Health-Related Quality of Life and Mental Health after Surgical Treatment of Hepatocellular Carcinoma in the Era of Minimal-Invasive Surgery: Resection versus Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Health-Related Quality Assessment Using the Short Form 36 (SF-36)
2.3. Hospital Anxiety and Depression Scale (HADS)
2.4. Clinical Database
2.5. Statistics
3. Results
3.1. Laparoscopic Liver Resection for HCC: Patients’ Characteristics and Postoperative Outcomes
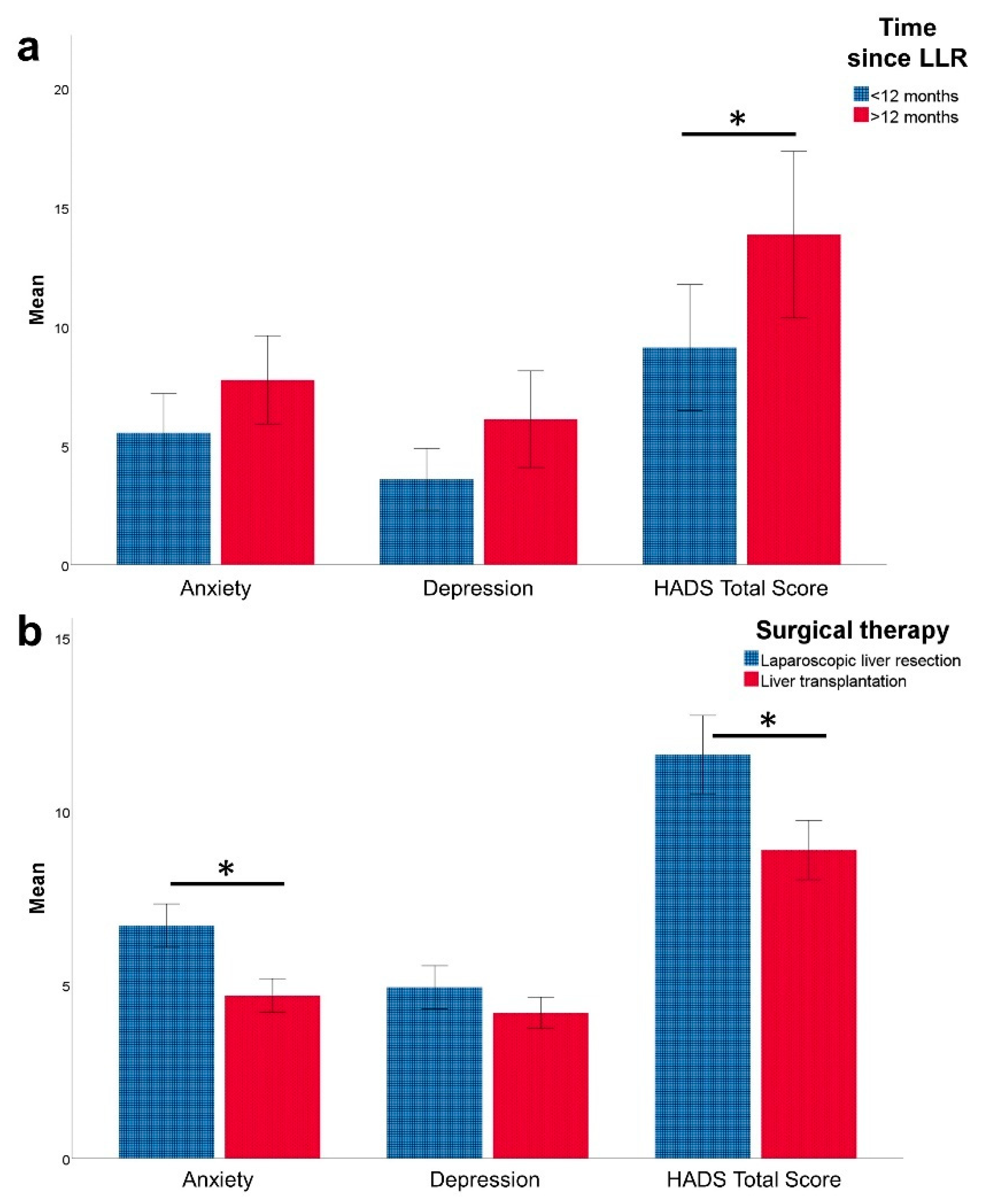
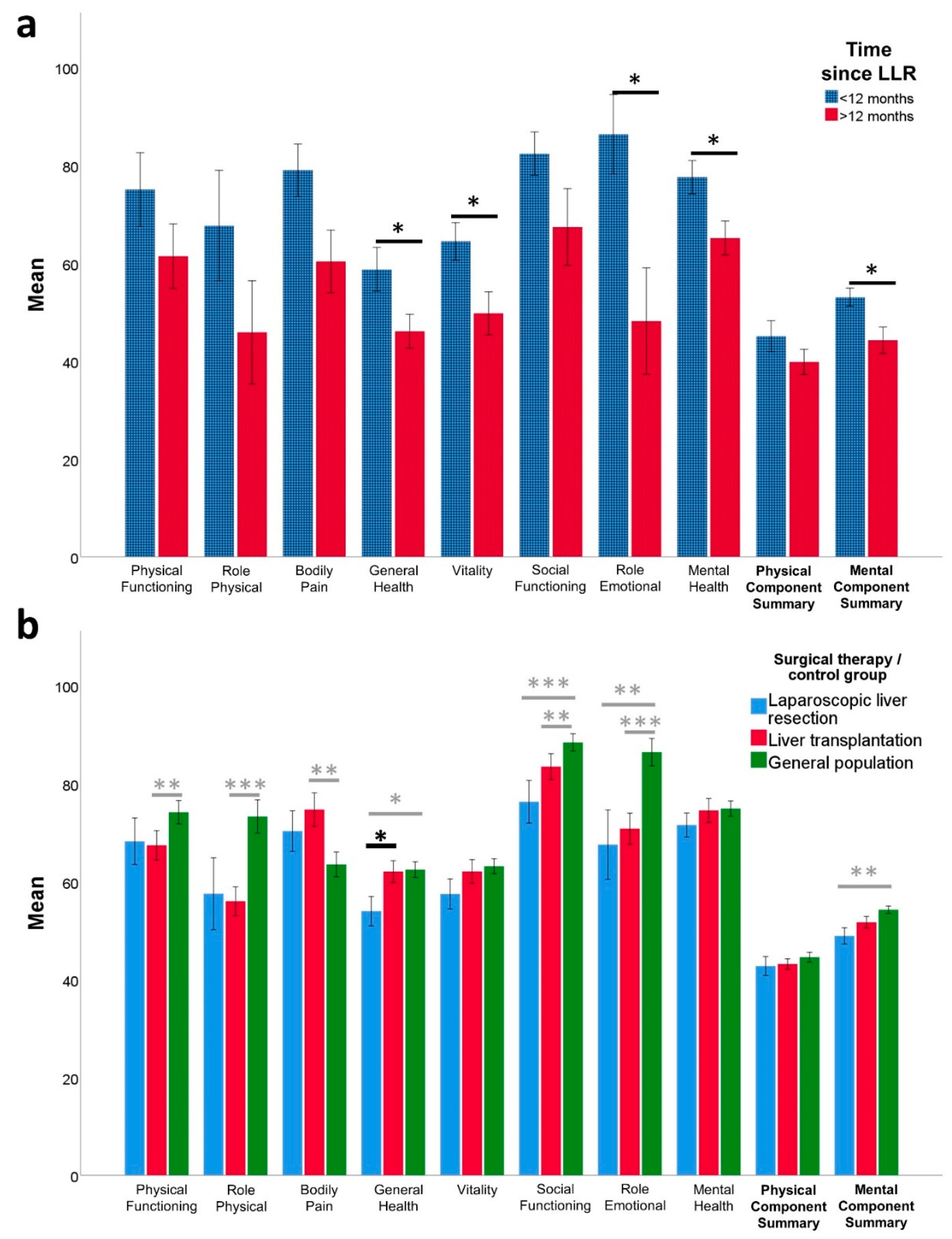
3.2. HRQoL (SF-36) and Mental Health (HADS) after Laparoscopic Liver Resection
3.3. Factors Influencing HRQoL and Mental Health after Laparoscopic Liver Resection
3.4. Laparoscopic Liver Resection vs. Liver Transplantation for HCC—Impact on HRQoL and Mental Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.G.; Homer, T.; Ternent, L.; Newton, J.; McNeil, C.J.; Hudson, M.; Jones, D.E. Health related quality of life in people with advanced chronic liver disease. J. Hepatol. 2014, 61, 1158–1165. [Google Scholar] [CrossRef]
- Peng, J.-K.; Hepgul, N.; Higginson, I.J.; Gao, W. Symptom prevalence and quality of life of patients with end-stage liver disease: A systematic review and meta-analysis. Palliat. Med. 2019, 33, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, T.; Terashi, T.; Shiotani, S.; Soejima, Y.; Sugimachi, K. Liver transplantation for hepatocellular carcinoma. Surgery 2002, 131, S190–S194. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Conci, S.; Cescon, M.; Fava, C.; Capelli, P.; D’Errico, A.; Torzilli, G.; Di Tommaso, L.; Giuliante, F.; Vecchio, F.M.; et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. J. Hepatol. 2015, 63, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Benzing, C.; Krezdorn, N.; Hinz, A.; Glaesmer, H.; Brahler, E.; Forster, J.; Wiltberger, G.; Krenzien, F.; Schmelzle, M.; Bartels, M. Mental Status in Patients Before and After Liver Transplantation. Ann. Transplant. 2015, 20, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Benzing, C.; Krezdorn, N.; Forster, J.; Hinz, A.; Krenzien, F.; Atanasov, G.; Schmelzle, M.; Hau, H.M.; Bartels, M. Health-related quality of life and affective status in liver transplant recipients and patients on the waiting list with low MELD scores. HPB (Oxford) 2016, 18, 449–455. [Google Scholar] [CrossRef]
- Broschewitz, J.; Wiltberger, G.; Krezdorn, N.; Krenzien, F.; Forster, J.; Atanasov, G.; Hau, H.M.; Schmelzle, M.; Hinz, A.; Bartels, M.; et al. Primary liver transplantation and liver retransplantation: Comparison of health-related quality of life and mental status—A cross-sectional study. Health Qual. Life Outcomes 2017, 15, 147. [Google Scholar] [CrossRef]
- Sebaaly, J.C.; Fleming, J.; Pilch, N.; Meadows, H.; Finn, A.; Chavin, K.; Baliga, P.; Bratton, C.F.; McGillicuddy, J.W.; Nadig, S.; et al. Depression, Resource Utilization, and Outcomes Following Liver Transplant. Prog. Transplant. 2016, 26, 270–276. [Google Scholar] [CrossRef]
- Krenzien, F.; Wabitsch, S.; Haber, P.; Kamali, C.; Brunnbauer, P.; Benzing, C.; Atanasov, G.; Wakabayashi, G.; Ollinger, R.; Pratschke, J.; et al. Validity of the Iwate criteria for patients with hepatocellular carcinoma undergoing minimally invasive liver resection. J. Hepatobiliary Pancreat. Sci. 2018, 25, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Feldbrugge, L.; Wabitsch, S.; Benzing, C.; Krenzien, F.; Kastner, A.; Haber, P.K.; Atanasov, G.; Andreou, A.; Ollinger, R.; Pratschke, J.; et al. Safety and feasibility of laparoscopic liver resection in patients with a history of abdominal surgeries. HPB (Oxford) 2019. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, S.; Haber, P.K.; Ekwelle, N.; Kastner, A.; Krenzien, F.; Benzing, C.; Atanasov, G.; Bellingrath, J.S.; Bauer, G.; Schoning, W.; et al. Minimally Invasive Liver Surgery in Elderly Patients-A Single-Center Experience. J. Surg. Res. 2019, 239, 92–97. [Google Scholar] [CrossRef]
- Krenzien, F.; Schmelzle, M.; Struecker, B.; Raschzok, N.; Benzing, C.; Jara, M.; Bahra, M.; Ollinger, R.; Sauer, I.M.; Pascher, A.; et al. Liver Transplantation and Liver Resection for Cirrhotic Patients with Hepatocellular Carcinoma: Comparison of Long-Term Survivals. J. Gastrointest. Surg. 2018, 22, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Lauerer, M.; Kaiser, K.; Nagel, E. Organ Transplantation in the Face of Donor Shortage — Ethical Implications with a Focus on Liver Allocation. Visc. Med. 2016, 32, 278–285. [Google Scholar] [CrossRef] [PubMed]
- DSO Jahresbericht. 2010. Available online: https://www.klinikum-stuttgart.de/fileadmin/user_upload/dso_jb2010_d_www.pdf (accessed on 2 May 2021).
- DSO Jahresbericht. 2019. Available online: https://dso.de/SiteCollectionDocuments/DSO-Jahresbericht%202019.pdf (accessed on 2 May 2021).
- Chiu, C.C.; Lee, K.T.; Wang, J.J.; Sun, D.P.; Lee, H.H.; Shi, H.Y. Health-Related Quality of Life before and after Surgical Resection of Hepatocellular Carcinoma: A Prospective Study. Asian Pac. J. Cancer Prev. 2018, 19, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Lee, K.T.; Lee, H.H.; Wang, J.J.; Sun, D.P.; Huang, C.C.; Shi, H.Y. Comparison of Models for Predicting Quality of Life After Surgical Resection of Hepatocellular Carcinoma: A Prospective Study. J. Gastrointest. Surg. 2018, 22, 1724–1731. [Google Scholar] [CrossRef]
- He, Q.; Jiang, J.J.; Jiang, Y.X.; Wang, W.T.; Yang, L. Health-Related Quality of Life Comparisons After Radical Therapy for Early-Stage Hepatocellular Carcinoma. Transplant. Proc. 2018, 50, 1470–1474. [Google Scholar] [CrossRef]
- Akcam, A.T.; Saritas, A.G.; Ulku, A.; Rencuzogullari, A. Oncological Outcomes of Hepatic Resection vs Transplantation for Localized Hepatocellular Carcinoma. Transplant. Proc. 2019, 51, 1147–1152. [Google Scholar] [CrossRef]
- Zaydfudim, V.M.; Vachharajani, N.; Klintmalm, G.B.; Jarnagin, W.R.; Hemming, A.W.; Doyle, M.B.; Cavaness, K.M.; Chapman, W.C.; Nagorney, D.M. Liver Resection and Transplantation for Patients With Hepatocellular Carcinoma Beyond Milan Criteria. Ann. Surg. 2016, 264, 650–658. [Google Scholar] [CrossRef]
- Benzing, C.; Krenzien, F.; Gohlke, D.; Andreou, A.; Haber, P.; Wabitsch, S.; Biebl, M.; Zorron, R.; Atanasov, G.; Strucker, B.; et al. Health-related quality of life after laparoscopic liver resection. J. Minim. Access. Surg. 2017. [Google Scholar] [CrossRef]
- Benzing, C.; Krezdorn, N.; Forster, J.; Hinz, A.; Atanasov, G.; Wiltberger, G.; Morgul, M.H.; Lange, U.G.; Schmelzle, M.; Hau, H.M.; et al. Impact of different immunosuppressive regimens on the health-related quality of life following orthotopic liver transplantation. Clin. Transplant. 2015, 29, 1081–1089. [Google Scholar] [CrossRef]
- Bellach, B.; Ellert, U.; Radoschewski, M. Der SF-36 im Bundes-Gesundheitssurvey Erste Ergebnisse und neue Fragen. Bundesgesundheitsblatt Gesundh. Gesundh. 2000, 43, 210–216. [Google Scholar] [CrossRef]
- Morfeld, M.K.I.; Bullinger, M. SF 36—Fragebogen zum Gesundheitszustand; Hogrefe: Gottingen, Germany, 2011. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Gandek, B.; Aaronson, N.K.; Apolone, G.; Bech, P.; Brazier, J.; Bullinger, M.; Kaasa, S.; Leplège, A.; et al. The factor structure of the SF-36 Health Survey in 10 countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1159–1165. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta. Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.B.U.; Snait, R.P. HADS-D: Hospital Anxiety and Depression Scale-Deutsche Version [Ein Fragebogen zur Erfassung von Angst und Depressivität in der somatischen Medizin]; Bern Huber: Berlin, Germany, 1995. [Google Scholar]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, M.; Wabitsch, S.; Haber, P.K.; Krenzien, F.; Kästner, A.; Biebl, M.; Öllinger, R.; Pratschke, J. Laparoskopische Leberchirurgie – Berliner Zentrumserfahrungen aus 250 konsekutiven Fällen. Zentralblatt. Chir. 2019, 144, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann.Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Fretland, A.A.; Dagenborg, V.J.; Waaler Bjornelv, G.M.; Aghayan, D.L.; Kazaryan, A.M.; Barkhatov, L.; Kristiansen, R.; Fagerland, M.W.; Edwin, B.; Andersen, M.H. Quality of life from a randomized trial of laparoscopic or open liver resection for colorectal liver metastases. Br. J. Surg. 2019, 106, 1372–1380. [Google Scholar] [CrossRef]
- Seehofer, D.; Sucher, R.; Schmelzle, M.; Ollinger, R.; Lederer, A.; Denecke, T.; Schott, E.; Pratschke, J. Evolution of laparoscopic liver surgery as standard procedure for HCC in cirrhosis? Z. Gastroenterol. 2017, 55, 453–460. [Google Scholar] [CrossRef]
- Haber, P.K.; Wabitsch, S.; Krenzien, F.; Benzing, C.; Andreou, A.; Schöning, W.; Öllinger, R.; Pratschke, J.; Schmelzle, M. Laparoscopic liver surgery in cirrhosis—Addressing lesions in posterosuperior segments. Surg. Oncol. 2019, 28, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Loguercio, C.; Sgarbi, D.; Abbiati, R.; Brunetti, N.; De Simone, T.; Zoli, M.; Marchesini, G. Reduced quality of life of patients with hepatocellular carcinoma. Dig. Liver Dis. 2003, 35, 46–54. [Google Scholar] [CrossRef]
- Shun, S.-C.; Chen, C.-H.; Sheu, J.-C.; Liang, J.-D.; Yang, J.-C.; Lai, Y.-H. Quality of life and its associated factors in patients with hepatocellular carcinoma receiving one course of transarterial chemoembolization treatment: A longitudinal study. Oncologist 2012, 17, 732–739. [Google Scholar] [CrossRef]
- Wible, B.C.; Rilling, W.S.; Drescher, P.; Hieb, R.A.; Saeian, K.; Frangakis, C.; Chen, Y.; Eastwood, D.; Kim, H.S. Longitudinal quality of life assessment of patients with hepatocellular carcinoma after primary transarterial chemoembolization. J. Vasc. Interv. Radiol. 2010, 21, 1024–1030. [Google Scholar] [CrossRef]
- Mise, Y.; Satou, S.; Ishizawa, T.; Kaneko, J.; Aoki, T.; Hasegawa, K.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Impact of surgery on quality of life in patients with hepatocellular carcinoma. World J. Surg. 2014, 38, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Chie, W.C.; Yu, F.; Li, M.; Baccaglini, L.; Blazeby, J.M.; Hsiao, C.F.; Chiu, H.C.; Poon, R.T.; Mikoshiba, N.; Al-Kadhimi, G.; et al. Quality of life changes in patients undergoing treatment for hepatocellular carcinoma. Qual. Life Res. 2015, 24, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Garratt, A.M.; Stavem, K. Measurement properties and normative data for the Norwegian SF-36: Results from a general population survey. Health Qual. Life Outcomes 2017, 15, 51. [Google Scholar] [CrossRef]
- Sobhonslidsuk, A.; Silpakit, C.; Kongsakon, R.; Satitpornkul, P.; Sripetch, C.; Khanthavit, A. Factors influencing health-related quality of life in chronic liver disease. World J. Gastroenterol. 2006, 12, 7786–7791. [Google Scholar] [CrossRef]
- Lei, J.Y.; Yan, L.N.; Wang, W.T.; Zhu, J.Q.; Li, D.J. Health-Related Quality of Life and Psychological Distress in Patients with Early-Stage Hepatocellular Carcinoma after Hepatic Resection or Transplantation. Transplant. Proc. 2016, 48, 2107–2111. [Google Scholar] [CrossRef]
| Laparoscopic Resection of Hepatocellular Carcinoma | |
|---|---|
| n = 98 | |
| Age a | 70.5 (50–85) |
| Body mass index (kg/m2) a | 26.5 (18–45) |
| Gender (male) b | 71 (72) |
| Presence of liver cirrhosis b | 72 (74) |
| Missing | 1 (1) |
| ASA score for physical status b | |
| <3 | 34 (35) |
| ≥3 | 52 (53) |
| Missing | 12 (12) |
| Surgical technique b | |
| Multiport laparoscopy | 64 (65) |
| Hand assisted laparoscopy | 25 (26) |
| Single incision laparoscopy | 5 (5) |
| Robotic surgery | 4 (4) |
| Extent of resection b | |
| Minor liver resection | 83 (85) |
| Major liver resection | 15 (15) |
| Length of operation (minutes) a | 209 (49–461) |
| Complications (Clavien-Dindo) b, c | |
| None | 73 (75) |
| I–II | 17 (17) |
| III | 7 (7) |
| IV–V | 1 (1) |
| Intensive Care Unit stay (days) a | 1 (0–8) |
| Hospital stay (days) a | 9 (3–27) |
| Laparoscopic Resection of Hepatocellular Carcinoma | |
|---|---|
| n = 43 * | |
| Physical Functioning | 80 (5–100) |
| Role Physical | 85 (0–100) |
| Bodily Pain | 64 (21–100) |
| General Health Perceptions | 52 (25–100) |
| Physical Component Summary | 47 (21–59) |
| Vitality | 55 (10–90) |
| Social Functioning | 87.5 (0–100) |
| Role Emotional | 100 (0–100) |
| Mental Health | 70 (40–100) |
| Mental Component Summary | 51 (28–67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldbrügge, L.; Langenscheidt, A.; Krenzien, F.; Schulz, M.; Krezdorn, N.; Kamali, K.; Hinz, A.; Bartels, M.; Fikatas, P.; Schmelzle, M.; et al. Health-Related Quality of Life and Mental Health after Surgical Treatment of Hepatocellular Carcinoma in the Era of Minimal-Invasive Surgery: Resection versus Transplantation. Healthcare 2021, 9, 694. https://doi.org/10.3390/healthcare9060694
Feldbrügge L, Langenscheidt A, Krenzien F, Schulz M, Krezdorn N, Kamali K, Hinz A, Bartels M, Fikatas P, Schmelzle M, et al. Health-Related Quality of Life and Mental Health after Surgical Treatment of Hepatocellular Carcinoma in the Era of Minimal-Invasive Surgery: Resection versus Transplantation. Healthcare. 2021; 9(6):694. https://doi.org/10.3390/healthcare9060694
Chicago/Turabian StyleFeldbrügge, Linda, Alexander Langenscheidt, Felix Krenzien, Mareike Schulz, Nicco Krezdorn, Kaan Kamali, Andreas Hinz, Michael Bartels, Panagiotis Fikatas, Moritz Schmelzle, and et al. 2021. "Health-Related Quality of Life and Mental Health after Surgical Treatment of Hepatocellular Carcinoma in the Era of Minimal-Invasive Surgery: Resection versus Transplantation" Healthcare 9, no. 6: 694. https://doi.org/10.3390/healthcare9060694
APA StyleFeldbrügge, L., Langenscheidt, A., Krenzien, F., Schulz, M., Krezdorn, N., Kamali, K., Hinz, A., Bartels, M., Fikatas, P., Schmelzle, M., Pratschke, J., & Benzing, C. (2021). Health-Related Quality of Life and Mental Health after Surgical Treatment of Hepatocellular Carcinoma in the Era of Minimal-Invasive Surgery: Resection versus Transplantation. Healthcare, 9(6), 694. https://doi.org/10.3390/healthcare9060694







