Hepatitis C Virus Prevalence and Risk Factors in a Village in Northeastern Romania—A Population-Based Screening—The First Step to Viral Micro-Elimination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Assessments
2.3. Ethics
2.4. Statistical Analysis
3. Results
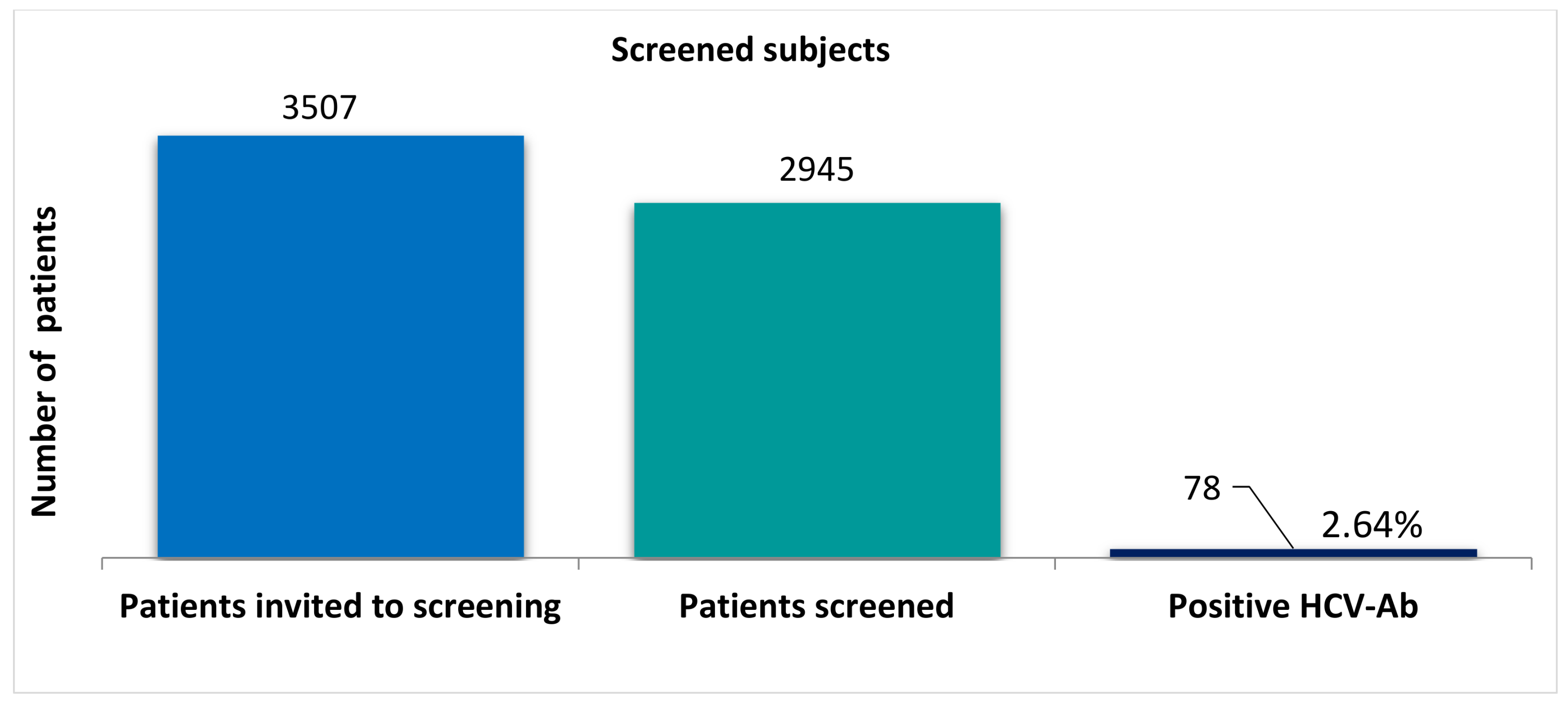
3.1. Prevalence of HCV Infection
3.2. Cascade of Care
3.3. Association between Risk Factors and Chronic HCV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Questions |
|---|
Part 1—Demographic data
|
Part 2—Risk factors for HCV infection (answer from the questionnaires)
|
References
- Lombardi, A.; Mondelli, M.U.; ESCMID Study Group for Viral Hepatitis (ESGVH). Hepatitis C: Is eradication possible? Liver Int. 2019, 39, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Hollande, C.; Parlati, L.; Pol, S. Micro-elimination of hepatitis C virus. Liver Int. 2020, 40, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef] [Green Version]
- Madaliński, K.; Zakrzewska, K.; Kołakowska, A.; Godzik, P. Epidemiology of HCV infection in Central and Eastern Europe. Prezgl. Epidemiol. 2015, 69, 459–464. [Google Scholar]
- Molnar, G.; Popa, S.; Jebeleanu, L.; Damian, C. Studiul prevalentei markerilor serici ai infectiei cu virusurile hepatitelor in anamneza epidemiologica a populatiei. Bacteriol. Virusol. Parazitol. Epidemiol. 1994, 30, 141–149. [Google Scholar]
- Gheorghe, L.; Csiki, I.E.; Iacob, S.; Gheorghe, C.; Smira, G.; Regep, L. The prevalence and risk factors of hepatitis C virus infection in adult population in Romania: A nationwide survey 2006–2008. J. Gastrointest. Liver Dis. 2010, 19, 373–379. [Google Scholar]
- Asselah, T.; Marcellin, P.; Schinazi, R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018, 38, 7–13. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, C.; Trifan, A. Hepatitis C Virus Treatment Revolution: Eastern European Story. Hepat. Mon. 2015, 15, e28969. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Birerdinc, A.; Henry, L. Hepatitis C infection: A multifaceted systemic disease with clinical, patient reported and economic consequences. J. Hepatol. 2016, 65 (Suppl. S1), S109–S119. [Google Scholar] [CrossRef] [Green Version]
- Vietri, J.; Prajapati, G.; El Khoury, A.C. The burden of hepatitis C in Europe from the patients’ perspective: A survey in 5 countries. BMC Gastroenterol. 2013, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Papatheodoridis, G.V.; Hatzakis, A.; Cholongitas, E.; Baptista-Leite, R.; Baskozos, I.; Chhatwal, J.; Colombo, M.; Cortez-Pinto, H.; Craxi, A.; Goldberg, D.; et al. Hepatitis C: The beginning of the end-key elements for successful European and national strategies to eliminate HCV in Europe. J. Viral Hepat. 2018, 25, 6–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis, 2016–2021: Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf (accessed on 26 April 2018).
- Idilman, R.; Razavi, H.; Robbins-Scott, S.; Akarca, U.S.; Örmeci, N.; Kaymakoglu, S.; Aygen, B.; Tozun, N.; Güner, R.; Bodur, H.; et al. A micro-elimination approach to addressing hepatitis C in Turkey. BMC Health Serv. Res. 2020, 20, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, H.; Baptista-Leite, R.; Franco, D.; Eclemea, I.; Bratu, E.C.; Furtunescu, F.L.; Pop, C.S.; Pana, B.C. Modeling the Puzzle of Hepatitis C Epidemiology in Romania: A Pathway to Control. J. Gastrointest. Liver Dis. 2020, 29, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Safreed-Harmon, K.; Thursz, M.R.; Dillon, J.F.; El-Sayed, M.H.; Elsharkawy, A.M.; Hatzakis, A.; Jadoul, M.; Prestileo, T.; Razavi, H.; et al. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin. Liver Dis. 2018, 38, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, D.M.; Ricart, O.C. Hepatitis C virus infection and new treatment strategies. Enferm. Infecc. Microbiol. Clin. 2019, 37, 15–19. [Google Scholar] [CrossRef]
- Razavi, H.; Sanchez, G.Y.; Yuen, C.; Cornberg, M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020, 40, 522–529. [Google Scholar] [CrossRef]
- Martínez, I.; Ryan, P.; Valencia, J.; Resino, S. The Challenging Road to Hepatitis C Virus Eradication. J. Clin. Med. 2021, 10, 611. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Combating Hepatitis B and C to Reach Elimination by 2030; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization (WHO). Monitoring and Evaluation for Viral Hepatitis B and C: Recommended Indicators and Framework; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization (WHO). Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection; Updated Version; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Matičič, M.; Lombardi, A.; Mondelli, M.U.; Colombo, M.; ESCMID Study Group for Viral Hepatitis (ESGVH). Elimination of hepatitis C in Europe: Can WHO targets be achieved? Clin. Microbiol. Infect. 2020, 26, 818–823. [Google Scholar] [CrossRef]
- Chowdhury, J.F.; Winston, A.; Zeina, T.; Shim, H.G.; Vindenes, T. Hepatitis C Virus Care Cascade Assessment—One Step Closer to Micro-Elimination. Open Forum Infect. Dis. 2020, 7, S561–S562. [Google Scholar] [CrossRef]
- Gheorghe, L.; Iacob, S.; Csiki, I.E.; Huiban, L.; Cojocaru, M.; Cojocariu, C.; Nemteanu, R.; Girleanu, I.; Sirli, R.; Singeap, A.M.; et al. The Prevalence of HCV Infection and Risk Factors in a Hospital-Based Population Screening, a First Step to the Micro-Elimination of HCV Infection in Medical Institutions from Romania—Results of the HepC ALERT Study. J. Gastrointest. Liver Dis. 2020, 29, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Kracht, P.A.M.; Arends, J.E.; van Erpecum, K.J.; Urbanus, A.; Willemse, J.A.; Hoepelman, A.I.M.; Croes, E.A. Strategies for achieving viral hepatitis C micro-elimination in the Netherlands. Hepatol. Med. Policy 2018, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Søholm, J.; Holm, D.K.; Mössner, B.; Madsen, L.W.; Hansen, J.F.; Weis, N.; Sauer, A.P.; Awad, T.; Christensen, P.B. Incidence, prevalence and risk factors forhepatitis C in Danish prisons. PLoS ONE 2019, 14, e0220297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busschots, D.; Toghanian, S.; Bielen, R.; Salomonsson, S.; Koc, Ö.M.; Hendrickx, G.; Jadoul, M.; Nevens, F.; Sokal, E.; Brixko, C.; et al. Eliminating viral hepatitis C in Belgium: The micro-elimination approach. BMC Infect. Dis. 2020, 20, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriano, L.E.; Goldhaber-Fiebert, J.D. Population Health and Cost-Effectiveness Implications of a “Treat All” Recommendation for HCV: A Review of the Model-Based Evidence. MDM Policy Pract. 2018, 3, 2381468318776634. [Google Scholar] [CrossRef] [Green Version]
- Shiha, G.; Soliman, R.; Mikhail, N.N.H.; Easterbrook, P. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J. Hepatol. 2020, 72, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Olafsson, S.; Fridriksdottir, R.H.; Tyrfingsson, T.; Runarsdottir, V.; Hansdottir, I.; Bergmann, O.M.; Björnsson, E.S.; Johannsson, B.; Sigurdardottir, B.; Löve, A.; et al. Iceland may already have reached the WHO 2030 targets for diagnosis and treatment of hepatitis C virus infection. Results from the Treatment as Prevention for Hepatitis C (TrapHepC) program. J. Hepatol. 2019, 70, e337-8. [Google Scholar] [CrossRef]
- Schröeder, S.E.; Pedrana, A.; Scott, N.; Wilson, D.; Kuschel, C.; Aufegger, L.; Atun, R.; Baptista-Leite, R.; Butsashvili, M.; El-Sayed, M.; et al. Innovative strategies for the elimination of viral hepatitis at a national level: A country case series. Liver Int. 2019, 39, 1818–1836. [Google Scholar] [CrossRef] [Green Version]
- PEAHC Plan Estrategico para el Abordaje de la Hepatitis C en el Sistema Nacional de Salud. 2020. Available online: http://www.msssi.goves/ciudadanos/enfLesiones/enfTrasmisibles/docs/plan_estrategico_hepatitis_C.pdf (accessed on 10 March 2021).
- Cuadrado, A.; Llerena, S.; Cobo, C.; Pallás, J.R.; Mateo, M.; Cabezas, J.; Fortea, J.I.; Alvarez, S.; Pellón, R.; Crespo, J.; et al. Microenvironment Eradication of Hepatitis C: A Novel Treatment Paradigm. Am. J. Gastroenterol. 2018, 113, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Mangia, A.; Cotugno, R.; Cocomazzi, G.; Squillante, M.M.; Piazzolla, V. Hepatitis C virus micro-elimination: Where do we stand? World J. Gastroenterol. 2021, 27, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Cozzolongo, R.; Osella, A.R.; Elba, S.; Petruzzi, J.; Buongiorno, G.; Giannuzzi, V.; Leone, G.; Bonfiglio, C.; Lanzilotta, E.; Manghisi, O.G.; et al. Epidemiology of HCV infection in the general population: A survey in a southern Italian town. Am. J. Gastroenterol. 2009, 104, 2740–2746. [Google Scholar] [CrossRef]
- Guadagnino, V.; Stroffolini, T.; Rapicetta, M.; Costantino, A.; Kondili, L.A.; Menniti-Ippolito, F.; Caroleo, B.; Costa, C.; Griffo, G.; Loiacono, L.; et al. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: A community-based survey in southern Italy. Hepatology 1997, 26, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tajes, S.; Domínguez, Á.; Carrión, J.A.; Buti, M.; Quer, J.C.; Morillas, R.M.; López, C.; Torras, X.; Baliellas, C.; Vergara, M.; et al. Significant decrease in the prevalence of hepatitis C infection after the introduction of direct acting antivirals. J. Gastroenterol. Hepatol. 2020, 35, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Pawlotsky, J.M.; Lazarus, J.V.; Aghemo, A.; Dore, G.J.; Grebely, J. Theremoval of DAA restrictions in Europe—One step closer to eliminating HCV as a major public health threat. J. Hepatol. 2018, 69, 1188–1196. [Google Scholar] [CrossRef]
- Safreed-Harmon, K.; Blach, S.; Aleman, S.; Bollerup, S.; Cooke, G.; Dalgard, O.; Dillon, J.F.; Dore, G.J.; Duberg, A.S.; Grebely, J.; et al. The consensus hepatitis C cascade of care: Standardized reporting to monitor progress toward elimination. Clin. Infect. Dis. 2019, 69, 2218–2227. [Google Scholar] [CrossRef]


| Variable | Patients with Negative HCV Ab (n = 2867) | Patients with Positive HCV Ab (n = 78) | p-Value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 947 (33) | 25 (32.1) | 0.815 |
| Female | 1920 (67) | 53 (67.9) | |
| Age, years, n (%) | |||
| 18–29 | 284 (9.9) | 1 (1.3) | 0.163 |
| 30–39 | 211 (7.4) | 4 (5.1) | |
| 40–49 | 358 (12.5) | 6 (7.7) | |
| 50–59 | 552 (19.2) | 11 (14.1) | |
| 60–69 | 614 (21.4) | 14 (17.9) | |
| 70–79 | 443 (15.5) | 18 (23.1) | |
| 80–97 | 405 (14.1) | 24 (30.8) | |
| Marital status, n (%) | |||
| Widowed or divorced | 353 (12.3) | 67 (85.9) | 0.037 |
| Married or living together as a couple | 2514 (87.7) | 11 (14.1) | |
| Social status, n (%) | |||
| Retired persons | 1277 (44.5) | 44 (56.4) | 0.26 |
| Housewives | 810 (28.3) | 28 (35.9) | |
| Employees | 780 (27.2) | 6 (7.7) | |
| Educational status, n (%) | |||
| Subjects without university studies | 2510 (87.5) | 73 (93.6) | 0.188 |
| Subjects with university studies | 357 (12.5) | 5 (6.4) |
| Risk Factors | HCV Negative (N = 2867) n (%) | HCV Positive (N = 78) n (%) | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Known HBV/HDV | 46 (1.60) | 3 (3.84) | 0.62 | 0.3–1.31 | 0.302 |
| Known HCV (+) family members | 47 (1.63) | 5 (6.41) | 2.23 | 1.37–3.5 | 0.0001 |
| Professional exposure to blood products | 78 (2.72) | 0 (0.00) | 0.25 | 0.11–0.53 | 0.0001 |
| Abortion before 1990 | 141 (4.91) | 8 (10.25) | 1.35 | 1.02–1.9 | 0.023 |
| Multiple surgeries | 83 (2.89) | 6 (7.69) | 1.32 | 1.05–1.72 | 0.038 |
| Blood transfusions before 1992 | 45 (1.56) | 4 (5.12) | 3.21 | 2.25–4.52 | 0.0001 |
| Multiple dental interventions | 67 (2.33) | 11 (14.10) | 1.12 | 0.67–1.45 | 0.303 |
| Hemodialysis | 33 (1.15) | 0 (0.00) | 0.34 | 0.06–1.03 | 0.062 |
| Sexual contacts with multiple partners | 133 (4.63) | 2 (2.56) | 0.88 | 0.52–1.38 | 0.615 |
| Intravenous drugs | 78 (2.72) | 1 (1.28) | 0.71 | 0.4–1.44 | 0.302 |
| Tattooing/piercing | 81 (2.82) | 3 (3.84) | 1.25 | 0.72–1.84 | 0.251 |
| Sharing personal hygiene objects | 103 (3.59) | 8 (10.25) | 1.45 | 1.12–1.73 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huiban, L.; Stanciu, C.; Muzica, C.M.; Cuciureanu, T.; Chiriac, S.; Zenovia, S.; Burduloi, V.M.; Petrea, O.; Sîngeap, A.M.; Gîrleanu, I.; et al. Hepatitis C Virus Prevalence and Risk Factors in a Village in Northeastern Romania—A Population-Based Screening—The First Step to Viral Micro-Elimination. Healthcare 2021, 9, 651. https://doi.org/10.3390/healthcare9060651
Huiban L, Stanciu C, Muzica CM, Cuciureanu T, Chiriac S, Zenovia S, Burduloi VM, Petrea O, Sîngeap AM, Gîrleanu I, et al. Hepatitis C Virus Prevalence and Risk Factors in a Village in Northeastern Romania—A Population-Based Screening—The First Step to Viral Micro-Elimination. Healthcare. 2021; 9(6):651. https://doi.org/10.3390/healthcare9060651
Chicago/Turabian StyleHuiban, Laura, Carol Stanciu, Cristina Maria Muzica, Tudor Cuciureanu, Stefan Chiriac, Sebastian Zenovia, Vladut Mirel Burduloi, Oana Petrea, Ana Maria Sîngeap, Irina Gîrleanu, and et al. 2021. "Hepatitis C Virus Prevalence and Risk Factors in a Village in Northeastern Romania—A Population-Based Screening—The First Step to Viral Micro-Elimination" Healthcare 9, no. 6: 651. https://doi.org/10.3390/healthcare9060651
APA StyleHuiban, L., Stanciu, C., Muzica, C. M., Cuciureanu, T., Chiriac, S., Zenovia, S., Burduloi, V. M., Petrea, O., Sîngeap, A. M., Gîrleanu, I., Sfarti, C., Cojocariu, C., & Trifan, A. (2021). Hepatitis C Virus Prevalence and Risk Factors in a Village in Northeastern Romania—A Population-Based Screening—The First Step to Viral Micro-Elimination. Healthcare, 9(6), 651. https://doi.org/10.3390/healthcare9060651










