Tracking the CAR-T Revolution: Analysis of Clinical Trials of CAR-T and TCR-T Therapies for the Treatment of Cancer (1997–2020)
Abstract
:1. Introduction
2. Data Sources
3. Cellular Immunotherapies for Cancer: Overview of Clinical Trials
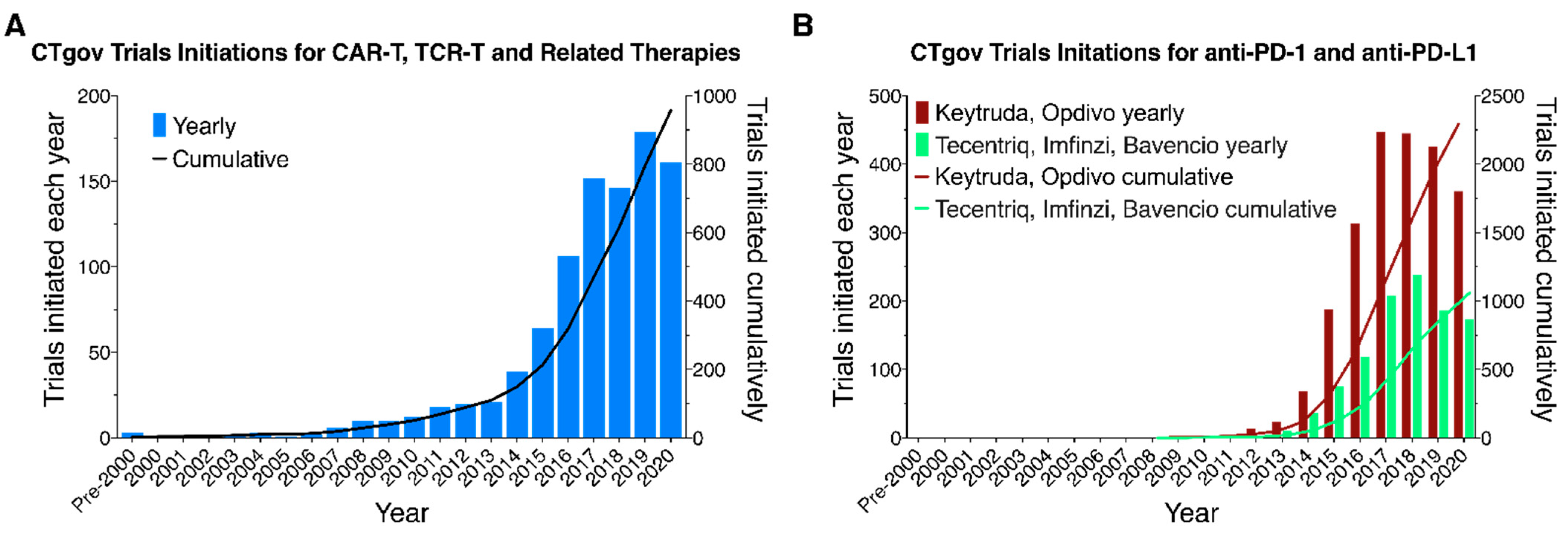
3.1. Clinical Trials for CAR-T/TCR-T and Related Therapies
3.2. Tissues and Antigens Targeted
4. Discussion
4.1. Rapid Rise in the Number of CAR-T/TCR-T Bioparallel Products
4.2. Effects of Clinical Success or Failure in the CAR-T/TCR-T Market
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical development of CAR T cells—Challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef]
- Davenport, A.J.; Cross, R.S.; Watson, K.A.; Liao, Y.; Shi, W.; Prince, H.M.; Beavis, P.A.; Trapani, J.A.; Kershaw, M.H.; Ritchie, D.S.; et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2068–E2076. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Wei, Q.; Brzostek, J.; Gascoigne, N.R.J. Signaling from T cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cell. Mol. Immunol. 2020, 17, 600–612. [Google Scholar] [CrossRef]
- Taylor, M.J.; Husain, K.; Gartner, Z.J.; Mayor, S.; Vale, R.D. A DNA-Based T Cell Receptor Reveals a Role for Receptor Clustering in Ligand Discrimination. Cell 2017, 169, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinchcombe, J.C.; Majorovits, E.; Bossi, G.; Fuller, S.; Griffiths, G.M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 2006, 443, 462–465. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Lee, Y.G.; Shestova, O.; Ravikumar, P.; Hayer, K.E.; Hong, S.J.; Lu, X.M.; Pajarillo, R.; Agarwal, S.; Kuramitsu, S.; et al. Impaired Death Receptor Signaling in Leukemia Causes Antigen-Independent Resistance by Inducing CAR T-cell Dysfunction. Cancer Discov. 2020, 10, 552–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufva, O.; Koski, J.; Maliniemi, P.; Ianevski, A.; Klievink, J.; Leitner, J.; Pölönen, P.; Hohtari, H.; Saeed, K.; Hannunen, T.; et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood 2020, 135, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Locke, F.L.; Rossi, J.M.; Neelapu, S.S.; Jacobson, C.A.; Miklos, D.B.; Ghobadi, A.; Oluwole, O.O.; Reagan, P.M.; Lekakis, L.J.; Lin, Y.; et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020, 4, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Huzair, F.; Kale, D. Biosimilars and the long game. Trends Biotechnol. 2015, 33, 250–252. [Google Scholar] [CrossRef]
- Moore, C. Biosimilar monoclonal antibodies (mAbs) in oncology. Br. J. Nurs. 2017, 26, S26–S32. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.A.N.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T Cells Targeting Carcinoembryonic Antigen Can Mediate Regression of Metastatic Colorectal Cancer but Induce Severe Transient Colitis. Mol. Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Chinnasamy, N.; Abate-Daga, D.; Gros, A.; Robbins, P.F.; Zheng, Z.; Dudley, M.E.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; et al. Cancer Regression and Neurological Toxicity Following Anti-MAGE-A3 TCR Gene Therapy. J. Immunother. 2013, 36, 133–151. [Google Scholar] [CrossRef] [Green Version]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L. The Emerging World of TCR-T Cell Trials Against Cancer: A Systematic Review. Technol. Cancer Res. Treat. 2019, 18, 1533033819831068. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Forero, J.V.; Lengerke-Diaz, P.A.; Castro, J.E. Chimeric antigen receptor T cell therapy in oncology—Pipeline at a glance: Analysis of the ClinicalTrials.gov database. Crit. Rev. Oncol. Hematol. 2021, 159, 103239. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Upadhaya, S.; Tatake, R.; Barkalow, F.; Hubbard-Lucey, V.M. Cancer cell therapies: The clinical trial landscape. Nat. Rev. Drug Discov. 2020, 19, 583–584. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Anderson, K.C. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy 2015, 7, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef]
- Labanieh, L.; Majzner, R.G.; Mackall, C.L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2018, 2, 377–391. [Google Scholar] [CrossRef]
- Kaida-Yip, F.; Deshpande, K.; Saran, T.; Vyas, D. Biosimilars: Review of current applications, obstacles, and their future in medicine. World J. Clin. Cases 2018, 6, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. mAbs 2015, 7, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, J.L. Trials and Terminations: Learning from Competitors’ R&D Failures. Manag. Sci. 2021, 1–19. [Google Scholar] [CrossRef]
- Ploessl, C.; Pan, A.; Maples, K.T.; Lowe, D.K. Dinutuximab: An anti-GD2 monoclonal antibody for high-risk neuroblastoma. Ann. Pharmacother. 2016, 50, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, R.; Novak, N.; Šerbec, V.Č.; Rajcevic, U. Targeting Malignant Brain Tumors with Antibodies. Front. Immunol. 2017, 8, 1181. [Google Scholar] [CrossRef] [Green Version]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Morgan, R.A.; Johnson, L.A.; Davis, J.L.; Zheng, Z.; Woolard, K.D.; Reap, E.A.; Feldman, S.A.; Chinnasamy, N.; Kuan, C.-T.; Song, H.; et al. Recognition of Glioma Stem Cells by Genetically Modified T Cells Targeting EGFRvIII and Development of Adoptive Cell Therapy for Glioma. Hum. Gene Ther. 2012, 23, 1043–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- D’Aloia, M.M.; Zizzari, I.G.; Sacchetti, B.; Pierelli, L.; Alimandi, M. CAR-T cells: The long and winding road to solid tumors. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Watanabe, K.; June, C.H.; Kloss, C.C.; Posey, A.D. Driving cars to the clinic for solid tumors. Gene Ther. 2018, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]





| NCT Trials Classifications | Early Phase 1 | Phase 1 | Phase 1 Phase 2 | Phase 2 | Phase 2 Phase 3 | Phase 3 | Not Applicable | Total |
|---|---|---|---|---|---|---|---|---|
| Not yet recruiting | 18 | 22 | 10 | 0 | 0 | 0 | 0 | 50 |
| Recruiting | 48 | 262 | 142 | 38 | 3 | 6 | 2 | 501 |
| 7.7% | 42.1% | 22.8% | 6.1% | 0.5% | 1.0% | |||
| Active, not recruiting | 3 | 74 | 26 | 16 | 0 | 1 | 1 | 121 |
| 0.5% | 11.9% | 4.2% | 2.6% | 0.0% | 0.2% | |||
| Enrolling by invitation | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 5 |
| 0.2% | 0.3% | 0.0% | 0.0% | 0.0% | 0.0% | |||
| Completed | 4 | 49 | 14 | 9 | 1 | 1 | 3 | 81 |
| Withdrawn | 0 | 15 | 11 | 11 | 0 | 1 | 0 | 38 |
| Terminated | 6 | 21 | 10 | 12 | 0 | 0 | 1 | 50 |
| Suspended | 0 | 7 | 5 | 2 | 0 | 0 | 0 | 14 |
| Unknown | 15 | 59 | 63 | 6 | 3 | 0 | 1 | 147 |
| Total | 95 | 511 | 281 | 94 | 7 | 9 | 10 | 1007 |
| Country of Origin | Total Trials | Initiated | Currently Active | Currently Inactive |
|---|---|---|---|---|
| China | 470 | 433 | 273 | 197 |
| US | 418 | 408 | 280 | 138 |
| United Kingdom | 43 | 43 | 26 | 17 |
| Germany | 15 | 15 | 11 | 4 |
| Belgium | 10 | 10 | 7 | 3 |
| France | 8 | 8 | 4 | 4 |
| Japan | 7 | 7 | 4 | 3 |
| Singapore | 9 | 7 | 3 | 6 |
| Australia | 3 | 3 | 2 | 1 |
| Italy | 4 | 4 | 4 | 0 |
| Netherlands | 3 | 3 | 2 | 1 |
| Spain | 3 | 3 | 3 | 0 |
| Israel | 2 | 2 | 2 | 0 |
| Russia | 2 | 2 | 2 | 0 |
| Sweden | 2 | 2 | 1 | 1 |
| Switzerland | 2 | 2 | 0 | 2 |
| Canada | 2 | 1 | 1 | 1 |
| Czech Republic | 1 | 1 | 0 | 1 |
| Malaysia | 1 | 1 | 1 | 0 |
| Norway | 1 | 1 | 0 | 1 |
| Turkey | 1 | 1 | 1 | 0 |
| Total | 1007 | 957 | 627 | 380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivica, N.A.; Young, C.M. Tracking the CAR-T Revolution: Analysis of Clinical Trials of CAR-T and TCR-T Therapies for the Treatment of Cancer (1997–2020). Healthcare 2021, 9, 1062. https://doi.org/10.3390/healthcare9081062
Ivica NA, Young CM. Tracking the CAR-T Revolution: Analysis of Clinical Trials of CAR-T and TCR-T Therapies for the Treatment of Cancer (1997–2020). Healthcare. 2021; 9(8):1062. https://doi.org/10.3390/healthcare9081062
Chicago/Turabian StyleIvica, Nikola A., and Colin M. Young. 2021. "Tracking the CAR-T Revolution: Analysis of Clinical Trials of CAR-T and TCR-T Therapies for the Treatment of Cancer (1997–2020)" Healthcare 9, no. 8: 1062. https://doi.org/10.3390/healthcare9081062
APA StyleIvica, N. A., & Young, C. M. (2021). Tracking the CAR-T Revolution: Analysis of Clinical Trials of CAR-T and TCR-T Therapies for the Treatment of Cancer (1997–2020). Healthcare, 9(8), 1062. https://doi.org/10.3390/healthcare9081062






