Lipid-Free Parenteral Nutrition Is Associated with an Increased Risk of Hepatic Dysfunction in Surgical Critically Ill Patients: A Retrospective Observational Study
Abstract
:1. Introduction
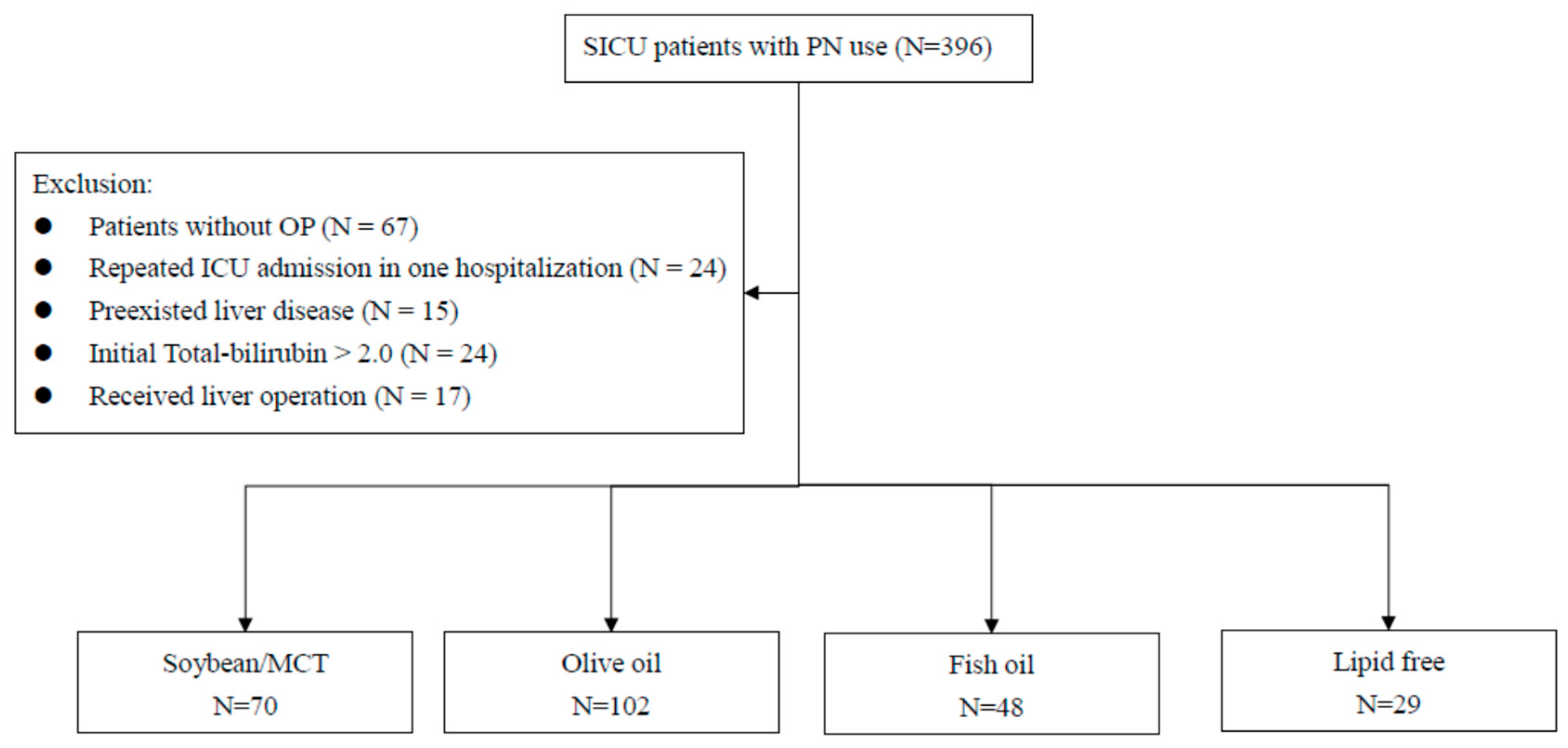
2. Materials and Methods
3. Definition of Hepatic Dysfunction
4. Assessment of Patient Severity
5. Measurements
6. Statistical Analysis
7. Result
8. Discussion
9. Limitation of the Study
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Raman, M.; Almutairdi, A.; Mulesa, L.; Alberda, C.; Beattie, C.; Gramlich, L. Parenteral Nutrition and Lipids. Nutrients 2017, 9, 388. [Google Scholar] [CrossRef] [Green Version]
- Bielawska, B.; Allard, J.P. Parenteral Nutrition and Intestinal Failure. Nutrients 2017, 9, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C.; Jensen, G.L.; Koletzko, B.V. Lipid emulsions in parenteral nutrition of intensive care patients: Current thinking and future directions. Intensive Care Med. 2010, 36, 735–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fell, G.L.; Nandivada, P.; Gura, K.M.; Puder, M. Intravenous Lipid Emulsions in Parenteral Nutrition. Adv. Nutr. 2015, 6, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, C.E.; Brody, R.A.; Parrott, J.S. The effects of different IV fat emulsions on clinical outcomes in criti-cally ill pa-tients. Crit. Care Med. 2014, 42, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. 2009, 28, 378–386. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miloudi, K.; Comte, B.; Rouleau, T.; Montoudis, A.; Levy, E.; Lavoie, J.-C. The mode of administration of total parenteral nutrition and nature of lipid content influence the generation of peroxides and aldehydes. Clin. Nutr. 2012, 31, 526–534. [Google Scholar] [CrossRef]
- Vanek, V.W.; Seidner, D.L.; Allen, P. Novel Nutrient Task Force, Intravenous Fat Emulsions Workgroup; Amer-ican Socie-ty for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors: A.S.P.E.N. position paper: Clinical role for alternative intravenous fat emulsions. Nutr. Clin. Pract. 2012, 27, 150–192. [Google Scholar] [CrossRef] [PubMed]
- Waitzberg, D.L.; Torrinhas, R.S.; Jacintho, T.M. New Parenteral Lipid Emulsions for Clinical Use. J. Parenter Enter. Nutr. 2006, 30, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nardi, L.; Bellinati-Pires, R.; Torrinhas, R.S.; Bacchi, C.E.; Arias, V.; Waitzberg, D.L. Effect of fish oil containing parenteral lipid emulsions on neu-trophil chemotaxis and resident-macrophages’ phagocytosis in rats. Clin. Nutr. 2008, 27, 283–288. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llop-Talaveron, J.M.; Badia-Tahull, M.B.; Leiva-Badosa, E.; Ramon-Torrel, J.M. Parenteral fish oil and liver function tests in hospi-talized adult patients receiving parenteral nutrition: A propensity score-matched analysis. Clin. Nutr. 2017, 36, 1082–1088. [Google Scholar] [CrossRef]
- Kelly, D.A. Intestinal Failure–Associated Liver Disease: What Do We Know Today? Gastroenterology 2006, 130, S70–S77. [Google Scholar] [CrossRef]
- Wales, P.W.; Allen, N.; Worthington, P.; George, D.; Compher, C.; American Society for Parenteral and Enteral Nutrition. Clinical guidelines: Support of pediatric patients with intestinal failure at risk of parenteral nutrition-associated liver disease. J. Parenter Enter. Nutr. 2014, 38, 538–557. [Google Scholar] [CrossRef]
- Nandivada, P.; Carlson, S.J.; Chang, M.I.; Cowan, E.; Gura, K.M.; Puder, M. Treatment of Parenteral Nutrition-Associated Liver Disease: The Role of Lipid Emulsions. Adv. Nutr. 2013, 4, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Tillman, E.M. Review and Clinical Update on Parenteral Nutrition–Associated Liver Disease. Nutr. Clin. Pr. 2012, 28, 30–39. [Google Scholar] [CrossRef]
- Xu, Z.-W.; Li, Y.-S. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 586–593. [Google Scholar] [CrossRef]
- Kumpf, V.J. Parenteral Nutrition-Associated Liver Disease in Adult and Pediatric Patients. Nutr. Clin. Pr. 2006, 21, 279–290. [Google Scholar] [CrossRef]
- Rollins, M.D.; Ward, R.M.; Jackson, W.D.; Mulroy, C.W.; Spencer, C.P.; Ying, J.; Greene, T.; Book, L.S. Effect of decreased parenteral soybean lipid emulsion on hepatic function in infants at risk for parenteral nutrition-associated liver disease: A pilot study. J. Pediatr. Surg. 2013, 48, 1348–1356. [Google Scholar] [CrossRef]
- Nandivada, P.; Fell, G.L.; Gura, K.M.; Puder, M. Lipid emulsions in the treatment and prevention of parenteral nutrition-associated liver disease in infants and children. Am. J. Clin. Nutr. 2016, 103, 629S–634S. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, S.; Braun, L.P.; Mercer, L.D.; Sherrill, M.; Stevens, J.; Javid, P.J. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J. Pediatr. Surg. 2013, 48, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Badia-Tahull, M.B.; Talaveron, J.L.; Leiva-Badosa, E. Impact of intravenous lipid emulsions on liver function tests: Contribution of parenteral fish oil. Nutrition 2015, 31, 1109–1116. [Google Scholar] [CrossRef]
- Buchman, A.L.; Iyer, K.; Fryer, J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology 2005, 43, 9–19. [Google Scholar] [CrossRef]
- Slattery, E.; Rumore, M.M.; Douglas, J.S.; Seres, D.S. 3-in-1 vs 2-in-1 parenteral nutrition in adults: A review. Nutr. Clin. Pract. 2014, 29, 631–635. [Google Scholar] [CrossRef]
- Patkova, A.; Josková, V.; Havel, E.; Kovařík, M.; Kuchařová, M.; Zadak, Z.; Hronek, M. Energy, Protein, Carbohydrate, and Lipid Intakes and Their Effects on Morbidity and Mortality in Critically Ill Adult Patients: A Systematic Review. Adv. Nutr. 2017, 8, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Pastor, C.M.; Suter, P.M. Hepatic hemodynamics and cell functions in human and experimental sepsis. Anesth. Analg. 1999, 89, 344–352. [Google Scholar] [PubMed]
- Sands, K.E.; Bates, D.W.; Lanken, P.N.; Graman, P.S.; Hibberd, P.L.; Kahn, K.L.; Parsonnet, J.; Panzer, R.; Orav, E.J.; Snydman, D.; et al. Epidemiology of Sepsis Syndrome in 8 Academic Medical Centers. JAMA 1997, 278, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Con-ference. Crit. Care Med. 2003, 16, 1250–1256. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe or-gan dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Mohil, R.S.; Bhatnagar, D.; Bahadur, L. POSSUM and P-POSSUM for risk-adjusted audit of patients undergo-ing emergency laparotomy. Br. J. Surg. 2004, 91, 500–503. [Google Scholar] [CrossRef]
- Parizkova, R.; Cerny, V.; Dostal, P. The cost in different subgroups of critically ill patients: A multicentric study in Czech Republic. Crit. Care 2001, 5, P259. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Adolph, M.; Deutz, N.E. Lipids in the intensive care unit: Recommendations from the ESPEN Ex-pert Group. Clin Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.E.; McClave, S.A.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit. Care Med. 2016, 44, 390–438. [Google Scholar] [CrossRef] [PubMed]
- Critical Care Nutrition. Canadian Clinical Practice Guidelines, Composition of Parenteral Nutrition: Type of Lipids 2013. Available online: www.criticalcarenutrition.com (accessed on 27 January 2021).
- Weimann, A.; Singer, P. Avoiding underfeeding in severely ill patients. Lancet 2013, 381, 1811. [Google Scholar] [CrossRef]
- Vogel, J.A.; Liao, M.M.; Hopkins, E. Prediction of postinjury multiple-organ failure in the emergency depart-ment: Development of the Denver Emergency Department Trauma Organ Failure score. J. Trauma Acute Care Surg. 2014, 76, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Soultati, A.; Dourakis, S.P. Liver dysfunction in the intensive care unit. Ann. Gastroenterol. 2005, 18, 35–45. [Google Scholar]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Buenestado, A.; Cortijo, J.; Sanz, M.J. Olive oil-based lipid emulsion’s neutral effects on neutrophil functions and leukocyte-endothelial cell interactions. JPEN J. Parenter Enter. Nutr. 2006, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Puder, M.; Valim, C.; Meisel, J.A.; Le, H.D.; de Meijer, V.E.; Robinson, E.M.; Zhou, J.; Duggan, C.; Gura, K.M. Parenteral Fish Oil Improves Outcomes in Patients With Parenteral Nutrition-Associated Liver Injury. Ann. Surg. 2009, 250, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, A.R.; Rößler, S.; Litz, R.J.; Stehr, S.N.; Heller, S.C.; Koch, R.; Koch, T. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit. Care Med. 2006, 34, 972–979. [Google Scholar] [CrossRef]
- Manzanares, W.; Langlois, P.L.; Hardy, G. Intravenous lipid emulsions in the critically ill: An update. Curr. Opin. Crit. Care 2016, 22, 308–315. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Soybean Oil/MCT | Olive Oil Based | Fish Oil Contained | |
|---|---|---|---|---|
| Commercial Products | Lipovenoes® | Lipofundin® | ClinOleic 20%® | SMOF® |
| Lipid source (%) | ||||
| Soybean oil | 50 | 50 | 20 | 30 |
| MCT | 50 | 50 | 0 | 30 |
| olive oil | 0 | 0 | 80 | 25 |
| Fish oil | 0 | 0 | 0 | 15 |
| Variable | Soybean Oil /MCT (N = 70) | Olive Oil Based (N = 102) | Fish Oil Contained (N = 48) | Lipid Free (N = 29) | p-Value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (y), mean (SD) | 66.2 (18.8) | 69.0 (15.8) | 66.5 (15.9) | 63.1 (18.1) | 0.4239 |
| Sex, n (%) | |||||
| Female | 32 (45.71) | 47 (46.08) | 14 (29.17) | 12 (41.4) | 0.2275 |
| Male | 38 (54.29) | 55 (53.92) | 34 (70.83) | 17 (58.6) | |
| Body weight (kg), mean (SD) | 60.1 (15.3) | 57.9 (12.6) | 62.8 (12.5) | 62.2 (18.2) | 0.1375 |
| Clinical, mean (SD) | |||||
| Severity score of illness | |||||
| Admission APACHEII score | 16.5 (7.27) | 17.9 (8.26) | 17.0 (7.73) | 18.82 (8.47) | 0.5243 |
| SOFA score | 3.88 (2.54) | 3.91 (2.97) | 3.52 (2.67) | 5.45 (3.37) | 0.0676 |
| Total POSSUM score | 46.7 (9.92) | 44.9 (9.78) | 45.0 (10.7) | 50.62 (11.80) | 0.0728 |
| ISS † | 28.3 (12.7) | 21.8 (12.5) | 22.3 (12.1) | 30.8 (3.76) | 0.2171 |
| Days of PN start, mean (SD) | 3.74 (5.53) | 3.59 (3.78) | 3.46 (5.24) | 4.21 (4.45) | 0.4031 |
| Days of EN establish, mean (SD) | 3.58 (2.13) | 4.18 (3.45) | 5.12 (3.95) | 4.39 (4.44) | 0.1279 |
| Days of PN use, mean (SD) | 12.1 (10.7) | 14.1 (17.7) | 18.3 (18.1) | 8.21 (6.50) | 0.0008 |
| Admission biochemical variables, mean (SD) | |||||
| Admission initial serum T-bilirubin | 0.92(0.50) | 0.91(0.47) | 1.07(0.51) | 1.14 (0.60) | 0.1286 |
| BUN | 38.1 (30.2) | 34.9 (26.5) | 32.7 (30.5) | 30.9 (27.3) | 0.2480 |
| Creatinine | 2.20 (2.05) | 2.21 (2.28) | 1.87 (1.86) | 2.45 (2.77) | 0.5874 |
| Serum albumin | 2.88 (0.66) | 2.9 (0.65) | 2.66 (0.68) | 2.74 (0.64) | 0.3543 |
| Serum lactate (mg/dL) | 42.8 (34.7) | 38.6 (29.7) | 39.5 (36.9) | 49.9 (47.2) | 0.6090 |
| Serum GPT | 67.6 (175) | 51.8 (104) | 33.1 (26.6) | 97.6 (136) | 0.1257 |
| Blood transfusion | 8.14 (11.3) | 5.85 (7.76) | 8.65 (17.9) | 12.5 (16.5) | 0.3670 |
| Comorbidity, n (%) | |||||
| Sepsis | 15 (21.43) | 20 (19.61) | 13 (27.08) | 4 (13.8) | 0.5802 |
| Trauma | 13 (18.57) | 17 (16.67) | 7 (14.58) | 6 (20.7) | 0.8668 |
| Type II diabetes | 36 (51.43) | 51 (50.00) | 28 (58.33) | 16 (55.2) | 0.7952 |
| Hypertension | 30 (42.86) | 46 (45.10) | 17 (35.42) | 12 (41.4) | 0.7345 |
| Heart disease | 7 (10.00) | 12 (11.76) | 6 (12.50) | 3 (10.3) | 0.9815 |
| Chronic kidney disease | 14 (20.00) | 21 (20.59) | 6 (12.50) | 6 (20.7) | 0.6617 |
| COPD | 1 (1.43) | 3 (2.94) | 1 (2.08) | 2 (6.90) | 0.5061 |
| Malignancy | 12 (17.14) | 21 (20.59) | 11 (22.92) | 3 (10.3) | 0.5449 |
| Post TAE | 6 (8.57) | 7 (6.86) | 2 (4.17) | 4 (13.8) | 0.4554 |
| Hemodynamic unstable on admission, n (%) | 26 (37.14) | 36 (35.29) | 21 (43.75) | 13 (44.8) | 0.6719 |
| Received abdominal operation, n (%) | 64 (91.43) | 91 (89.22) | 45 (93.75) | 25 (86.2) | 0.6872 |
| Vasopressor use at ER, n (%) | 12 (17.14) | 15 (14.71) | 7 (14.58) | 9 (31.0) | 0.2071 |
| Received CVVH, n (%) | 5 (7.14) | 15 (14.71) | 7 (14.58) | 7 (24.1) | 0.1478 |
| Received HD, n (%) | 6 (8.57) | 15 (14.71) | 5 (10.42) | 8 (27.6) | 0.0775 |
| Blood stream infection, n (%) | 32 (45.71) | 34 (33.33) | 18 (37.50) | 12 (41.4) | 0.4221 |
| Length of stay (day), mean (SD) | 35.6 (26.1) | 37.6 (28.5) | 40.0 (28.0) | 37.0 (29.4) | 0.7693 |
| Duration of ventilator days, mean (SD) | 14.6 (14.4) | 19.6 (21.6) | 17.6 (15.3) | 21.8 (23.6) | 0.2495 |
| Outcome | No. of Event | Person-Days | Incidence † | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| Mortality at discharge Soybean oil/MCT | 13 | 2494 | 5.21 | 0.48 (0.22–1.05) | 0.0665 | Ref. | |
| Olive oil-based | 17 | 3839 | 4.43 | 0.39 (0.18–0.84) | 0.0156 | 0.82 (0.39–1.69) | 0.5828 |
| Fish oil containing | 13 | 1918 | 6.78 | 0.65 (0.29–1.47) | 0.3043 | 1.32 (0.60–2.91) | 0.4854 |
| Lipid free | 12 | 1074 | 11.17 | Ref. | |||
| 30-day mortality | |||||||
| Soybean oil/MCT | 9 | 1623 | 5.55 | 0.53 (0.20–1.38) | 0.1899 | Ref. | |
| Olive oil-based | 6 | 2371 | 2.53 | 0.25 (0.09–0.72) | 0.0102 | 0.47 (0.17–1.31) | 0.1488 |
| Fish oil containing | 6 | 1204 | 4.98 | 0.55 (0.18–1.65) | 0.2888 | 1.03 (0.36–2.93) | 0.9587 |
| Lipid free | 8 | 647 | 12.36 | Ref. | |||
| 90-day mortality | |||||||
| Soybean oil/MCT | 13 | 2433 | 5.34 | 0.53 (0.24–1.19) | 0.1223 | Ref. | |
| Olive oil-based | 15 | 3691 | 4.06 | 0.41 (0.19–0.89) | 0.0242 | 0.76 (0.36–1.60) | 0.4671 |
| Fish oil containing | 13 | 1823 | 7.13 | 0.76 (0.33–1.75) | 0.5222 | 1.42 (0.65–3.10) | 0.3840 |
| Lipid free | 11 | 1031 | 10.67 | Ref. | |||
| T-bilirubin > 6 mg/dL | |||||||
| Soybean oil/MCT | 10 | 2163 | 4.62 | 0.25 (0.11–0.59) | 0.0014 | Ref. | |
| Olive oil-based | 18 | 3312 | 5.43 | 0.30 (0.14–0.63) | 0.0015 | 1.15 (0.52–2.51) | 0.7335 |
| Fish oil containing | 12 | 1455 | 8.25 | 0.39 (0.17–0.89) | 0.0248 | 1.53 (0.66–3.57) | 0.3230 |
| Lipid free | 12 | 666 | 18.02 | Ref. |
| Variable | N | No. of Event | Person-Days | Incidence † | HR (95% CI) * | p-Value |
|---|---|---|---|---|---|---|
| Soybean | ||||||
| None | 29 | 12 | 666 | 18.02 | Ref. | |
| ≤median (0.15 g/kg/day) | 110 | 31 | 3159 | 9.81 | 0.52 (0.26–1.02) | 0.0583 |
| >median | 110 | 9 | 3771 | 2.39 | 0.14 (0.06–0.32) | <0.0001 |
| MCT | ||||||
| None | 99 | 23 | 2449 | 9.39 | Ref. | |
| ≤median (0.22 g/kg/day) | 75 | 23 | 2732 | 8.42 | 0.96 (0.50–1.85) | 0.9107 |
| >median | 75 | 6 | 2415 | 2.48 | 0.28 (0.11–1.69) | 0.0057 |
| Olive oil | ||||||
| None | 88 | 20 | 2379 | 8.41 | Ref. | |
| ≤median (0.24 g/kg/day) | 80 | 25 | 2704 | 9.25 | 1.10 (0.59–2.04) | 0.7709 |
| >median | 81 | 7 | 2513 | 2.79 | 0.34 (0.14–0.80) | 0.0133 |
| Fish oil | ||||||
| None | 172 | 33 | 4722 | 6.99 | Ref. | |
| ≤median (0.05 g/kg/day) | 38 | 16 | 1531 | 10.45 | 1.72 (0.88–3.35) | 0.1139 |
| >median | 39 | 3 | 1343 | 2.23 | 0.33 (0.10–1.06) | 0.0628 |
| Variable | N | No. of Event | Person-Days | Incidence † | HR (95% CI) * | p-Value |
|---|---|---|---|---|---|---|
| Soybean | ||||||
| None | 29 | 12 | 1074 | 11.17 | Ref. | |
| ≤median (0.15 g/kg/day) | 110 | 24 | 4299 | 5.58 | 0.51 (0.25–1.04) | 0.0641 |
| >median | 110 | 19 | 3952 | 4.81 | 0.44 (0.21–0.91) | 0.0289 |
| MCT | ||||||
| None | 99 | 21 | 3183 | 6.60 | Ref. | |
| ≤median (0.22 g/kg/day) | 75 | 20 | 3664 | 5.46 | 0.82 (0.40–1.66) | 0.5748 |
| >median | 75 | 14 | 2478 | 5.65 | 0.87 (0.44–1.73) | 0.6983 |
| Olive oil | ||||||
| None | 88 | 23 | 3046 | 7.55 | Ref. | |
| ≤median (0.24 g/kg/day) | 80 | 17 | 3629 | 4.68 | 0.60 (0.31–1.16) | 0.1286 |
| >median | 81 | 15 | 2650 | 5.66 | 0.76 (0.40–1.47) | 0.4161 |
| Fish oil | ||||||
| None | 172 | 33 | 5799 | 5.69 | Ref. | |
| ≤median (0.05 g/kg/day) | 38 | 15 | 2078 | 7.22 | 1.39 (0.70–2.73) | 0.3464 |
| >median | 39 | 7 | 1448 | 4.83 | 0.91 (0.40–2.07) | 0.8212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-C.; Chen, T.-A.; Tsai, I.-J.; Wang, Y.-C.; Cheng, H.-T.; Tzeng, C.-W.; Hsu, C.-H.; Muo, C.-H. Lipid-Free Parenteral Nutrition Is Associated with an Increased Risk of Hepatic Dysfunction in Surgical Critically Ill Patients: A Retrospective Observational Study. Healthcare 2021, 9, 1096. https://doi.org/10.3390/healthcare9091096
Wu S-C, Chen T-A, Tsai I-J, Wang Y-C, Cheng H-T, Tzeng C-W, Hsu C-H, Muo C-H. Lipid-Free Parenteral Nutrition Is Associated with an Increased Risk of Hepatic Dysfunction in Surgical Critically Ill Patients: A Retrospective Observational Study. Healthcare. 2021; 9(9):1096. https://doi.org/10.3390/healthcare9091096
Chicago/Turabian StyleWu, Shih-Chi, Te-An Chen, I-Ju Tsai, Yu-Chun Wang, Han-Tsung Cheng, Chia-Wei Tzeng, Chia-Hao Hsu, and Chih-Hsin Muo. 2021. "Lipid-Free Parenteral Nutrition Is Associated with an Increased Risk of Hepatic Dysfunction in Surgical Critically Ill Patients: A Retrospective Observational Study" Healthcare 9, no. 9: 1096. https://doi.org/10.3390/healthcare9091096






