The Potential Impact of Salivary IL-1 on the Diagnosis of Periodontal Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Saliva Collection
2.3. Cytokine Array in Saliva Samples
2.4. Multiplex Fluorescent Bead Immunoassay of Saliva Samples
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Screening of Periodontal Disease-Related Biomarkers in Saliva Samples
3.3. Potential Salivary Biomarkers for Predicting Gingival Bleeding
3.4. Potential Salivary Biomarkers for Predicting Periodontal Disease
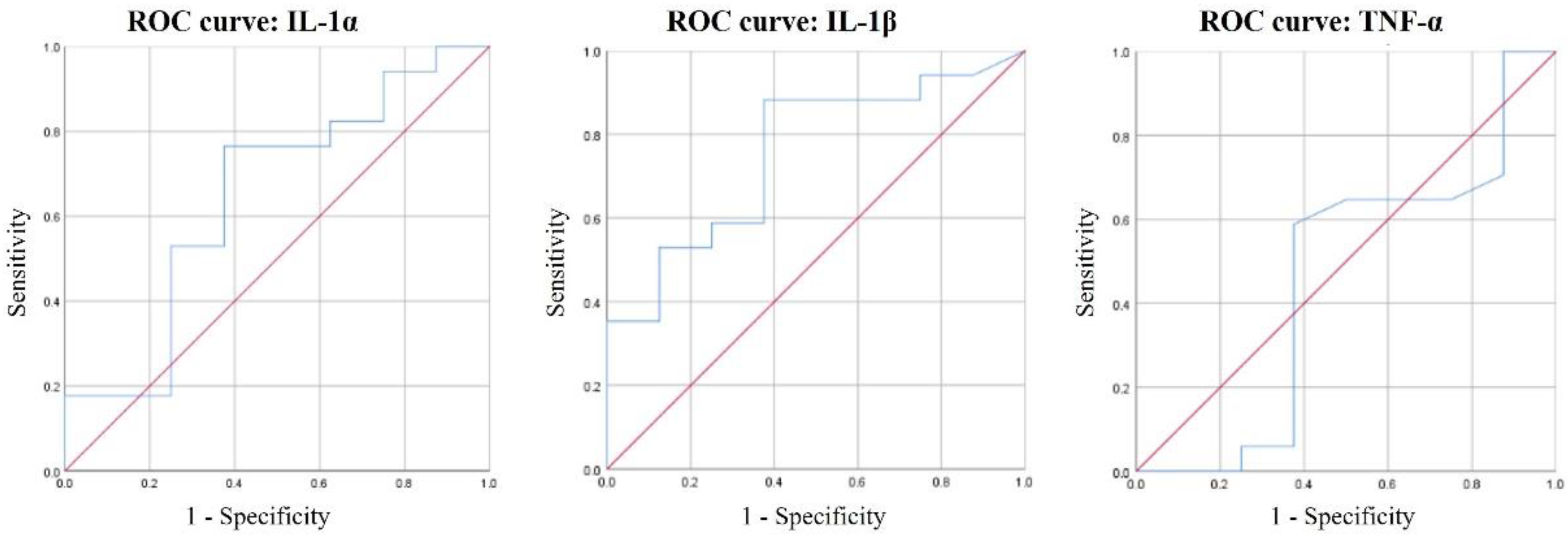
3.5. ROC Curves of IL-1α, IL-1β, and TNF-α for Periodontal Disease Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Wei, L.; Borgnakke, W.S.; Thornton-Evans, G.; Zhang, X.Y.; Lu, H.; Mcguire, L.C.; Genco, R.J. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontol. 2000 2016, 72, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef]
- Lianidou, E. Detection and relevance of epigenetic markers on ctDNA: Recent advances and future outlook. Mol. Oncol. 2021. [Google Scholar] [CrossRef]
- Riis, J.L.; Granger, D.A.; DiPietro, J.A.; Bandeen-Roche, K.; Johnson, S.B. Salivary Cytokines as a Minimally-Invasive Measure of Immune Functioning in Young Children: Correlates of Individual Differences and Sensitivity to Laboratory Stress. Dev. Psychobiol. 2015, 57, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Arregger, A.L.; Cardoso, E.M.L.; TUmilasci, O.; Contreras, L.N. Diagnostic value of salivary cortisol in end stage renal disease. Steroids 2008, 73, 77–82. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.H.; Meijer, J.; Leong, S.; Xie, Y.M.; Yu, T.W.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary proteomic and genomic biomarkers for primary Sjogren’s syndrome. Arthritis Rheumatol. 2007, 56, 3588–3600. [Google Scholar] [CrossRef]
- Novak, B.J.; Blake, D.R.; Meinardi, S.; Rowland, F.S.; Pontello, A.; Cooper, D.M.; Galassetti, P.R. Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15613–15618. [Google Scholar] [CrossRef]
- Rao, P.V.; Reddy, A.P.; Lu, X.; Dasari, S.; Krishnaprasad, A.; Biggs, E.; Roberts, C.T.; Nagalla, S.R. Proteomic Identification of Salivary Biomarkers of Type-2 Diabetes. J. Proteome Res. 2009, 8, 239–245. [Google Scholar] [CrossRef]
- Savica, V.; Calo, L.; Santoro, D.; Monardo, P.; Granata, A.; Bellinghieri, G. Salivary phosphate secretion in chronic kidney disease. J. Renal Nutr. 2008, 18, 87–90. [Google Scholar] [CrossRef]
- Streckfus, C.; Bigler, L.; Tucci, M.; Thigpen, J.T. A preliminary study of CA15-3, c-erbB-2, epidermal growth factor receptor, cathepsin-D, and p53 in saliva among women with breast carcinoma. Cancer Investig. 2000, 18, 101–109. [Google Scholar] [CrossRef]
- Ongoz Dede, F.; Balli, U.; Bozkurt Dogan, S.; Guven, B. Interleukin-32 levels in gingival crevicular fluid and saliva of patients with chronic periodontitis after periodontal treatment. J. Periodontal Res. 2017, 52, 397–407. [Google Scholar] [CrossRef]
- Riis, J.L.; Out, D.; Dorn, L.D.; Beal, S.J.; Denson, L.A.; Pabst, S.; Jaedicke, K.; Granger, D.A. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Dev. Psychobiol. 2014, 56, 797–811. [Google Scholar] [CrossRef]
- Giannobile, W.V.; McDevitt, J.T.; Niedbala, R.S.; Malamud, D. Translational and clinical applications of salivary diagnostics. Adv. Dent. Res. 2011, 23, 375–380. [Google Scholar] [CrossRef]
- Zhang, L.; Henson, B.S.; Camargo, P.M.; Wong, D.T. The clinical value of salivary biomarkers for periodontal disease. Periodontol. 2000 2009, 51, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Yeltiwar, R.K.; Pushpanshu, K. Salivary interleukin-1β levels in patients with chronic periodontitis before and after periodontal phase I therapy and healthy controls: A case-control study. J. Periodontol. 2011, 82, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Rangbulla, V.; Nirola, A.; Gupta, M.; Batra, P.; Gupta, M. Salivary IgA, Interleukin-1β and MMP-8 as Salivary Biomarkers in Chronic Periodontitis Patients. Chin. J. Dent. Res. 2017, 20, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.A.; Miozza, V.A.; Delgado, A.; Busch, L. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. J. Clin. Periodontol. 2013, 40, 1112–1117. [Google Scholar] [CrossRef]
- Agarwal, N.; Kumar, V.S.; Gujjari, S.A. Effect of periodontal therapy on hemoglobin and erythrocyte levels in chronic generalized periodontitis patients: An interventional study. J. Indian Soc. Periodontol. 2009, 13, 6–11. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 2–18. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis. Markers 2019, 2019, 8761860. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Pan, Y. The Influences of Periodontal Status and Periodontal Pathogen Quantity on Salivary 8-Hydroxydeoxyguanosine and Interleukin-17 Levels. J. Periodontol. 2016, 87, 591–600. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y. Point-of-care diagnosis of periodontitis using saliva: Technically feasible but still a challenge. Front. Cell. Infect. Microbiol. 2015, 5, 65. [Google Scholar] [CrossRef]
- Koshi, E.; Rajesh, S.; Koshi, P.; Arunima, P.R. Risk assessment for periodontal disease. J. Indian Soc. Periodontol. 2012, 16, 324–328. [Google Scholar] [CrossRef]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. (Zagreb) 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Campbell, J.L.; Cooper-White, J.; Dimeski, G.; Punyadeera, C. The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. Clin. Transl. Med. 2012, 1, 19. [Google Scholar] [CrossRef]
- Ray, C.A.; Bowsher, R.R.; Smith, W.C.; Devanarayan, V.; Willey, M.B.; Brandt, J.T.; Dean, R.A. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. J. Pharm. Biomed. Anal. 2005, 36, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Navidad, J.F.; Griswold, D.J.; Gradus, M.S.; Bhattacharyya, S. Evaluation of Luminex xTAG gastrointestinal pathogen analyte-specific reagents for high-throughput, simultaneous detection of bacteria, viruses, and parasites of clinical and public health importance. J. Clin. Microbiol. 2013, 51, 3018–3024. [Google Scholar] [CrossRef]
- Arellano-Garcia, M.E.; Hu, S.; Wang, J.; Henson, B.; Zhou, H.; Chia, D.; Wong, D.T. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008, 14, 705–712. [Google Scholar] [CrossRef]
- Augustine, S.A.; Eason, T.N.; Simmons, K.J.; Curioso, C.L.; Griffin, S.M.; Ramudit, M.K.; Plunkett, T.R. Developing a Salivary Antibody Multiplex Immunoassay to Measure Human Exposure to Environmental Pathogens. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.C.; Wang, K.; Wadhwa, P.D.; Culhane, J.F.; Nelson, E.L. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J. Reprod. Immunol. 2005, 66, 175–191. [Google Scholar] [CrossRef]
- Offenbacher, S. Periodontal diseases: Pathogenesis. Ann. Periodontol. 1996, 1, 821–878. [Google Scholar] [CrossRef]
- Reynolds, J.J.; Meikle, M.C. Mechanisms of connective tissue matrix destruction in periodontitis. Periodontol. 2000 1997, 14, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. Interleukin-4: A prototypic immunoregulatory lymphokine. Blood 1991, 77, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Gamonal, J.; Acevedo, A.; Bascones, A.; Jorge, O.; Silva, A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J. Periodontol. 2000, 71, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Gamonal, J.; Acevedo, A.; Bascones, A.; Jorge, O.; Silva, A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J. Periodontal Res. 2001, 36, 194–203. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.; Radzikowska, A.; Jakubaszek, M.; Maślińska, M.; Kwiatkowska, B.; Maśliński, W. Monocyte-related biomarkers of rheumatoid arthritis development in undifferentiated arthritis patients—A pilot study. Reumatologia 2018, 56, 10–16. [Google Scholar] [CrossRef][Green Version]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Jain, P.; Ved, A.; Dubey, R.; Singh, N.; Parihar, A.S.; Maytreyee, R. Comparative Evaluation of Serum Tumor Necrosis Factor α in Health and Chronic Periodontitis: A Case-Control Study. Contemp. Clin. Dent. 2020, 11, 342–349. [Google Scholar] [CrossRef]
- Lee, A.; Ghaname, C.B.; Braun, T.M.; Sugai, J.V.; Teles, R.P.; Loesche, W.J.; Kornman, K.S.; Giannobile, W.V.; Kinney, J.S. Bacterial and Salivary Biomarkers Predict the Gingival Inflammatory Profile. J. Periodontol. 2012, 83, 79–89. [Google Scholar] [CrossRef]
- Syndergaard, B.; Al-Sabbagh, M.; Kryscio, R.J.; Xi, J.; Ding, X.; Ebersole, J.L.; Miller, C.S. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014, 85, e295–e303. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, H.N. Changes in Inflammatory Cytokines in Saliva after Non-Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Romero-Castro, N.S.; Vázquez-Villamar, M.; Muñoz-Valle, J.F.; Reyes-Fernández, S.; Serna-Radilla, V.O.; García-Arellano, S.; Castro-Alarcón, N. Relationship between TNF-α, MMP-8, and MMP-9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology 2020, 108, 25–33. [Google Scholar] [CrossRef]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine gene polymorphisms associate with microbiogical agents in periodontal disease: Our experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gupta, N.D.; Bey, A.; Khan, S. Salivary TNF-alpha: A potential marker of periodontal destruction. J. Indian Soc. Periodontol. 2014, 18, 306–310. [Google Scholar] [CrossRef]
- Varghese, S.S.; Thomas, H.; Jayakumar, N.D.; Sankari, M.; Lakshmanan, R. Estimation of salivary tumor necrosis factor-alpha in chronic and aggressive periodontitis patients. Contemp. Clin. Dent. 2015, 6, S152–S156. [Google Scholar] [CrossRef]
- Bostanci, N.; Mitsakakis, K.; Afacan, B.; Bao, K.; Johannsen, B.; Baumgartner, D.; Müller, L.; Kotolová, H.; Emingil, G.; Karpíšek, M. Validation and verification of predictive salivary biomarkers for oral health. Sci. Rep. 2021, 11, 6406. [Google Scholar] [CrossRef] [PubMed]
- Kc, S.; Wang, X.Z.; Gallagher, J.E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. J. Clin. Periodontol. 2020, 47, 289–308. [Google Scholar] [CrossRef]

| Variables | Items | N |
|---|---|---|
| Gender | Male Female | 19 14 |
| Age (year) | ≤39 40–49 50–59 ≥60 | 6 7 14 6 |
| Periodontal disease | Yes No | 21 12 |
| Gingival bleeding status | High Low | 25 8 |
| Systemic disease | Yes No | 4 29 |
| Total | 33 |
| Biomarkers | Gingival Bleeding Status | Mean (pg/mL) | SD | p-Value * | p-Value ** |
|---|---|---|---|---|---|
| IL-1α | High | 729.22 | 497.16 | 0.170 | 0.333 |
| Low | 438.89 | 254.64 | |||
| IL-1β | High | 265.31 | 199.52 | 0.025 | 0.049 |
| Low | 81.80 | 46.60 | |||
| IL-8 | High | 444.94 | 273.73 | 0.442 | 0.752 |
| Low | 349.88 | 187.71 | |||
| TNF-α | High | 13.46 | 3.80 | 0.028 | 0.374 |
| Low | 15.40 | 6.76 | |||
| IL-4 | High | 3.46 | 1.17 | 0.683 | 0.826 |
| Low | 3.19 | 1.31 | |||
| CCL2/MCP-1 | High | 268.60 | 152.37 | 0.992 | 0.593 |
| Low | 269.35 | 136.38 | |||
| CCL3/MIP-1α | High | 25.50 | 9.92 | 0.556 | 0.957 |
| Low | 29.12 | 12.83 |
| Biomarkers | Periodontal Disease | Mean (pg/mL) | SD | p-Value * | p-Value ** |
|---|---|---|---|---|---|
| IL-1α | Y | 590.78 | 344.72 | 0.048 | 0.037 |
| N | 343.60 | 188.30 | |||
| IL-1β | Y | 216.98 | 180.81 | 0.106 | 0.095 |
| N | 94.55 | 96.93 | |||
| IL-8 | Y | 459.89 | 389.10 | 0.881 | 0.622 |
| N | 435.10 | 303.45 | |||
| TNF-α | Y | 13.64 | 4.07 | 0.045 | 0.837 |
| N | 15.25 | 6.82 | |||
| IL-4 | Y | 3.54 | 1.30 | 0.547 | 0.777 |
| N | 3.16 | 1.61 | |||
| CCL2/MCP-1 | Y | 315.12 | 203.11 | 0.335 | 0.460 |
| N | 407.98 | 264.82 | |||
| CCL3/MIP-1 | Y | 30.52 | 10.23 | 0.402 | 0.302 |
| N | 34.56 | 11.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kim, K.-R.; Kim, H.-N. The Potential Impact of Salivary IL-1 on the Diagnosis of Periodontal Disease: A Pilot Study. Healthcare 2021, 9, 729. https://doi.org/10.3390/healthcare9060729
Kim J-Y, Kim K-R, Kim H-N. The Potential Impact of Salivary IL-1 on the Diagnosis of Periodontal Disease: A Pilot Study. Healthcare. 2021; 9(6):729. https://doi.org/10.3390/healthcare9060729
Chicago/Turabian StyleKim, Ji-Youn, Ki-Rim Kim, and Han-Na Kim. 2021. "The Potential Impact of Salivary IL-1 on the Diagnosis of Periodontal Disease: A Pilot Study" Healthcare 9, no. 6: 729. https://doi.org/10.3390/healthcare9060729
APA StyleKim, J.-Y., Kim, K.-R., & Kim, H.-N. (2021). The Potential Impact of Salivary IL-1 on the Diagnosis of Periodontal Disease: A Pilot Study. Healthcare, 9(6), 729. https://doi.org/10.3390/healthcare9060729






