov, V.N.; Aronson, K.J.; Bhatti, P.; Cocco, P.; Costa, G.; et al. Carcinogenicity of night shift work. Lancet Oncol. 2019, 20, 1058–1059. [Google Scholar] [CrossRef]
- McElvenny, D.M.; Crawford, J.O.; Davis, A.; Dixon, K.; Alexander, C.; Cowie, H.; Cherrie, J.W. A Review of the Impact of Shift Work on Occupational Cancer. Available online: https://www.iosh.co.uk/shiftworkreview (accessed on 31 March 2021).
- Copertaro, A.; Bracci, M. Working against the biological clock: A review for the Occupational Physician. Ind. Health 2019, 57, 557–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, T.; Bordin-Junior, N.A.; De Almeida, E.A.; Zuccari, D.A.P.D.C. Evaluation of melatonin and AFMK levels in women with breast cancer. Endocrine 2018, 62, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Trudel-Fitzgerald, C.; Zhou, E.S.; Poole, E.M.; Zhang, X.; Michels, K.B.; Eliassen, A.H.; Chen, W.Y.; Holmes, M.D.; Tworoger, S.S.; Schernhammer, E.S. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br. J. Cancer 2017, 116, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Toxicology Program. Draft Report on Carcinogens Monograph on Night Shift Work and Light at Night; U.S. Department of Health and Human Services: Washington, WA, USA, 2018.
- González-González, A.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules 2018, 23, 336. [Google Scholar] [CrossRef] [Green Version]
- Rutters, F.; Lemmens, S.G.; Adam, T.C.; Bremmer, M.; Elders, P.J.; Nijpels, G.; Dekker, J.M. Is Social Jetlag Associated with an Adverse Endocrine, Behavioral, and Cardiovascular Risk Profile? J. Biol. Rhythm. 2014, 29, 377–383. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Ki, J.; Ryu, J.; Baek, J.; Huh, I.; Choi-Kwon, S. Association between Health Problems and Turnover Intention in Shift Work Nurses: Health Problem Clustering. Int. J. Environ. Res. Public Health 2020, 17, 4532. [Google Scholar] [CrossRef]
- Costa, G.; Sartori, S. Ageing, working hours and work ability. Ergonomics 2007, 50, 1914–1930. [Google Scholar] [CrossRef] [PubMed]
- Di Muzio, M.; Reda, F.; Diella, G.; Di Simone, E.; Novelli, L.; D’Atri, A.; Giannini, A.; De Gennaro, L. Not only a Problem of Fatigue and Sleepiness: Changes in Psychomotor Performance in Italian Nurses across 8-h Rapidly Rotating Shifts. J. Clin. Med. 2019, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Jã¸rgensen, J.T.; Karlsen, S.; Stayner, L.; Andersen, J.; Andersen, Z.J. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scand. J. Work. Environ. Health 2016, 43, 117–126. [Google Scholar] [CrossRef]
- Lee, G.-J.; Kim, K.; Kim, S.-Y.; Kim, J.-H.; Suh, C.; Son, B.-C.; Lee, C.-K.; Choi, J. Effects of shift work on abdominal obesity among 20–39-year-old female nurses: A 5-year retrospective longitudinal study. Ann. Occup. Environ. Med. 2016, 28, 69. [Google Scholar] [CrossRef] [Green Version]
- Ramin, C.; Devore, E.; Wang, W.; Pierre-Paul, J.; Wegrzyn, L.R.; Schernhammer, E.S. Night shift work at specific age ranges and chronic disease risk factors. Occup. Environ. Med. 2015, 72, 100–107. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568. [Google Scholar] [CrossRef] [Green Version]
- Stimpfel, A.W.; Aiken, L.H. Hospital Staff Nurses’ Shift Length Associated with Safety and Quality of Care. J. Nurs. Care Qual. 2013, 28, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.; Sims, S.; Parr, J.; Davies, N. Impact of 12h shift patterns in nursing: A scoping review. Int. J. Nurs. Stud. 2015, 52, 605–634. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, J.; Tolley, C.; Lamond, N.; Heuvel, C.V.D.; Pincombe, J.; Rogers, A.E.; Drew, D. Sleep and errors in a group of Australian hospital nurses at work and during the commute. Appl. Ergon. 2008, 39, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Clendon, J.; Gibbons, V. 12 h shifts and rates of error among nurses: A systematic review. Int. J. Nurs. Stud. 2015, 52, 1231–1242. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística (INE). Profesionales Sanitarios en España en 2019. Available online: https://www.ine.es/uc/EpbTZETN (accessed on 31 March 2021).
- Stevens, R.G.; Hansen, J.; Costa, G.; Haus, E.; Kauppinen, T.; Aronson, K.J.; Castaño-Vinyals, G.; Davis, S.; Frings-Dresen, M.H.W.; Fritschi, L.; et al. Considerations of circadian impact for defining ’shift work’ in cancer studies: IARC Working Group Report. Occup. Environ. Med. 2010, 68, 154–162. [Google Scholar] [CrossRef]
- Directiva 2003/88/CE del Parlamento Europeo y del Consejo, de 4 de Noviembre de 2003, Relativa a Determinados Aspectos de la Ordenación del Tiempo de Trabajo. Available online: https://www.boe.es/doue/2003/299/L00009-00019.pdf (accessed on 31 March 2021).
- Nebot, M.; Manzanares, S.; López, M.J.; Ariza, C.; Galán, I.; Moncada, A.; Montes, A.; Pérez-Ríos, M.; Schiaffino, A.; Fernández, E. Estimación de la exposición al humo ambiental de tabaco: Revisión de cuestionarios utilizados en España. Gac. Sanit. 2011, 25, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagundo-Rivera, J.; Gómez-Salgado, J.; García-Iglesias, J.; Gómez-Salgado, C.; Camacho-Martín, S.; Ruiz-Frutos, C. Relationship between Night Shifts and Risk of Breast Cancer among Nurses: A Systematic Review. Medicina 2020, 56, 680. [Google Scholar] [CrossRef] [PubMed]
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Routledge: Boca Raton, FL, USA, 2017. [Google Scholar]
- Krzywinski, M.; Altman, N. Classification and regression trees. Nat. Methods 2017, 14, 757–758. [Google Scholar] [CrossRef]
- Szkiela, M.; Kusideł, E.; Makowiec-Dąbrowska, T.; Kaleta, D. Night Shift Work—A Risk Factor for Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 659. [Google Scholar] [CrossRef] [Green Version]
- Bustamante-Montes, L.P.; Flores-Meza, B.; Hernández-Valero, M.A.; Cárdenas-López, A.; Dolores-Velázquez, R.; Borja-Bustamante, P.; Borja-Aburto, V.H. Night Shift Work and Risk of Breast Cancer in Women. Arch. Med. Res. 2019, 50, 393–399. [Google Scholar] [CrossRef]
- López-Abente, G.; Aragonés, N.; Pérez-Gómez, B.; Pollán, M.; García-Pérez, J.; Ramis, R.; Fernández-Navarro, P. Time trends in municipal distribution patterns of cancer mortality in Spain. BMC Cancer 2014, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Schernhammer, E.S.; Hankinson, S.E.; Rosner, B.; Kroenke, C.H.; Willett, W.C.; Colditz, G.A.; Kawachi, I. Job Stress and Breast Cancer Risk: The Nurses’ Health Study. Am. J. Epidemiol. 2004, 160, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossek, E.E.; Lee, K.-H. Work-Family Conflict and Work-Life Conflict. Oxf. Res. Encycl. Bus. Manag. 2017, 1–23. [Google Scholar] [CrossRef]
- Labrague, L.; Ballad, C.; Fronda, D. Predictors and outcomes of work–family conflict among nurses. Int. Nurs. Rev. 2020. [Google Scholar] [CrossRef]
- Masuda, A.D.; Sortheix, F.; Beham, B.; Naidoo, L.J. Cultural value orientations and work–family conflict: The mediating role of work and family demands. J. Vocat. Behav. 2019, 112, 294–310. [Google Scholar] [CrossRef]
- Arlinghaus, A.; Bohle, P.; Iskra-Golec, I.; Jansen, N.; Jay, S.; Rotenberg, L. Working Time Society consensus statements: Evidence-based effects of shift work and non-standard working hours on workers, family and community. Ind. Health 2019, 57, 184–200. [Google Scholar] [CrossRef] [Green Version]
- Pinto, K.A.; Menezes, G.M.D.S.; Griep, R.H.; Lima, K.T.R.D.S.; Almeida, M.D.C.C.D.; Aquino, E.M.L. Work-family conflict and time use: Psychometric assessment of an instrument in ELSA-Brazil. Rev. Saúde Públ. 2016, 50, 39. [Google Scholar] [CrossRef]
- Svedberg, P.; Mather, L.; Bergström, G.; Lindfors, P.; Blom, V. Time pressure and sleep problems due to thoughts about work as risk factors for future sickness absence. Int. Arch. Occup. Environ. Health 2018, 91, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Savard, J.; Morin, C.M. Insomnia in the Context of Cancer: A Review of a Neglected Problem. J. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Haus, E.; Stevens, R. Shift work and cancer—Considerations on rationale, mechanisms, and epidemiology. Scand. J. Work. Environ. Health 2010, 36, 163–179. [Google Scholar] [CrossRef]
- Khan, W.A.A.; Jackson, M.L.; Kennedy, G.A.; Conduit, R. A field investigation of the relationship between rotating shifts, sleep, mental health and physical activity of Australian paramedics. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Neri, A.; Somma, G.; Coppeta, L.; Iavicoli, I.; Bergamaschi, A.; Magrini, A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup. Environ. Med. 2009, 67, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Eldevik, M.F.; Flo, E.; Moen, B.E.; Pallesen, S.; Bjorvatn, B. Insomnia, Excessive Sleepiness, Excessive Fatigue, Anxiety, Depression and Shift Work Disorder in Nurses Having Less than 11 Hours In-Between Shifts. PLoS ONE 2013, 8, e70882. [Google Scholar] [CrossRef]
- Costa, G.; Anelli, M.M.; Castellini, G.; Fustinoni, S.; Neri, L. Stress and sleep in nurses employed in “3 × 8” and “2 × 12” fast rotating shift schedules. Chrono Int. 2014, 31, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, M.; Brown, J.A.; Breen, L.J.; Kane, R.; Hegney, D.; Rees, C.S. A comparison of nurse shift workers’ and non-shift workers’ psychological functioning and resilience. J. Adv. Nurs. 2019, 75, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Risquez, M.I.; Godoy-Fernández, C. Association between occupational satisfaction and perceived general health in emergency nurses. Enferm. Clin. 2008, 18, 134–141. [Google Scholar] [CrossRef]
- McHugh, M.D.; Kutney-Lee, A.; Cimiotti, J.P.; Sloane, D.M.; Aiken, L.H. Nurses’ Widespread Job Dissatisfaction, Burnout, and Frustration with Health Benefits Signal Problems for Patient Care. Health Aff. 2011, 30, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Ferri, P.; Guadi, M.; Marcheselli, L.; Balduzzi, S.; Magnani, D.; Di Lorenzo, R. The impact of shift work on the psychological and physical health of nurses in a general hospital: A comparison between rotating night shifts and day shifts. Health Policy 2016, 9, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bogaert, P.; Kowalski, C.; Weeks, S.M.; Van Heusden, D.; Clarke, S.P. The relationship between nurse practice environment, nurse work characteristics, burnout and job outcome and quality of nursing care: A cross-sectional survey. Int. J. Nurs. Stud. 2013, 50, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Stimpfel, A.W.; Sloane, D.M.; Aiken, L.H. The Longer the Shifts for Hospital Nurses, the Higher the Levels of Burnout and Patient Dissatisfaction. Health Aff. 2012, 31, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Stimpfel, A.W.; Lake, E.T.; Barton, S.; Gorman, K.C.; Aiken, L.H. How Differing Shift Lengths Relate to Quality Outcomes in Pediatrics. JONA 2013, 43, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Ramoo, V.; Abdullah, K.L.; Piaw, C.Y. The relationship between job satisfaction and intention to leave current employment among registered nurses in a teaching hospital. J. Clin. Nurs. 2013, 22, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Aiken, L.H.; Sermeus, W.; Heede, K.V.D.; Sloane, D.M.; Busse, R.; McKee, M.; Bruyneel, L.; Rafferty, A.M.; Griffiths, P.; Moreno-Casbas, M.T.; et al. Patient safety, satisfaction, and quality of hospital care: Cross sectional surveys of nurses and patients in 12 countries in Europe and the United States. BMJ 2012, 344, e1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiken, L.H.; Sloane, D.M.; Bruyneel, L.; Heede, K.V.D.; Sermeus, W. Nurses’ reports of working conditions and hospital quality of care in 12 countries in Europe. Int. J. Nurs. Stud. 2013, 50, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project—Systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Brum, M.C.B.; Filho, F.F.D.; Schnorr, C.C.; Bottega, G.B.; Rodrigues, T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peplonska, B.; Bukowska, A.; Sobala, W. Association of Rotating Night Shift Work with BMI and Abdominal Obesity among Nurses and Midwives. PLoS ONE 2015, 10, e0133761. [Google Scholar] [CrossRef]
- Di Sibio, A.; Abriata, G.; Buffa, R.; Viniegra, M.; Forman, D.; Sierra, M.S. Etiology of breast cancer (C50) in Central and South America. In Cancer in Central and South America; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Dossus, L.; Boutron-Ruault, M.-C.; Kaaks, R.; Gram, I.T.; Vilier, A.; Fervers, B.; Manjer, J.; Tjonneland, A.; Olsen, A.; Overvad, K.; et al. Active and passive cigarette smoking and breast cancer risk: Results from the EPIC cohort. Int. J. Cancer 2013, 134, 1871–1888. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Margolis, K.L.; Wactawski-Wende, J.; Horn, K.; Messina, C.; Stefanick, M.L.; Tindle, H.; Tong, E.; Rohan, T. Association of active and passive smoking with risk of breast cancer among postmenopausal women: A prospective cohort study. BMJ 2011, 342, d1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Guillén, A.G.; Pardo, J.M.V. Return to Work after Breast Cancer. Med. Segur. Trap. 2017, 63, 51–67. [Google Scholar]
- Guinan, E.M.; Connolly, E.M.; Kennedy, M.J.; Hussey, J. The presentation of metabolic dysfunction and the relationship with energy output in breast cancer survivors: A cross-sectional study. Nutr. J. 2013, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Bruno, E.; Gargano, G.; Villarini, A.; Traina, A.; Johansson, H.; Mano, M.P.; De Magistris, M.S.; Simeoni, M.; Consolaro, E.; Mercandino, A.; et al. Adherence to WCRF/AICR cancer prevention recommendations and metabolic syndrome in breast cancer patients. Int. J. Cancer 2015, 138, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Toribio, M.J.; Lope, V.; Castelló, A.; Salas, D.; Vidal, C.; Ascunce, N.; Santamariña, C.; Moreo, P.; Pedraz-Pingarrón, C.; Sánchez-Contador, C.; et al. Prevalence of healthy lifestyles against cancer in Spanish women. Sci. Rep. 2019, 9, 10638. [Google Scholar] [CrossRef]
- Barrios-Rodríguez, R.; Toledo, E.; Martinez-Gonzalez, M.A.; Aguilera-Buenosvinos, I.; Romanos-Nanclares, A.; Jiménez-Moleón, J.J. Adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research Recommendations and Breast Cancer in the SUN Project. Nutrients 2020, 12, 2076. [Google Scholar] [CrossRef] [PubMed]
- Albala, L.; Bober, T.; Hale, G.; Warfield, B.; Collins, M.L.; Merritt, Z.; Steimetz, E.; Nadler, S.; Lev, Y.; Hanifin, J. Effect on nurse and patient experience: Overnight use of blue-depleted illumination. BMJ Open Qual. 2019, 8, e000692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, G. Borg’s Perceived Exertion and Pan Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Sala, E.; Bonfiglioli, R.; Fostinellil, J.; Tomasi, C.; Graziosi, F.; Violante, F.S.; Apostoli, P. Risk assessment comparison of biomechanical overloading of the musculoskeletal system: 10 years’ applied experience. Ital. Med. Lav. Ergon. 2014, 36, 260–266. [Google Scholar]
- Sala, E.; Lopomo, N.F.; Tomasi, C.; Romagnoli, F.; Morotti, A.; Apostoli, P.; De Palma, G. Importance of Work-Related Psychosocial Factors in Exertion Perception Using the Borg Scale Among Workers Subjected to Heavy Physical Work. Front. Public Health 2021, 9. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A.G.; Cull, A.; Bjordal, K.; Groenvold, M.; Aaronson, N.K. The European Organization for Research and treatment of cancer approach to quality of life assessment: Guidelines for developing questionnaire modules. Qual. Life Res. 1993, 2, 287–295. [Google Scholar] [CrossRef]
- Sprangers, M.; Groenvold, M.; I Arraras, J.; Franklin, J.; Velde, A.T.; Muller, M.; Franzini, L.; Williams, A.; De Haes, H.C.; Hopwood, P.; et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J. Clin. Oncol. 1996, 14, 2756–2768. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Randler, C.; Díaz-Morales, J.F.; Rahafar, A.; Vollmer, C. Morningness–eveningness and amplitude—Development and validation of an improved composite scale to measure circadian preference and stability (MESSi). Chrono Int. 2016, 33, 832–848. [Google Scholar] [CrossRef]

| Cases | Percentage | |
|---|---|---|
| Permanent Shift | 107 | 19.2% |
| Only morning | 73 | 68.2% |
| Only afternoon/evening | 5 | 4.7% |
| Only night | 29 | 27.1% |
| Rotative 3 Shifts/24 h Cycles (M/AE/N) | 212 | 37.9% |
| Rotative 2 Shifts/24 h Cycles | 204 | 36.6% |
| Only morning + eventual extra-duty (+17 h) | 33 | 16.2% |
| 12-h shifts | 71 | 34.8% |
| Rotative M and N | 31 | 15.2% |
| Rotative M and AE | 62 | 30.4% |
| Rotative AE and N | 7 | 3.4% |
| 24-h Shifts | 21 | 3.8% |
| Irregular | 14 | 2.5% |
| Total general | 558 | 100% |
| N (%) | Healthy Cases (%) (N = 502) | Breast Cancer Cases (%) (N = 56) | χ2 | p | Odds Ratio (CI = 95%) | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 58 (10.4) | 53 (10.6) | 5 (8.9) | 0.144 | 0.705 | 1.203 (0.460, 3.145) |
| Female | 500 (89.6) | 449 (89.4) | 51 (91.1) | |||
| Age | ||||||
| 41 or younger | 281 (50.4) | 256 (51.0) | 25 (44.6) | 0.813 | 0.367 | 1.290 (0.741, 2.247) |
| Older than 41 | 277 (49.6) | 246 (49.0) | 31 (55.4) | |||
| Marital relationship | ||||||
| With partner | 317 (56.8) | 278 (55.4) | 39 (69.6) | 4.178 | 0.041 | 0.541 (0.298, 0.982) |
| Single | 241 (43.2) | 224 (44.6) | 17 (30.4) | |||
| Children under 14 years | ||||||
| Yes | 225 (40.3) | 201 (40.0) | 24 (43.0) | 0.166 | 0.684 | 0.890 (0.509, 1.558) |
| No | 333 (59.7) | 301 (60.0) | 32 (57.1) | |||
| Care for dependents at home | ||||||
| Yes | 58 (10.4) | 44 (8.8) | 14 (25.0) | 14.257 | <0.001 | 0.288 (0.146, 0.569) |
| No | 500 (89.6) | 458 (91.2) | 42 (75.0) | |||
| Mammography (N = 497) | ||||||
| Yes | 211 (42.5) | 156 (35.3) | 55 (100) | * | <0.001 | 1.353 (1.248, 1.466) |
| Never | 286 (57.5) | 286 (64.7) | 0 (0) | |||
| Family history of cancer (N = 551) | ||||||
| Yes | 72 (13.1) | 58 (11.7) | 14 (25.0) | 7.814 | 0.005 | 0.398 (0.205, 0.773) |
| No | 479 (86.9) | 437 (88.3) | 42 (75.0) | |||
| BMI | ||||||
| Underweight | 10 (1.8) | 8 (1.6) | 2 (3.6) | 8.074 | 0.045 | - |
| Normal | 376 (67.4) | 347 (69.1) | 29 (51.8) | |||
| Overweight | 128 (22.9) | 111 (22.1) | 17 (30.4) | |||
| Obese | 44 (7.9) | 36 (7.2) | 8 (14.3) | |||
| Physical activity at work | ||||||
| Light | 124 (22.2) | 114 (22.7) | 10 (17.9) | 30.175 | <0.001 | - |
| Moderate | 313 (56.1) | 283 (56.4) | 30 (53.6) | |||
| Hard | 113 (20.3) | 103 (20.5) | 10 (17.9) | |||
| Very hard | 8 (1.4) | 2 (0.4) | 6 (10.7) | |||
| Physical activity during leisure time | ||||||
| Two hours or less | 286 (51.25) | 259 (51.6) | 27 (48.2) | 0.230 | 0.631 | 1.144 (0.659, 1.887) |
| More than 2 h | 272 (28.75) | 243 (48.4) | 29 (51.8) | |||
| Tobacco consumption | ||||||
| Yes | 301 (53.9) | 271 (54.0) | 30 (53.6) | 0.003 | 0.953 | 1.016 (0.584, 1.770) |
| No | 257 (46.1) | 231 (46.0) | 26 (46.4) | |||
| Compliance with the smoking ban at work | ||||||
| Totally | 124 (22.2) | 104 (20.7) | 20 (35.7) | 11.377 | 0.010 | - |
| Almost always | 239 (42.8) | 213 (42.4) | 26 (46.4) | |||
| Hardly ever | 141 (25.3) | 132 (26.3) | 9 (16.1) | |||
| Never | 54 (9.7) | 53 (10.6) | 1 (1.8) | |||
| Exposure to tobacco smoke at home | ||||||
| More than 5 h a day | 22 (3.9) | 15 (3.0) | 7 (12.5) | 15.967 | 0.001 | - |
| Between 1 and 5 h a day | 36 (6.5) | 36 (7.2) | 0 (0) | |||
| Less than 1 h a day | 42 (7.5) | 39 (7.8) | 3 (5.4) | |||
| Never or hardly ever | 458 (82.1) | 412 (82.1) | 46 (82.1) | |||
| Use of medication to sleep | ||||||
| Yes | 116 (20.8) | 83 (16.5) | 33 (58.9) | 54.988 | <0.001 | 0.138 (0.077, 0.247) |
| No | 442 (79.2) | 419 (83.5) | 23 (41.1) | |||
| Hormone-based oral contraceptives (women only) (N = 504) | ||||||
| Yes | 334 (66.3) | 295 (65.7) | 39 (70.9) | 0.594 | 0.441 | 0.786 (0.425, 1.451) |
| Never | 170 (33.7) | 154 (34.3) | 16 (29.1) | |||
| N (%) | Healthy Cases (%) (N = 502) | Breast Cancer Cases (%) (N = 56) | χ2 | p | Odds Ratio (CI = 95%) | |
|---|---|---|---|---|---|---|
| Shift work at this moment | ||||||
| Yes | 444 (79.6) | 411 (81.9) | 33 (58.9) | 16.315 | <0.001 | 3.148 (1.765, 5.615) |
| No | 114 (20.4) | 91 (18.1) | 23 (41.1) | |||
| Night work at this moment | ||||||
| Yes | 378 (67.7) | 352 (70.1) | 26 (46.4) | 12.940 | <0.001 | 2.708 (1.548, 4.735) |
| No | 180 (32.3) | 150 (29.9) | 30 (53.6) | |||
| Working experience | ||||||
| 16 years or less | 280 (50.2) | 275 (54.8) | 5 (8.9) | 42.369 | <0.001 | 12.346 (4.854, 31.250) |
| More than 16 years | 278 (49.8) | 227 (45.2) | 51 (91.1) | |||
| Total years performing more than 3 nights a month | ||||||
| 10 years or less | 317 (56.8) | 302 (60.2) | 15 (26.8) | 22.870 | <0.001 | 4.132 (2.227, 7.634) |
| More than 10 years | 241 (43.2) | 200 (39.8) | 41 (73.2) | |||
| Total worked nights | ||||||
| Less than 500 nights | 265 (47.5) | 254 (50.6) | 11 (19.6) | 19.358 | <0.001 | 4.190 (2.118, 8.287) |
| 500 nights or more | 293 (52.5) | 248 (49.4) | 45 (80.4) | |||
| Total sick leaves over lifespan (N = 550) | ||||||
| 2 or less | 342 (62.2) | 329 (66.3) | 13 (24.1) | 36.977 | <0.001 | 6.211 (3.236, 11.905) |
| More than 2 | 208 (37.8) | 167 (33.7) | 41 (75.9) | |||
| Sick leaves in the last year (N = 554) | ||||||
| Without sick leave | 385 (69.5) | 368 (73.6) | 17 (31.4) | 40.782 | <0.001 | 6.061 (3.300, 11.111) |
| With sick leave | 169 (30.5) | 132 (26.4) | 37 (68.5) | |||
| From 1 to 10… | M (SD) (N = 558) | Breast Cancer Cases (N = 56) | Non Cases (N = 502) | Mann Whitney-U | p |
|---|---|---|---|---|---|
| How do you value your overall health? | 7.94 (1.26) | 6.45 (1.61) | 8.11 (1.09) | 5920.500 | <0.001 |
| How do you value your sleepingquality? | 6.28 (1.96) | 5.29 (2.06) | 6.39 (1.91) | 9741.500 | <0.001 |
| How do you value the effect shift work has on your health? | 9.08 (1.37) | 9.16 (1.60) | 9.07 (1.35) | 15,223.500 | 0.262 |
| How do you value your level of work stress? | 7.57 (1.86) | 8.23 (1.67) | 7.49 (1.87) | 17,571.000 | 0.002 |
| How do you value your satisfaction with your current job? | 7.28 (1.87) | 7.02 (2.09) | 7.31 (1.85) | 12,903.500 | 0.305 |
| Coefficient | OR | CI = 95% for OR | ||
|---|---|---|---|---|
| Inferior | Superior | |||
| Number of working years 1 | 2.167 ** | 8.733 | 2.811 | 27.134 |
| Medication to sleep | 1.765 ** | 5.841 | 2.848 | 11.978 |
| Night work at this moment | 1.701 ** | 5.479 | 2.520 | 11.915 |
| Sick leave last year | 1.684 ** | 5.387 | 2.527 | 11.484 |
| Total years performing more than 3 nights per month 2 | 0.830 * | 2.294 | 1.008 | 5.220 |
| Constant | −1.814 ** | 0.163 | ||
| Sensitivity/Specificity | 52.8%/95.9% | |||
| Correctly classified percentage | 91.3% | |||
| R2 Cox and Snell/R2 Nagelkerke | 0.228/0.461 | |||
| Hosmer-Lemeshov Test | 0.811 | |||
| Omnibus test | < 0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagundo-Rivera, J.; Allande-Cussó, R.; Ortega-Moreno, M.; García-Iglesias, J.J.; Romero, A.; Ruiz-Frutos, C.; Gómez-Salgado, J. Implications of Lifestyle and Occupational Factors on the Risk of Breast Cancer in Shiftwork Nurses. Healthcare 2021, 9, 649. https://doi.org/10.3390/healthcare9060649
Fagundo-Rivera J, Allande-Cussó R, Ortega-Moreno M, García-Iglesias JJ, Romero A, Ruiz-Frutos C, Gómez-Salgado J. Implications of Lifestyle and Occupational Factors on the Risk of Breast Cancer in Shiftwork Nurses. Healthcare. 2021; 9(6):649. https://doi.org/10.3390/healthcare9060649
Chicago/Turabian StyleFagundo-Rivera, Javier, Regina Allande-Cussó, Mónica Ortega-Moreno, Juan Jesús García-Iglesias, Adolfo Romero, Carlos Ruiz-Frutos, and Juan Gómez-Salgado. 2021. "Implications of Lifestyle and Occupational Factors on the Risk of Breast Cancer in Shiftwork Nurses" Healthcare 9, no. 6: 649. https://doi.org/10.3390/healthcare9060649









