Cost-Effectiveness of an Exercise Programme That Provided Group or Individual Training to Reduce the Fall Risk in Healthy Community-Dwelling People Aged 65–80: A Secondary Data Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Group and Individual Interventions
2.3. Variable Collection
2.4. Economic Assessment
2.5. Statistical Analysis
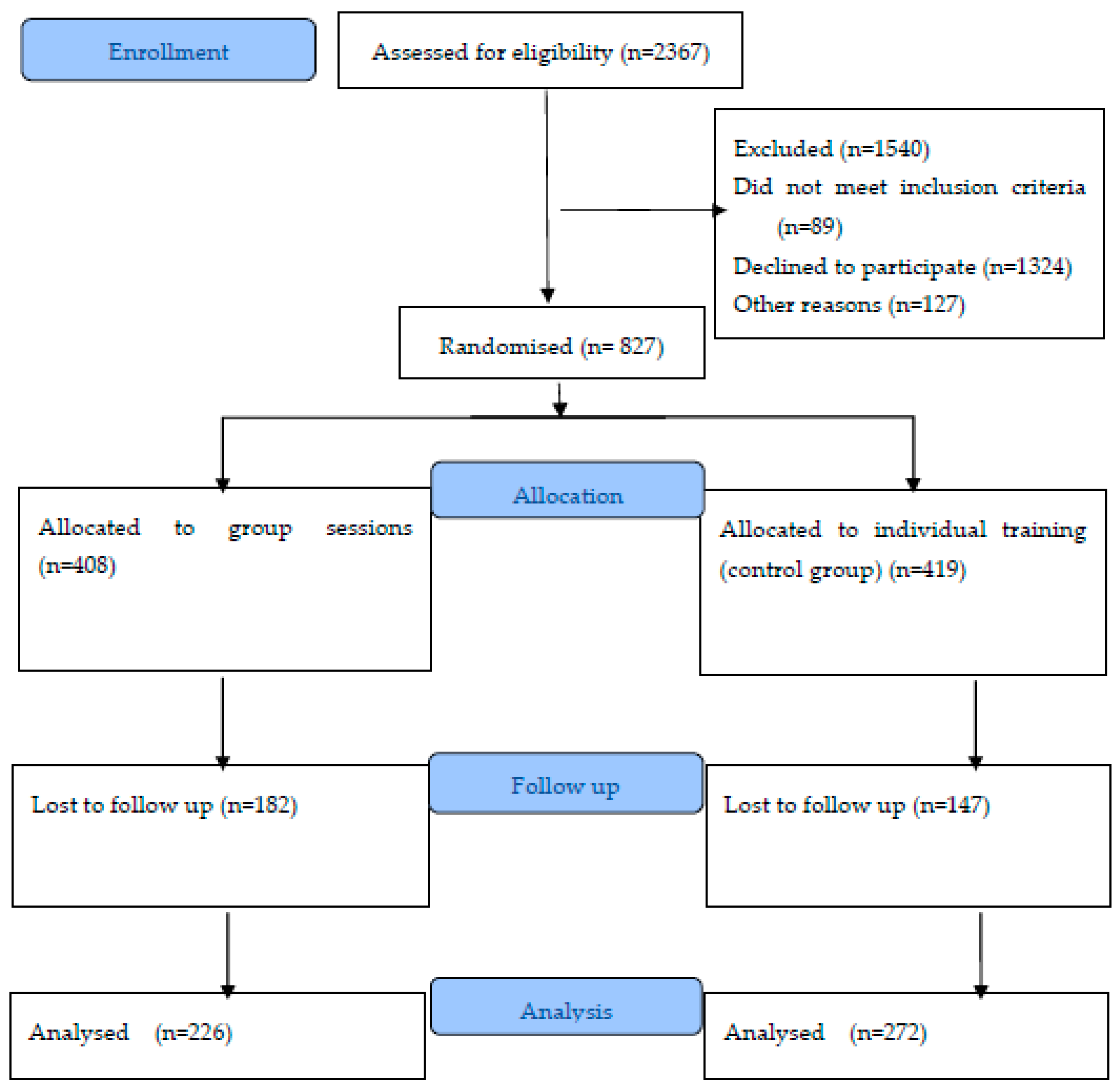
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and fall injuries among adults aged ≥65 years United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef]
- Miake-Lye, E.M.; Hempel, S.; Ganz, D.A.; Shekelle, P.G. Inpatient fall prevention programs as a patient safety strategy: A systematic review. Ann. Intern. Med. 2013, 158 Pt 2, 390–396. [Google Scholar] [CrossRef]
- Parachute. The Cost of Injury in Canada; Parachute: Toronto, ON, Canada, 2015. [Google Scholar]
- Kelsey, J.L.; Procter-Gray, E.; Hannan, M.T.; Li, W. Heterogeneity of falls among older adults: Implications for public health prevention. Am. J. Public Health 2012, 102, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.R.; Stevens, J.A.; Lee, R. The direct costs of fatal and non-fatal falls among older adults United States. J. Saf. Res. 2016, 58, 99–103. [Google Scholar] [CrossRef]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Carande-Kulis, V.; Stevens, J.A.; Florence, C.S.; Beattie, B.L.; Arias, I. A cost-benefit analysis of three older adult fall prevention interventions. J. Saf. Res. 2015, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Robertson, M.C.; Ashe, M.C.; Liu-Ambrose, T.; Khan, K.M.; Marra, C.A. International comparison of cost of falls in older adults living in the community: A systematic review. Osteoporos. Int. 2010, 21, 1295–1306. [Google Scholar] [CrossRef]
- ESA on Falls. Active Ageing through Preventing Falls: “Falls Prevention Is Everyone’s Business”. Available online: https://eupha.org/repository/sections/ipsp/Joint_Declaration_Sept_2015.pdf (accessed on 30 January 2021).
- Pearce, L. Preventing falls in hospital. Nurs. Manag. 2017, 23, 11. [Google Scholar]
- Lamb, S.E.; Hauer, K.; Becker, C. Manual for the Fall Prevention Classification System. Version 1. Available online: http://www.profane.eu.org/documents/Falls_Taxonomy.pdf (accessed on 30 January 2021).
- Lamb, S.E.; Becker, C.; Gillespie, L.D.; Smith, J.L.; Finnegan, S.; Potter, R.; Pfeiffer, K. Reporting of complex interventions in clinical trials: Development of a taxonomy to classify and describe fall-prevention interventions. Trials 2011, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, F.; Cassou, B.; Charles, M.A.; Dargent-Molina, P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f6234. [Google Scholar] [CrossRef]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.; Lamb, S.E. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 12, CD007146. [Google Scholar] [CrossRef]
- Lacroix, A.; Kressig, R.W.; Muehlbauer, T.; Gschwind, Y.J.; Pfenninger, B.; Bruegger, O.; Granacher, U. Effects of a Supervised versus an Unsupervised Combined Balance and Strength Training Program on Balance and Muscle Power in Healthy Older Adults: A Randomized Controlled Trial. Gerontology 2016, 62, 275–288. [Google Scholar] [CrossRef]
- Morris, J.N.; Howard, E.P.; Steel, K.; Berg, K.; Tchalla, A.; Munankarmi, A.; David, D. Strategies to reduce the risk of falling: Cohort study analysis with 1-year follow-up in community dwelling older adults. BMC Geriatr. 2016, 29, 92. [Google Scholar] [CrossRef]
- Phelan, E.A.; Mahoney, J.E.; Voit, J.C.; Stevens, J.A. Assessment and management of fall risk in primary care settings. Med. Clin. N. Am. 2015, 99, 281–293. [Google Scholar] [CrossRef]
- Sherrington, C.; Whitney, J.C.; Lord, S.R.; Herbert, R.D.; Cumming, R.G.; Close, J.C.T. Effective exercise for the prevention of falls: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2008, 56, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. N. S. W. Public Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1750–1758. [Google Scholar] [CrossRef]
- Benavent-Caballer, V.; Rosado-Calatayud, P.; Segura-Ortí, E.; Amer-Cuenca, J.J.; Lisón, J.F. The effectiveness of a video-supported group-based Otago exercise programme on physical performance in community-dwelling older adults: A preliminary study. Physiotherapy 2016, 102, 280–286. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Moen, K.; Røysland, A.S.; Helbostad, J.L. The Otago Exercise Program performed as group training versus home training in fall-prone older people: A randomized controlled Trial. Physiother. Res. Int. 2014, 19, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Skelton, D.; Dinan, S.; Campbell, M.; Rutherford, O. Tailored group exercise (Falls Management Exercise FaME) reduces falls in community-dwelling older frequent fallers (an RCT). Age Ageing 2005, 34, 636–639. [Google Scholar] [CrossRef] [PubMed]
- De Vries, N.M.; van Ravensberg, C.D.; Hobbelen, J.S.; Olde Rikkert, M.G.M.; Staal, J.B.; Nijhuis-van der Sanden, M.W.G. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: A meta-analysis. Ageing Res. Rev. 2012, 11, 136–149. [Google Scholar] [CrossRef]
- Cyarto, E.V.; Brown, W.J.; Shall, A.L.; Trost, S.G. Comparative effects of home- and group-based exercise on balance confidence and balance ability in older adults: Cluster randomized trial. Gerontology 2008, 54, 272–280. [Google Scholar] [CrossRef]
- Helbostad, J.L.; Sletvold, O.; Moe-Nilssen, R. Home training with and without additional group training in physically frail old people living at home: Effect on health-related quality of life and ambulation. Clin. Rehabil. 2004, 18, 498–508. [Google Scholar] [CrossRef]
- Davis, J.C.; Robertson, M.C.; Ashe, M.C.; Liu-Ambrose, T.; Khan, K.M.; Marra, C.A. Does a home-based strength and balance programme in people aged > or =80 years provide the best value for money to prevent falls? A systematic review of economic evaluations of falls prevention interventions. Br. J. Sports Med. 2010, 44, 80–89, Erratum in Br. J. Sports Med. 2011, 45, e3. [Google Scholar] [CrossRef]
- Albornos-Muñoz, L.; Moreno-Casbas, M.T.; Sánchez-Pablo, C.; Bays-Moneo, A.; Fernández-Domínguez, J.C.; Rich-Ruiz, M.; Gea-Sánchez, M.; the Otago Project Working Group. Efficacy of the Otago Exercise Programme to reduce falls in community-dwelling adults aged 65–80 years old when delivered as group or individual training. J. Adv. Nurs. 2018, 74, 1700–1711. [Google Scholar] [CrossRef]
- Campbell, A.J.; Robertson, M.C. Otago Exercise Programme to Prevent Falls in Older Adults: A Home-Based, Individually Tailored Strength and Balance Retraining Programme; Otago Medical School, University of Otago: Otago, New Zealand, 2003. [Google Scholar]
- Newman-Beinart, N.A.; Norton, S.; Dowling, D.; Gavriloff, D.; Vari, C.; Weinman, J.A.; Godfrey, E.L. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: The Exercise Adherence Rating Scale (EARS). Physiotherapy 2017, 103, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.Y.; Au-Yeung, S.S.; Lo, S.K. A comparison of four functional tests in discriminating fallers from non-fallers in older people. Disabil. Rehabil. 2003, 25, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, E.; Valenzuela, J.; Escobar, M. Rendimiento en las pruebas “Timed Up and Go” y “Estación Unipodal” en adultos mayores chilenos entre 60 y 89 años [Timed up and go right and left unipodal stance results in Chilean older people with different degrees of disability]. Rev. Med. Chile 2015, 143, 39–46. (In Spanish) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community dwelling older adults using the Time Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef]
- Rodríguez-Guevara, C.; Helena-Hugo, L. Validez y confiabilidad de la Escala de Tinetti para población colombiana. Rev. Colomb. Reumatol. 2012, 19, 218–233. [Google Scholar] [CrossRef]
- Lauretani, F.; Ticinesi, A.; Gionti, L.; Prati, B.; Nouvenne, A.; Tana, C.; Meschi, T.; Maggio, M. Short-Physical Performance Battery (SPPB) score is associated with falls in older outpatients. Aging Clin. Exp. Res. 2019, 31, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Dinan, S.; Gawler, S. The Manual LTT ‘Cómo Enseñar el Programa de Ejercicios de Otago’. Available online: http://profound.eu.com/resources/ (accessed on 30 January 2021).
- Van Asselt, A.D.; van Mastrigt, G.A.; Dirksen, C.D.; Arntz, A.; Severens, J.L.; Kessels, A.G.H. How to deal with cost differences at baseline. Pharmacoeconomics 2009, 27, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lung, T.; Si, L.; Hooper, R.; Di Tanna, G.L. Health Economic Evaluation alongside Stepped Wedge Trials: A Methodological Systematic Review. Pharmacoeconomics 2021, 39, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Willan, A.R.; Briggs, A.H.; Hoch, J.S. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ. 2004, 13, 461–475. [Google Scholar] [CrossRef]
- Fenwick, E.; Claxton, K.; Sculpher, M. Representing uncertainty: The role of cost-effectiveness acceptability curves. Health Econ. 2001, 10, 779–787. [Google Scholar] [CrossRef]
- Albert, S.M.; Raviotta, J.; Lin, C.J.; Edelstein, O.; Smith, K.J. Cost-effectiveness of a statewide falls prevention program in Pennsylvania: Healthy Steps for Older Adults. Am. J. Manag. Care 2016, 22, 638–644. [Google Scholar] [PubMed]
- Keall, M.D.; Pierse, N.; Howden-Chapman, P.; Guria, J.; Cunningham, C.W.; Baker, M.G. Cost–benefit analysis of fall injuries prevented by a programme of home modifications: A cluster randomised controlled trial. Inj. Prev. 2017, 23, 22–26. [Google Scholar] [CrossRef]
- Iliffe, S.; Kendrick, D.; Morris, R.; Masud, T.; Gage, H.; Skelton, D.; Dinan, S.; Bowling, A.; Griffin, M.; Haworth, D.; et al. Multi-centre cluster randomised trial comparing a community group exercise programme with home based exercise with usual care for people aged 65 and over in primary care. Health Technol. Assess 2014, 18, vii–xxvii, 1–105. [Google Scholar] [CrossRef]
- Abdulrazaq, S.; Oldham, J.; Skelton, D.A.; O’Neill, T.; Munford, L.; Gannon, B.; Pilling, M.; Todd, C.; Stanmore, E.K. A prospective cohort study measuring cost-benefit analysis of the Otago Exercise Programme in community dwelling adults with rheumatoid arthritis. BMC Health Serv. 2018, 18, 574. [Google Scholar] [CrossRef]
- Hewitt, J.; Saing, S.; Goodall, S.; Henwood, T.; Clemson, L.; Refshauge, K. An economic evaluation of the SUNBEAM programme: A falls-prevention randomized controlled trial in residential aged care. Clin. Rehabil. 2019, 33, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.C.; Campbell, A.J.; Gardner, M.M.; Devlin, N. Preventing injuries in older people by preventing falls: A meta-analysis of individual-level data. J. Am. Geriatr. Soc. 2002, 50, 905–911. [Google Scholar] [CrossRef]
- Mills, K.M.; Sadler, S.; Peterson, K.; Pang, L. An Economic Evaluation of Preventing Falls Using a New Exercise Program in Institutionalized Elderly. J. Phys. Act. Health 2018, 15, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Corbacho, B.; Cockayne, S.; Fairhurst, C.; Hewitt, C.E.; Hicks, K.; Kenan, A.-M.; Lamb, S.E.; MacIntosh, C.; Menz, H.B.; Redmond, A.C.; et al. Cost-Effectiveness of a Multifaceted Podiatry Intervention for the Prevention of Falls in Older People: The REducing Falls with Orthoses and a Multifaceted Podiatry Intervention Trial Findings. Gerontology 2018, 64, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Deverall, E.; Kvizhinadze, G.; Pega, F.; Blakely, T.; Wilson, N. Exercise programmes to prevent falls among older adults: Modelling health gain, cost-utility and equity impacts. Inj. Prev. 2019, 25, 258–263. [Google Scholar] [CrossRef]
- Kunigkeit, C.; Stock, S.; Müller, D. Cost-effectiveness of a home safety intervention to prevent falls in impaired elderly people living in the community. Arch. Osteoporos. 2018, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Matchar, D.B.; Eom, K.; Duncan, P.W.; Lee, M.; Sim, R.; Sivapragasam, N.R.; Lien, C.T.; Ong, M.E.H. A Cost-Effectiveness Analysis of a Randomized Control Trial of a Tailored, Multifactorial Program to Prevent Falls among the Community-Dwelling Elderly. Arch. Phys. Med. Rehabil. 2019, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Crandall, C.J.; Ganz, D.A. Cost-effectiveness of combined oral bisphosphonate therapy and falls prevention exercise for fracture prevention in the USA. Osteoporos. Int. 2017, 28, 585–595. [Google Scholar] [CrossRef]
- Pega, F.; Kvizhinadze, G.; Blakely, T.; Atkinson, J.; Wilson, N. Home safety assessment and modification to reduce injurious falls in community-dwelling older adults: Cost-utility and equity analysis. Inj. Prev. 2016, 22, 420–426. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L.; Laosa, O.; Vellas, B.; Paolisso, G.; Topinkova, E.; Oliva-Moreno, J.; Bourdel-Marchasson, I.; Izquierdo, M.; Hood, K.; Zeyfang, A.; et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J. Cachexia Sarcopenia Muscle 2019, 10, 721–733. [Google Scholar] [CrossRef]
- Isaranuwatchai, W.; Perdrizet, J.; Kle-Reid, M.; Hoch, J.S. Cost-effectiveness analysis of a multifactorial fall prevention intervention in older home care clients at risk for falling. BMC Geriatr. 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Eom, G.M.; Kim, C.S.; Kim, D.-H.; Lee, J.-H.; Park, B.K.; Hong, J. Sex differences in the postural sway characteristics of young and elderly subjects during quiet natural standing. Geriatr. Gerontol. Int. 2010, 10, 191–198. [Google Scholar] [CrossRef]
- Puszczalowska-Lizis, E.; Bujas, P.; Jandzis, S.; Omorczyk, J.; Zak, M. Inter-gender differences of balance indicators in persons 60–90 years of age. Clin. Interv. Aging 2018, 13, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Hardy, R.; Aihie Sayer, A.; Ben-Shlomo, Y.; Birnie, K.; Cooper, C.; Craig, L.; Deary, I.J.; Demakakos, P.; Gallacher, J.; et al. Age and gender differences in physical capability levels from mid-life onwards: The harmonisation and meta-analysis of data from eight UK cohort studies. PLoS ONE 2011, 6, e27899. [Google Scholar] [CrossRef]
- Aranda-Reneo, I.; Rodríguez-Sánchez, B.; Peña-Longobardo, L.M.; Oliva-Moreno, J.; López-Bastida, J. Can the Consideration of Societal Costs Change the Recommendation of Economic Evaluations in the Field of Rare Diseases? An Empirical Analysis. Value Health 2021, 24, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.; Papenburg, J.; Tan, S.S.; Brouwer, W.; Hakkaart, L. A noticeable difference? Productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur. J. Health Econ. 2016, 17, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Capri, S.; Ceci, A.; Terranova, L.; Merlo, F.; Mantovani, L. Guidelines for Economic Evaluations in Italy: Recommendations from the Italian Group of Pharmacoeconomic Studies. Drug Inf. J. 2001, 35, 189–201. [Google Scholar] [CrossRef]
- Lopez-Bastida, J.; Oliva, J.; Antonanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Spanish recommendations on economic evaluation of health technologies. Eur. J. Health Econ. 2010, 11, 513–520. [Google Scholar] [CrossRef]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Sáez de Asteasu, M.L.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of Exercise Intervention on Functionalline in Very Elderly Patients during Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 28–36, Erratum in JAMA Intern. Med. 2019, 179, 127. [Google Scholar] [CrossRef]
- Oh, B.; Cho, B.; Choi, H.C.; Son, K.Y.; Park, S.M.; Chun, S.; Cho, S.-I. The influence of lower-extremity function in elderly individuals’ quality of life (QOL): An analysis of the correlation between SPPB and EQ-5D. Arch. Gerontol. Geriatr. 2014, 58, 278–282. [Google Scholar] [CrossRef]
- Al, M.J.; van Hout, B.A.; Michel, B.C.; Rutten, F.F. Sample size calculation in economic evaluations. Health Econ. 1998, 7, 327–335. [Google Scholar] [CrossRef]
- Boyd, K.A.; Briggs, A.H.; Fenwick, E.; Norrie, J.; Stock, S. Power and sample size for cost-effectiveness analysis: fFN neonatal screening. Contemp. Clin. Trials 2011, 32, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Glick, H.A. Sample size and power for cost-effectiveness analysis (part 1). Pharmacoeconomics 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]



| Control (n = 272) | Intervention (n = 226) | |
|---|---|---|
| Age, mean (SD) | 71.67 (4.11 *) | 72.17 (4.18 *) |
| Modified Tinetti Scale, mean (SD) | 32.06 (3.94 *) | 31.68 (4.24 *) |
| SPPB Test, mean (SD) | 9.9 (1.9 *) | 10.17 (1.97 *) |
| Sex, n (%) | ||
| Male | 99 (36) | 65 (29) |
| Female | 173 (64) | 161 (71) |
| Education level, n (%) | ||
| None | 26 (10) | 17 (8) |
| Did not finish primary studies | 52 (19) | 54 (24) |
| Primary studies (0–11/12 years) | 122 (45) | 104 (46) |
| Secondary studies (11/12–17/18 years) | 43 (16) | 39 (17) |
| University studies | 29 (11) | 12 (5) |
| Marital status, n (%) | ||
| Single | 17 (6) | 11 (5) |
| Married | 177 (65) | 141 (62) |
| Widow/widower | 65 (24) | 63 (28) |
| Other | 13 (5) | 11 (5) |
| Costs in Euros | Intervention Mean (SD) (€) | Control Mean (SD) (€) | Mean Difference (95% CI) a(€) |
| Visits to family doctors | 5.74 (21.24) | 4.11 (17.78) | 1.63 (−1.8; 5.07) |
| Hospital costs | 7.82 (42.84) | 8.46 (54.94) | −0.64 (−9.45; 8.17) |
| Direct healthcare costs | 13.56 (54.1) | 12.57 (58.36) | 0.99 (−9; 10.98) |
| Intervention costs | 24.47 (10.24) | 75.75 (25.36) | −51.28 (−54.81; −47.75) |
| Total costs | 38.03 (55.85) | 88.32 (62.17) | −50.29 (−60.79; −39.79) |
| No-risk of Falling | Intervention Mean (SD) | Control Mean (SD) | Mean Difference (95% CI) b |
| Modified Tinetti | 0.98 (0.15) | 0.96 (0.19) | 0.01 (−0.02; 0.04) |
| Timed Up and Go | 0.80 (0.43) | 0.71 (0.46) | 0.09 (0.01; 0.17) |
| SPPB | 0.79 (0.41) | 0.75 (0.44) | 0.04 (−0.03; 0.12) |
| Outcome | Incremental Costs in Euros (€) a | Incremental Effects a | ICER |
|---|---|---|---|
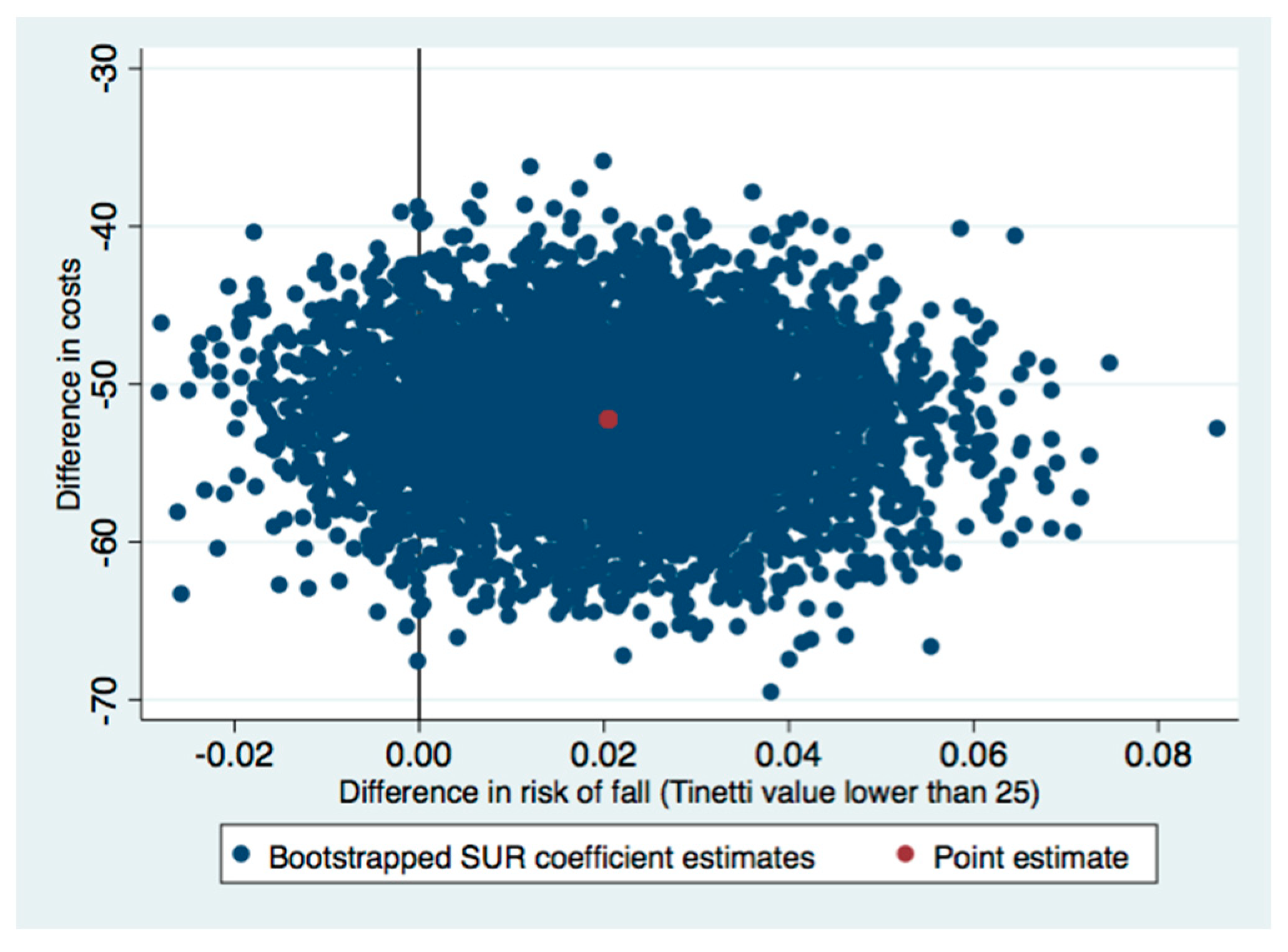
| Tinetti a | −52.19 (−61.46 to −42.92) | 0.02 (−0.01 to 0.05) | Dominant |
| Timed Up and Go | −52.19 (−61.50 to −42.88) | 0.11 (0.05 to 0.18) | Dominant |
| SPPB b | −52.35 (−62.32 to −42.06) | 0.08 (0.01 to 0.14) | Dominant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-Reneo, I.; Albornos-Muñoz, L.; Rich-Ruiz, M.; Cidoncha-Moreno, M.Á.; Pastor-López, Á.; Moreno-Casbas, T.; Otago Project Working Group. Cost-Effectiveness of an Exercise Programme That Provided Group or Individual Training to Reduce the Fall Risk in Healthy Community-Dwelling People Aged 65–80: A Secondary Data Analysis. Healthcare 2021, 9, 714. https://doi.org/10.3390/healthcare9060714
Aranda-Reneo I, Albornos-Muñoz L, Rich-Ruiz M, Cidoncha-Moreno MÁ, Pastor-López Á, Moreno-Casbas T, Otago Project Working Group. Cost-Effectiveness of an Exercise Programme That Provided Group or Individual Training to Reduce the Fall Risk in Healthy Community-Dwelling People Aged 65–80: A Secondary Data Analysis. Healthcare. 2021; 9(6):714. https://doi.org/10.3390/healthcare9060714
Chicago/Turabian StyleAranda-Reneo, Isaac, Laura Albornos-Muñoz, Manuel Rich-Ruiz, María Ángeles Cidoncha-Moreno, Ángeles Pastor-López, Teresa Moreno-Casbas, and Otago Project Working Group. 2021. "Cost-Effectiveness of an Exercise Programme That Provided Group or Individual Training to Reduce the Fall Risk in Healthy Community-Dwelling People Aged 65–80: A Secondary Data Analysis" Healthcare 9, no. 6: 714. https://doi.org/10.3390/healthcare9060714
APA StyleAranda-Reneo, I., Albornos-Muñoz, L., Rich-Ruiz, M., Cidoncha-Moreno, M. Á., Pastor-López, Á., Moreno-Casbas, T., & Otago Project Working Group. (2021). Cost-Effectiveness of an Exercise Programme That Provided Group or Individual Training to Reduce the Fall Risk in Healthy Community-Dwelling People Aged 65–80: A Secondary Data Analysis. Healthcare, 9(6), 714. https://doi.org/10.3390/healthcare9060714







