Recent Advances of Nanostructured Materials for Photoelectrochemical Bioanalysis
Abstract
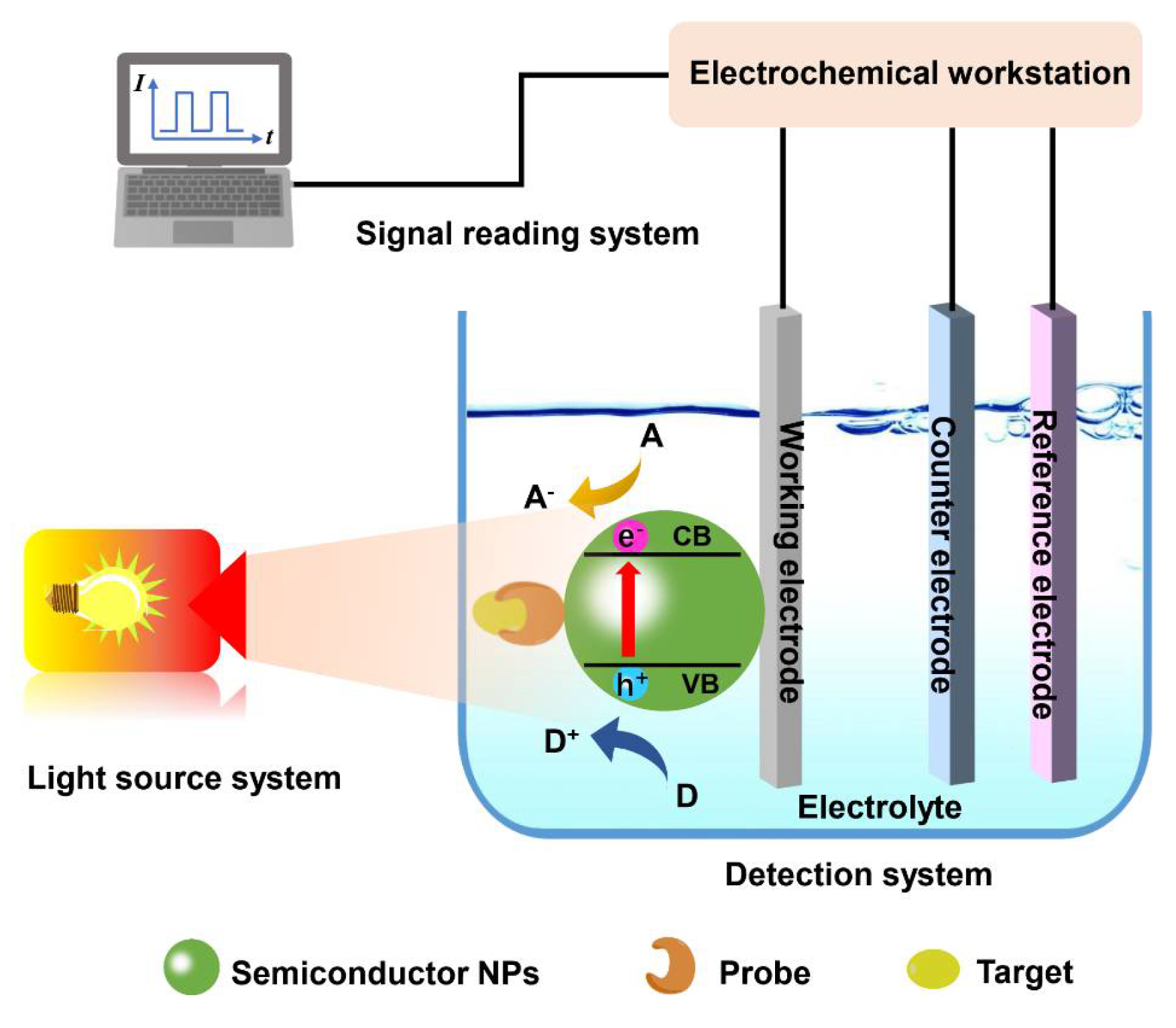
:1. Introduction
2. Functional Nanostructure-Based PEC Biosensing
2.1. 0D-Nanostructure Materials
2.1.1. QDs
2.1.2. Carbon-Based NPs
2.1.3. Noble-Metal NPs
2.2. 1D-Nanostructure Materials
2.2.1. Metal Oxide-Based Semiconductors
2.2.2. Metal Chalcogenide-Based Semiconductors
2.2.3. Carbon Nanotube-Based Semiconductors
2.3. 2D-Nanostructure Materials
2.3.1. Carbon-Based Graphene-like 2D Nanostructures
2.3.2. Transition Metal Dichalcogenides (TMDs)
2.4. 3D-Nanostructure Materials
3. Novel PEC Biosensing Patterns
3.1. Mimic Enzyme Assay
3.2. Self-Powered Sensing
3.3. Dual-Readout Assay
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becquerel, A.E. Recherches sur les effets de la radiation chimique de la lumiere solaire au moyen des courants electriques. CR Acad. Sci. 1839, 9, 1. [Google Scholar]
- Ke, J.; He, F.; Wu, H.; Lyu, S.; Liu, J.; Yang, B.; Li, Z.; Zhang, Q.; Chen, J.; Lei, L.; et al. Nanocarbon-Enhanced 2D Photoelectrodes: A New Paradigm in Photoelectrochemical Water Splitting. Nano-Micro Lett. 2021, 13, 24. [Google Scholar] [CrossRef]
- Mittal, H.; Khanuja, M. Hydrothermal in-situ synthesis of MoSe2-polypyrrole nanocomposite for efficient photocatalytic degradation of dyes under dark and visible light irradiation. Sep. Purif. Technol. 2021, 254, 117508. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Zhang, L.; Zhu, L.-B.; Gao, Y.; Fan, G.-C.; Han, D.-M.; Chen, G.; Zhao, W.-W. Bismuth-containing semiconductors for photoelectrochemical sensing and biosensing. Coord. Chem. Rev. 2019, 393, 9–20. [Google Scholar] [CrossRef]
- Svitková, V.; Palchetti, I. Functional polymers in photoelectrochemical biosensing. Bioelectrochemistry 2020, 136, 107590. [Google Scholar] [CrossRef]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Tu, W.; Wang, Z.; Dai, Z. Selective photoelectrochemical architectures for biosensing: Design, mechanism and responsibility. Trac Trends Anal. Chem. 2018, 105, 470–483. [Google Scholar] [CrossRef]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- Willner, I.; Patolsky, F.; Wasserman, J. Photoelectrochemistry with controlled DNA-cross-linked CdS nanoparticle arrays. Angew. Chem. 2001, 40, 1861–1864. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.; Abad-Fuentes, A. Applications of quantum dots as probes in immunosensing of small-sized analytes. Biosens. Bioelectron. 2013, 41, 12–29. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-W.; Yu, X.-D.; Xu, J.-J.; Chen, H.-Y. Recent advances in the use of quantum dots for photoelectrochemical bioanalysis. Nanoscale 2016, 8, 17407–17414. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Zhang, S. Quantum dot-based photoelectric conversion for biosensing applications. Trac Trends Anal. Chem. 2015, 67, 56–73. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Hildebrandt, N. Recent developments in lanthanide-to-quantum dot FRET using time-gated fluorescence detection and photon upconversion. TrAC Trends Anal. Chem. 2016, 84, 60–71. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Zhou, Q.; Pan, D.; Zhang, Y.; Zhang, Y.; Shen, Y. Boosting the Sensitivity of a Photoelectrochemical Immunoassay by Using SiO2@polydopamine Core–Shell Nanoparticles as a Highly Efficient Quencher. ACS Appl. Nano Mater. 2019, 2, 1579–1588. [Google Scholar] [CrossRef]
- Zhang, L.; Lib, P.; Fengbc, L.; Chenbc, X.; Jiangb, J.; Zhangb, S.; Zhangb, C.; Zhangd, A.; Chena, G.; Wang, H. Synergetic Ag2S and ZnS quantum dots as the sensitizer and recognition probe: A visible light-driven photoelectrochemical sensor for the “signal-on” analysis of mercury (II). J. Hazard. Mater. 2020, 387, 121715. [Google Scholar] [CrossRef]
- Li, M.; Xiong, C.; Zheng, Y.; Liang, W.; Yuan, R.; Chai, Y. Ultrasensitive Photoelectrochemical Biosensor Based on DNA Tetrahedron as Nanocarrier for Efficient Immobilization of CdTe QDs-Methylene Blue as Signal Probe with Near-Zero Background Noise. Anal. Chem. 2018, 90, 8211–8216. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Zhao, W.-W.; Jiang, D.-C.; Xu, J.-J.; Chen, H.-Y. Polymer Dots for Photoelectrochemical Bioanalysis. Anal. Chem. 2017, 89, 4945–4950. [Google Scholar] [CrossRef]
- Shi, X.-M.; Mei, L.-P.; Wang, Q.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Energy Transfer between Semiconducting Polymer Dots and Gold Nanoparticles in a Photoelectrochemical System: A Case Application for Cathodic Bioanalysis. Anal. Chem. 2018, 90, 4277–4281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Shi, X.-M.; Guo, H.-Q.; Zhao, X.-Z.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Gold Nanoparticle Couples with Entropy-Driven Toehold-Mediated DNA Strand Displacement Reaction on Magnetic Beads: Toward Ultrasensitive Energy-Transfer-Based Photoelectrochemical Detection of miRNA-141 in Real Blood Sample. Anal. Chem. 2018, 90, 11892–11898. [Google Scholar] [CrossRef]
- Shi, X.-M.; Mei, L.-P.; Zhang, N.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. A Polymer Dots-Based Photoelectrochemical pH Sensor: Simplicity, High Sensitivity, and Broad-Range pH Measurement. Anal. Chem. 2018, 90, 8300–8303. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shi, X.-M.; Xu, Y.-T.; Fan, G.-C.; Liang, Y.-Y.; Wang, C.; Zhao, W.-W. Gold Nanoparticle-Induced Photocurrent Quenching and Recovery of Polymer Dots: Toward Signal-On Energy-Transfer-Based Photocathodic Bioanalysis of Telomerase Activity in Cell Extracts. Anal. Chem. 2019, 91, 6403–6407. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ruan, Y.-F.; Zhao, W.-W.; Lin, P.; Xu, J.-J.; Chen, H.-Y. Semiconducting Organic–Inorganic Nanodots Heterojunctions: Platforms for General Photoelectrochemical Bioanalysis Application. Anal. Chem. 2018, 90, 3759–3765. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, S.; Tu, W.; Wang, X.; Wang, X.; Bao, J.; Wang, Y.; Han, M.; Dai, Z. A “Signal On” Photoelectrochemical Biosensor Based on Bismuth@N,O-Codoped-Carbon Core-Shell Nanohybrids for Ultrasensitive Detection of Telomerase in HeLa Cells. Chem. Eur. J. 2018, 24, 3677–3682. [Google Scholar] [CrossRef]
- You, F.H.; Wei, J.; Cheng, Y.; Wen, Z.R.; Ding, C.F.; Guo, Y.S.; Wang, K. A sensitive and stable visible-light-driven photoelectrochemical aptasensor for determination of oxytetracycline in tomato samples. J. Hazard. Mater. 2020, 398, 122944. [Google Scholar] [CrossRef]
- Dong, Y.-X.; Cao, J.-T.; Wang, B.; Ma, S.-H.; Liu, Y.-M. Spatial-Resolved Photoelectrochemical Biosensing Array Based on a CdS@g-C3N4 Heterojunction: A Universal Immunosensing Platform for Accurate Detection. ACS Appl. Mater. Interfaces 2018, 10, 3723–3731. [Google Scholar] [CrossRef]
- Hao, N.; Hua, R.; Zhang, K.; Lu, J.; Wang, K. A Sunlight Powered Portable Photoelectrochemical Biosensor Based on a Potentiometric Resolve Ratiometric Principle. Anal. Chem. 2018, 90, 13207–13211. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.; Wu, N.; Zhang, Z. Graphitic Carbon Nitride/Poly(3-Hexylthiophene) Nanocomposites for the Photoelectrochemical Detection of H2O2 in Living Cells. ACS Appl. Nano Mater. 2020, 3, 8598–8603. [Google Scholar] [CrossRef]
- Zheng, Y.-N.; Liang, W.-B.; Xiong, C.-Y.; Yuan, Y.-L.; Chai, Y.-Q.; Yuan, R. Self-Enhanced Ultrasensitive Photoelectrochemical Biosensor Based on Nanocapsule Packaging Both Donor–Acceptor-Type Photoactive Material and Its Sensitizer. Anal. Chem. 2016, 88, 8698–8705. [Google Scholar] [CrossRef]
- Shen, C.; Lou, Q.; Zang, J.; Liu, K.; Qu, S.; Dong, L.; Shan, C. Near-Infrared Chemiluminescent Carbon Nanodots and Their Application in Reactive Oxygen Species Bioimaging. Adv. Sci. 2020, 7, 1903525. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, Y.; Li, Z.; Yang, J.; Aryee, A.A.; Qu, L.; Du, D.; Lin, Y. Lysosome-targeted carbon dots for ratiometric imaging of formaldehyde in living cells. Nanoscale 2019, 11, 8458–8463. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Zhang, Z.; Yan, L.; Guo, L.; Niu, G.; Zhang, J.; Zhao, J.; Zhang, H.; Wang, P.; et al. Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy. Nano Res. 2017, 10, 3113–3123. [Google Scholar] [CrossRef]
- Campuzano, S.; Yanez-Sedeno, P.; Pingarron, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Ostermeyer, G.P.; Du, D.; Lin, Y. Carbon nanodot-hybridized silica nanospheres assisted immunoassay for sensitive detection of Escherichia coli. Sens. Actuators B Chem. 2021, 349, 130730. [Google Scholar] [CrossRef]
- Putri, F.A.R.; Mudasir, M.; Morita, K.; Suherman, S. Microwave-Assisted Synthesis of Amikacin Modified N,S co-Doped Carbon Dots for Escherichia coli Detection. Chemosensors 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.C.E.; Gonçalves, H.M. Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Zhang, Y.; Xu, C.; Zhang, L.; Cheng, X.; Liu, H.; Chen, X.; Yu, J. Editable TiO2 Nanomaterial-Modified Paper in Situ for Highly Efficient Detection of Carcinoembryonic Antigen by Photoelectrochemical Method. ACS Appl. Mater. Interfaces 2018, 10, 14594–14601. [Google Scholar] [CrossRef]
- Sui, C.; Wang, T.; Zhou, Y.; Yin, H.; Meng, X.; Zhang, S.; Waterhouse, G.I.; Xu, Q.; Zhuge, Y.; Ai, S. Photoelectrochemical biosensor for hydroxymethylated DNA detection and T4-β-glucosyltransferase activity assay based on WS2 nanosheets and carbon dots. Biosens. Bioelectron. 2019, 127, 38–44. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep Ultraviolet to Near-Infrared Emission and Photoresponse in Layered N-Doped Graphene Quantum Dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Teng, C.-Y.; Chen, S.-J.; Teng, H. Nitrogen-Doped Graphene Oxide Quantum Dots as Photocatalysts for Overall Water-Splitting under Visible Light Illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Q.; Jiang, D.; Du, X.; Qian, J.; Mao, H.; Wang, K. Atmospheric pressure synthesis of nitrogen doped graphene quantum dots for fabrication of BiOBr nanohybrids with enhanced visible-light photoactivity and photostability. Carbon 2016, 96, 1157–1165. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Zhou, L.; Li, H.; Wang, K. New Insights toward Efficient Charge-Separation Mechanism for High-Performance Photoelectrochemical Aptasensing: Enhanced Charge-Carrier Lifetime via Coupling Ultrathin MoS2 Nanoplates with Nitrogen-Doped Graphene Quantum Dots. Anal. Chem. 2017, 89, 4525–4531. [Google Scholar] [CrossRef]
- Ge, S.; Lan, F.; Liang, L.; Ren, N.; Li, L.; Liu, H.; Yan, M.; Yu, J. Ultrasensitive Photoelectrochemical Biosensing of Cell Surface N-Glycan Expression Based on the Enhancement of Nanogold-Assembled Mesoporous Silica Amplified by Graphene Quantum Dots and Hybridization Chain Reaction. ACS Appl. Mater. Interfaces 2017, 9, 6670–6678. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.C.; Shen, Z.; Chan, D.K.L.; Gu, T. g-C3N4 quantum dots: Direct synthesis, upconversion properties and photocatalytic application. Chem. Commun. 2014, 50, 10148–10150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.; Cui, C.; Su, M.; Wang, Y.; Wei, Q.; Tan, W. Construction of self-powered cytosensing device based on ZnO nanodisks@g-C3N4 quantum dots and application in the detection of CCRF-CEM cells. Nano Energy 2018, 46, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Victorious, A.; Saha, S.; Pandey, R.; Soleymani, L. Enhancing the Sensitivity of Photoelectrochemical DNA Biosensing Using Plasmonic DNA Barcodes and Differential Signal Readout. Angew. Chem. Int. Ed. 2021, 60, 7316–7322. [Google Scholar] [CrossRef]
- Cui, L.; Shen, J.; Li, C.-C.; Cui, P.-P.; Luo, X.; Wang, X.; Zhang, C.-Y. Construction of a Dye-Sensitized and Gold Plasmon-Enhanced Cathodic Photoelectrochemical Biosensor for Methyltransferase Activity Assay. Anal. Chem. 2021, 93, 10310–10316. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Zhou, J.; Wu, K.-W.; Ding, S.-N.; Xu, J.-J.; Chen, H.-Y. Plasmonic Enhanced Gold Nanoclusters-Based Photoelectrochemical Biosensor for Sensitive Alkaline Phosphatase Activity Analysis. Anal. Chem. 2020, 92, 6886–6892. [Google Scholar] [CrossRef]
- Ruan, Y.-F.; Zhang, N.; Zhu, Y.-C.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical Bioanalysis Platform of Gold Nanoparticles Equipped Perovskite Bi4NbO8Cl. Anal. Chem. 2017, 89, 7869–7875. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.-M.; Xu, Y.-T.; Fan, G.-C.; Yu, X.-D.; Liang, Y.-Y.; Zhao, W.-W. Binding-induced formation of DNAzyme on an Au@Ag nanoparticles/TiO2 nanorods electrode: Stimulating biocatalytic precipitation amplification for plasmonic photoelectrochemical bioanalysis. Biosens. Bioelectron. 2019, 134, 103–108. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Zhang, N.; Ruan, Y.-F.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Alkaline Phosphatase Tagged Antibodies on Gold Nanoparticles/TiO2 Nanotubes Electrode: A Plasmonic Strategy for Label-Free and Amplified Photoelectrochemical Immunoassay. Anal. Chem. 2016, 88, 5626–5630. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-W.; Yu, P.-P.; Shan, Y.; Wang, J.; Xu, J.-J.; Chen, H.-Y. Exciton-Plasmon Interactions between CdS Quantum Dots and Ag Nanoparticles in Photoelectrochemical System and Its Biosensing Application. Anal. Chem. 2012, 84, 5892–5897. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Zheng, H.; Yang, L.; Li, L.; Zhang, S.; Zhou, Y.; Alwarappan, S. A photoelectrochemical aptasensor for aflatoxin B1 detection based on an energy transfer strategy between Ce-TiO2@MoSe2 and Au nanoparticles. Nanoscale 2019, 11, 9115–9124. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Liang, Y.-Y.; He, J.-P.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Ag nanoclusters could efficiently quench the photoresponse of CdS quantum dots for novel energy transfer-based photoelectrochemical bioanalysis. Biosens. Bioelectron. 2016, 85, 930–934. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Xu, F.; Zhang, N.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. DNA sequence functionalized with heterogeneous core satellite nanoassembly for novel energy-transfer-based photoelectrochemical bioanalysis. Biosens. Bioelectron. 2017, 91, 293–298. [Google Scholar] [CrossRef]
- Hung, C.M.; Le, D.T.T.; Van Hieu, N. On-chip growth of semiconductor metal oxide nanowires for gas sensors: A review. J. Sci. Adv. Mater. Devices 2017, 2, 263–285. [Google Scholar] [CrossRef]
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Sci. 2004, 34, 151–180. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Wang, D.; Wang, X.; Liu, X.; Kang, Y.; Yu, H.; Zhang, H.; Hu, W.; He, J.; Sun, H.; et al. Tuning the Charge Transfer Dynamics of the Nanostructured GaN Photoelectrodes for Efficient Photoelectrochemical Detection in the Ultraviolet Band. Adv. Funct. Mater. 2021, 31, 2103007. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Yang, M.; Du, H.; Qu, F. Dual signal amplification photoelectrochemical biosensor for highly sensitive human epidermal growth factor receptor-2 detection. Biosens. Bioelectron. 2019, 139, 111312. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Liu, J.; Guan, F.; Sun, R.; Jiang, L.; Feng, X. A Reliable Photoelectrochemical Bioassay System Based on Cathodic Reaction at a Solid-Liquid-Air Joint Interface. Adv. Funct. Mater. 2018, 28, 28. [Google Scholar] [CrossRef]
- Fan, B.; Fan, Q.; Hu, L.; Cui, M.; Wang, X.; Ma, H.; Wei, Q. Polydopamine-PEG–Folic Acid Conjugate Film Engineered TiO2 Nanotube Arrays for Photoelectrochemical Sensing of Folate Binding Protein. ACS Appl. Mater. Interfaces 2020, 12, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, D.; Pan, G.; Zhou, D.; Xu, W.; Zhu, J.; Wang, H.; Chen, C.; Song, H. All-inorganic perovskite quantum dot/TiO2 inverse opal electrode platform: Stable and efficient photoelectrochemical sensing of dopamine under visible irradiation. Nanoscale 2018, 10, 10505–10513. [Google Scholar] [CrossRef]
- Deng, H.; Chai, Y.; Yuan, R.; Yuan, Y. In Situ Formation of Multifunctional DNA Nanospheres for a Sensitive and Accurate Dual-Mode Biosensor for Photoelectrochemical and Electrochemical Assay. Anal. Chem. 2020, 92, 8364–8370. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, Z. Periodical 2D Photonic-Plasmonic Au/TiOx Nanocavity Resonators for Photoelectrochemical Applications. Small 2018, 14, e1703610. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Fan, B.; Li, L.; Sun, X.; Wang, X.; Ma, H.; Wei, Q.; Ju, H. Label-Free Antifouling Photoelectrochemical Sensing Strategy for Detecting Breast Tumor Cells Based on Ligand–Receptor Interactions. ACS Appl. Bio Mater. 2021, 4, 4479–4485. [Google Scholar] [CrossRef]
- Sheng, X.; He, D.; Yang, J.; Zhu, K.; Feng, X. Oriented Assembled TiO2 Hierarchical Nanowire Arrays with Fast Electron Transport Properties. Nano Lett. 2014, 14, 1848–1852. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, K.; Frank, A.J.; Grimes, C.A.; Mallouk, T.E. Rapid Charge Transport in Dye-Sensitized Solar Cells Made from Vertically Aligned Single-Crystal Rutile TiO2 Nanowires. Angew. Chem. Int. Ed. 2012, 51, 2727–2730. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, X.; Wang, D.; Liu, J.; Sun, R.; Jiang, L.; Feng, X. High-Performance Triphase Bio-Photoelectrochemical Assay System Based on Superhydrophobic Substrate-Supported TiO2 Nanowire Arrays. Adv. Funct. Mater. 2018, 28, 1801483. [Google Scholar] [CrossRef]
- Pang, X.H.; Bian, H.J.; Su, M.H.; Ren, Y.Y.; Qi, J.N.; Ma, H.M.; Wu, D.; Hu, L.H.; Du, B.; Wei, Q. Photoelectrochemical Cytosensing of RAW264.7 Macrophage Cells Based on a TiO2 Nanoneedls@MoO3 Array. Anal. Chem. 2017, 89, 7950–7957. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Zhang, Y.; Yan, M.; Zhang, L.; Ge, S.; Yu, J. Photoelectrochemical biosensor of HIV-1 based on cascaded photoactive materials and triple-helix molecular switch. Biosens. Bioelectron. 2019, 139, 111325. [Google Scholar] [CrossRef]
- Li, Z.; Su, C.; Wu, D.; Zhang, Z. Gold Nanoparticles Decorated Hematite Photoelectrode for Sensitive and Selective Photoelectrochemical Aptasensing of Lysozyme. Anal. Chem. 2018, 90, 961–967. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, W.; Tan, Y.; Xie, Q. CdS Quantum-Dots-Decorated V2O5 Nanosheets as Chemically Etchable Active Materials for Sensitive Photoelectrochemical Immunoassay of Carcinoembryonic Antigen. ACS Appl. Mater. Interfaces 2020, 12, 29066–29073. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Xu, Y.-T.; Xue, Y.; Fan, G.-C.; Zhang, P.-K.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Three-Dimensional CdS@Carbon Fiber Networks: Innovative Synthesis and Application as a General Platform for Photoelectrochemical Bioanalysis. Anal. Chem. 2019, 91, 6419–6423. [Google Scholar] [CrossRef] [Green Version]
- Vaquero, F.; Navarro, R.; Fierro, J. Influence of the solvent on the structure, morphology and performance for H2 evolution of CdS photocatalysts prepared by solvothermal method. Appl. Catal. B Environ. 2017, 203, 753–767. [Google Scholar] [CrossRef]
- Fan, J.; Zang, Y.; Jiang, J.; Lei, J.; Xue, H. Beta-cyclodextrin-functionalized CdS nanorods as building modules for ultrasensitive photoelectrochemical bioassay of HIV DNA. Biosens. Bioelectron. 2019, 142, 111557. [Google Scholar] [CrossRef]
- Li, Q.; Chen, W.; Xiao, H.; Gong, Y.; Li, Z.; Zheng, L.; Zheng, X.; Yan, W.; Cheong, W.; Shen, R.; et al. Fe Isolated Single Atoms on S, N Codoped Carbon by Copolymer Pyrolysis Strategy for Highly Efficient Oxygen Reduction Reaction. Adv. Mater. 2018, 30, e1800588. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiong, L.; Zhao, B.; Liu, M.; Huang, L. Densely Populated Single Atom Catalysts. Small Methods 2019, 4, 4. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, J.; Zheng, L.; Yan, H.; Jiao, L.; Wang, X.; Cai, X.; Wu, Y.; Chen, G.; Chen, L.; et al. Single-Atom-Based Heterojunction Coupling with Ion-Exchange Reaction for Sensitive Photoelectrochemical Immunoassay. Nano Lett. 2021, 21, 1879–1887. [Google Scholar] [CrossRef]
- You, F.; Zhu, M.; Ding, L.; Xu, Y.; Wang, K. Design and construction of Z-scheme Bi2S3/nitrogen-doped graphene quantum dots: Boosted photoelectric conversion efficiency for high-performance photoelectrochemical aptasensing of sulfadimethoxine. Biosens. Bioelectron. 2019, 130, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huan, J.; Hao, N.; Qian, J.; Mao, H.; Wang, K. Engineering of Heterojunction-Mediated Biointerface for Photoelectrochemical Aptasensing: Case of Direct Z-Scheme CdTe-Bi2S3 Heterojunction with Improved Visible-Light-Driven Photoelectrical Conversion Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 18369–18376. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hu, J.; Wang, M.; Diao, X.-K.; Li, C.-C.; Zhang, C.-Y. Mimic Peroxidase- and Bi2S3 Nanorod-Based Photoelectrochemical Biosensor for Signal-On Detection of Polynucleotide Kinase. Anal. Chem. 2018, 90, 11478–11485. [Google Scholar] [CrossRef]
- Li, D.; Xing, G.; Tang, S.; Li, X.; Fan, L.; Li, Y. Ultrathin ZnSe nanowires: One-pot synthesis via a heat-triggered precursor slow releasing route, controllable Mn doping and application in UV and near-visible light detection. Nanoscale 2017, 9, 15044–15055. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ding, X.; Yang, Q.; Wang, Y.; Zhao, G.; Yang, N. A pM leveled photoelectrochemical sensor for microcystin-LR based on surface molecularly imprinted TiO2@CNTs nanostructure. J. Hazard. Mater. 2017, 331, 309–320. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, T.; Wu, T.; Feng, Y.; Sun, M.; Yan, L.; Du, B.; Wei, Q. Fabrication of hierarchical MIL-68(In)-NH2/MWCNT/CdS composites for constructing label-free photoelectrochemical tetracycline aptasensor platform. Biosens. Bioelectron. 2019, 135, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, X.; Gu, S.; Shi, X.-M.; Fan, G.-C. Enhanced two-electrode photoelectrochemical biosensing platform amplified by bilirubin oxidase labelling. Sens. Actuators B Chem. 2021, 343, 130060. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Zhang, J. Mo-doped BiVO4 and graphene nanocomposites with enhanced photoelectrochemical performance for aptasensing of streptomycin. Carbon 2017, 120, 194–202. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Jiang, D.; Ding, L.; Wen, Z.; Guo, Y.; Ding, C.; Wang, K. Oxygen vacancy enhanced photoelectrochemical performance of Bi2MoO6/B, N co-doped graphene for fabricating lincomycin aptasensor. Biosens. Bioelectron. 2019, 135, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, J.; Li, Z.; Zhang, P.; Li, Y.; Liu, G.; Wang, Y.; Yue, Z. Photoelectrochemical determination of hydrogen peroxide using a gold electrode modified with fluorescent gold nanoclusters and graphene oxide. Microchim. Acta 2017, 184, 677–686. [Google Scholar] [CrossRef]
- Peng, M.; Guan, G.; Deng, H.; Han, B.; Tian, C.; Zhuang, J.; Xu, Y.; Liu, W.; Lin, Z. PCN-224/rGO nanocomposite based photoelectrochemical sensor with intrinsic recognition ability for efficient p-arsanilic acid detection. Environ. Sci. Nano 2019, 6, 207–215. [Google Scholar] [CrossRef]
- Qian, Y.; Feng, J.; Fan, D.; Zhang, Y.; Kuang, X.; Wang, H.; Wei, Q.; Ju, H. A sandwich-type photoelectrochemical immunosensor for NT-pro BNP detection based on F-Bi2WO6/Ag2S and GO/PDA for signal amplification. Biosens. Bioelectron. 2019, 131, 299–306. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, H.; Zhang, Y.; Lv, Y.; Li, H.; Liu, S.; Shen, Y.; Zhang, Y. Metal-Free All-Carbon Nanohybrid for Ultrasensitive Photoelectrochemical Immunosensing of alpha-Fetoprotein. ACS Sens. 2018, 3, 1385–1391. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Zhang, Z.; Cao, J.; Ma, T. Host–Guest Recognition on 2D Graphitic Carbon Nitride for Nanosensing. Adv. Mater. Interfaces 2019, 6, 6. [Google Scholar] [CrossRef]
- Mazhabi, R.M.; Ge, L.; Jiang, H.; Wang, X. A facile photoelectrochemical sensor for high sensitive ROS and AA detection based on graphitic carbon nitride nanosheets. Biosens. Bioelectron. 2018, 107, 54–61. [Google Scholar] [CrossRef]
- Yan, P.; Dong, J.; Mo, Z.; Xu, L.; Qian, J.; Xia, J.; Zhang, J.; Li, H. Enhanced photoelectrochemical sensing performance of graphitic carbon nitride by nitrogen vacancies engineering. Biosens. Bioelectron. 2020, 148, 111802. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Tang, L.; Zeng, G.; Fang, S.; Ouyang, X.; Long, B.; Zhou, Y.; Deng, Y.; Liu, Y.; Wang, J. Self-powered photoelectrochemical aptasensor based on phosphorus doped porous ultrathin g-C3N4 nanosheets enhanced by surface plasmon resonance effect. Biosens. Bioelectron. 2018, 121, 19–26. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Zhang, L.; Feng, L.; Zhang, C.; Jiang, J.; Wang, H. Turning on the Photoelectrochemical Responses of Cd Probe-Deposited g-C3N4 Nanosheets by Nitrogen Plasma Treatment toward a Selective Sensor for H2S. ACS Appl. Mater. Interfaces 2021, 13, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ouyang, X.; Peng, B.; Zeng, G.; Zhu, Y.; Yu, J.; Feng, C.; Fang, S.; Zhu, X.; Tan, J. Highly sensitive detection of microcystin-LR under visible light using a self-powered photoelectrochemical aptasensor based on a CoO/Au/g-C3N4 Z-scheme heterojunction. Nanoscale 2019, 11, 12198–12209. [Google Scholar] [CrossRef]
- Lv, S.; Li, Y.; Zhang, K.; Lin, Z.; Tang, D. Carbon Dots/g-C3N4 Nanoheterostructures-Based Signal-Generation Tags for Photoelectrochemical Immunoassay of Cancer Biomarkers Coupling with Copper Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 38336–38343. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Kang, Q.; Zhang, Y.; Wei, Q.; Wang, Y.; Ju, H. Ultrasensitive Photoelectrochemical Biosensing Platform for Detecting N-Terminal Pro-brain Natriuretic Peptide Based on SnO2/SnS2/mpg-C3N4 Amplified by PbS/SiO2. ACS Appl. Mater. Interfaces 2018, 10, 31080–31087. [Google Scholar] [CrossRef]
- Li, P.-P.; Cao, Y.; Mao, C.-J.; Jin, B.-K.; Zhu, J.-J. TiO2/g-C3N4/CdS Nanocomposite-Based Photoelectrochemical Biosensor for Ultrasensitive Evaluation of T4 Polynucleotide Kinase Activity. Anal. Chem. 2019, 91, 1563–1570. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Zhang, X.; Li, J.; Zhang, R.; Song, W. Liposomal Controlled Release Ag-Activated DNAzyme Cycle Amplification on a 2D Pyrene COF-Based Photocathode for α-Synuclein Immunosensing. Anal. Chem. 2021, 93, 8647–8655. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, L.; Wang, J.; Ma, K.; Lv, J. A Novel Biosensor Based on Molybdenum Disulfide (MoS2) Modified Porous Anodic Aluminum Oxide Nanochannels for Ultrasensitive microRNA-155 Detection. Small 2020, 16, e2001223. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Song, Z.; Nawaz, M.H.; Dai, M.; Han, D.; Han, L.; Fan, Y.; Xu, J.; Han, D.; Niu, L. MoS2/ZnO-Heterostructures-Based Label-Free, Visible-Light-Excited Photoelectrochemical Sensor for Sensitive and Selective Determination of Synthetic Antioxidant Propyl Gallate. Anal. Chem. 2019, 91, 10657–10662. [Google Scholar] [CrossRef]
- Lu, M.; Li, B.; Zhang, Y.; Liang, Q.; Li, X.; Xu, S.; Li, Z. Facile synthesis and characterization of a cobalt phthalocyanine sensitized SnIn4S8 composites toward enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 16680–16690. [Google Scholar] [CrossRef]
- Ding, L.; Jiang, D.; Wen, Z.; Xu, Y.; Guo, Y.; Ding, C.; Wang, K. Ultrasensitive and visible light-responsive photoelectrochemical aptasensor for edifenphos based on Zinc phthalocyanine sensitized MoS2 nanosheets. Biosens. Bioelectron. 2020, 150, 111867. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cui, H.; Wang, X.; Luo, F.; Qiu, B.; Cai, W.; Huang, H.; Wang, J.; Lin, Z. Highly Sensitive and Selective Photoelectrochemical Aptasensors for Cancer Biomarkers Based on MoS2/Au/GaN Photoelectrodes. Anal. Chem. 2021, 93, 7341–7347. [Google Scholar] [CrossRef]
- Raza, F.; Park, J.H.; Lee, H.-R.; Kim, H.-I.; Jeon, S.-J.; Kim, J.-H. Visible-Light-Driven Oxidative Coupling Reactions of Amines by Photoactive WS2 Nanosheets. ACS Catal. 2016, 6, 2754–2759. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Wang, S.; Yin, H.; Chen, Y.; Luo, H.; Ai, S. One step preparation of CN-WS2 nanocomposite with enhanced photoactivity and its application for photoelectrochemical detection of 5-formylcytosine in the genomic DNA of maize seedling. Biosens. Bioelectron. 2020, 151, 111973. [Google Scholar] [CrossRef]
- Wang, Q.; Yin, H.; Zhou, Y.; Wang, J.; Ai, S. Investigation of the inhibited biotoxicity of heavy metals towards 5- formylcytosine in rice by hydrochar based on photoelectrochemical biosensor. J. Hazard. Mater. 2021, 414, 125293. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yin, H.; Chen, Y.; Wang, S.; Li, J.; Zhang, Y.; Li, C.; Ai, S. Preparation of P-g-C3N4-WS2 nanocomposite and its application in photoelectrochemical detection of 5-formylcytosine. J. Colloid Interface Sci. 2020, 561, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, S.; Yin, H.; Chen, Y.; Zhou, Y.; Huang, J.; Ai, S. Photoelectrochemical Biosensor for DNA Formylation Detection in Genomic DNA of Maize Seedlings Based on Black TiO2-Enhanced Photoactivity of MoS2/WS2 Heterojunction. ACS Sens. 2020, 5, 1092–1101. [Google Scholar] [CrossRef]
- Qing, M.; Chen, S.L.; Han, L.; Yang, Y.Z.; Luo, H.Q.; Li, N.B. Three–dimensional donor–acceptor–type photoactive material/conducting polyaniline hydrogel complex for sensitive photocathodic enzymatic bioanalysis. Biosens. Bioelectron. 2020, 158, 112179. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Liu, Y.-L.; Xu, Y.-T.; Ruan, Y.-F.; Fan, G.-C.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Three-Dimensional TiO2@Cu2O@Nickel Foam Electrodes: Design, Characterization, and Validation of O2-Independent Photocathodic Enzymatic Bioanalysis. ACS Appl. Mater. Interfaces 2019, 11, 25702–25707. [Google Scholar] [CrossRef]
- Ge, L.; Li, H.; Du, X.; Zhu, M.; Chen, W.; Shi, T.; Hao, N.; Liu, Q.; Wang, K. Facile one-pot synthesis of visible light-responsive BiPO4/nitrogen doped graphene hydrogel for fabricating label-free photoelectrochemical tetracycline aptasensor. Biosens. Bioelectron. 2018, 111, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, P.; Tang, Y.; Yang, L.; Li, L.; Qi, Z.; Li, D.; Wong, D.K. A photoelectrochemical aptasensor based on a 3D flower-like TiO2-MoS2-gold nanoparticle heterostructure for detection of kanamycin. Biosens. Bioelectron. 2018, 112, 193–201. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Fu, C.; Sun, Y.; Zhao, J.; Li, N.; Liu, Y.; Ge, S.; Yu, J. AgInSe2-Sensitized ZnO Nanoflower Wide-Spectrum Response Photoelectrochemical/Visual Sensing Platform via Au@Nanorod-Anchored CeO2 Octahedron Regulated Signal. Anal. Chem. 2020, 92, 7604–7611. [Google Scholar] [CrossRef]
- Jalali, M.; Moakhar, R.S.; Abdelfattah, T.; Filine, E.; Mahshid, S.S.; Mahshid, S. Nanopattern-Assisted Direct Growth of Peony-like 3D MoS2/Au Composite for Nonenzymatic Photoelectrochemical Sensing. ACS Appl. Mater. Interfaces 2020, 12, 7411–7422. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, F.; Li, X.; Wang, Y.; Fan, D.; Du, B.; Li, Y.; Wei, Q. Label-free photoelectrochemical immunosensor for NT-proBNP detection based on La-CdS/3D ZnIn2S4/Au@ZnO sensitization structure. Biosens. Bioelectron. 2018, 117, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, C.; Zhang, L. Construction of 3D Bi/ZnSnO3 hollow microspheres for label-free highly selective photoelectrochemical recognition of norepinephrine. Nanoscale 2021, 13, 9270–9279. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Hua, R.; Chen, S.; Zhang, Y.; Zhou, Z.; Qian, J.; Liu, Q.; Wang, K. Multiple signal-amplification via Ag and TiO2 decorated 3D nitrogen doped graphene hydrogel for fabricating sensitive label-free photoelectrochemical thrombin aptasensor. Biosens. Bioelectron. 2018, 101, 14–20. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Pan, X.; Zhang, L.; Gong, J. Boosted photoelectrochemical immunosensing of metronidazole in tablet using coral-like g-C3N4 nanoarchitectures. Biosens. Bioelectron. 2019, 123, 7–13. [Google Scholar] [CrossRef]
- Lan, F.; Liang, L.; Zhang, Y.; Li, L.; Ren, N.; Yan, M.; Ge, S.; Yu, J. Internal Light Source-Driven Photoelectrochemical 3D-rGO/Cellulose Device Based on Cascade DNA Amplification Strategy Integrating Target Analog Chain and DNA Mimic Enzyme. ACS Appl. Mater. Interfaces 2017, 9, 37839–37847. [Google Scholar] [CrossRef]
- Ye, H.; Yang, K.; Tao, J.; Liu, Y.; Zhang, Q.; Habibi, S.; Nie, Z.; Xia, X. An Enzyme-Free Signal Amplification Technique for Ultrasensitive Colorimetric Assay of Disease Biomarkers. ACS Nano 2017, 11, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhao, S.; Wang, Q.; Wei, H. N-Doped Carbon As Peroxidase-Like Nanozymes for Total Antioxidant Capacity Assay. Anal. Chem. 2019, 91, 15267–15274. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Liao, W.-C.; Cazelles, R.; Wang, S.; Yu, X.; Gutkin, V.; Willner, I. Mimicking Horseradish Peroxidase Functions Using Cu2+-Modified Carbon Nitride Nanoparticles or Cu2+-Modified Carbon Dots as Heterogeneous Catalysts. ACS Nano 2017, 11, 3247–3253. [Google Scholar] [CrossRef]
- Chen, Y.; Yuchi, Q.; Li, T.; Yang, G.; Miao, J.; Huang, C.; Liu, J.; Li, A.; Qin, Y.; Zhang, L. Precise engineering of ultra-thin Fe2O3 decorated Pt-based nanozymes via atomic layer deposition to switch off undesired activity for enhanced sensing performance. Sens. Actuators B Chem. 2020, 305, 127436. [Google Scholar] [CrossRef]
- Huang, D.; Wang, L.; Zhan, Y.; Zou, L.; Ye, B. Photoelectrochemical biosensor for CEA detection based on SnS2-GR with multiple quenching effects of Au@CuS-GR. Biosens. Bioelectron. 2019, 140, 111358. [Google Scholar] [CrossRef]
- Kong, W.; Guo, X.; Jing, M.; Qu, F.; Lu, L. Highly sensitive photoelectrochemical detection of bleomycin based on Au/WS2 nanorod array as signal matrix and Ag/ZnMOF nanozyme as multifunctional amplifier. Biosens. Bioelectron. 2020, 150, 111875. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.-C.; Gao, F.; Cui, Y.; Wang, W.; Luo, X. High-activity Fe3O4 nanozyme as signal amplifier: A simple, low-cost but efficient strategy for ultrasensitive photoelectrochemical immunoassay. Biosens. Bioelectron. 2019, 127, 64–71. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, L.; Tang, L.; Peng, B.; Huang, H.; Wang, J.; Yu, J.; Ouyang, X.; Tan, J. Ultrathin PtNi nanozyme based self-powered photoelectrochemical aptasensor for ultrasensitive chloramphenicol detection. Biosens. Bioelectron. 2019, 146, 111756. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Y.; Tu, W.; Dai, Z. Photoelectrochemical Bioanalysis Platform for Cells Monitoring Based on Dual Signal Amplification Using in Situ Generation of Electron Acceptor Coupled with Heterojunction. ACS Appl. Mater. Interfaces 2017, 9, 22289–22297. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Newbigging, A.M.; Wang, Z.; Tao, J.; Deng, W.; Le, X.C.; Zhang, H. DNAzyme-Mediated Assays for Amplified Detection of Nucleic Acids and Proteins. Anal. Chem. 2017, 90, 190–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Zhao, Z.; Lv, Y.-F.; Huan, S.-Y.; Fu, T.; Zhang, X.-B.; Shen, G.-L.; Yu, R.-Q. DNAzyme-based biosensors and nanodevices. Chem. Commun. 2015, 51, 979–995. [Google Scholar] [CrossRef]
- Meng, L.; Liu, M.; Xiao, K.; Zhang, X.; Du, C.; Chen, J. Sensitive photoelectrochemical assay of Pb2+ based on DNAzyme-induced disassembly of the “Z-scheme” TiO2/Au/CdS QDs system. Chem. Commun. 2020, 56, 8261–8264. [Google Scholar] [CrossRef]
- Yan, K.; Ji, W.; Zhu, Y.; Chen, F.; Zhang, J. Photofuel cell coupling with redox cycling as a highly sensitive and selective self-powered sensing platform for the detection of tyrosinase activity. Chem. Commun. 2019, 55, 12040–12043. [Google Scholar] [CrossRef]
- Hun, X.; Meng, Y. Electron Acceptors Co-Regulated Self-Powered Photoelectrochemical Strategy and Its Application for Circulating Tumor Nucleic Acid Detection Coupled with Recombinase Polymerase Amplification. Anal. Chem. 2020, 92, 11771–11778. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, Z.; Tang, L.; Ouyang, X.; Zhu, X.; Chen, L.; Fan, X.; Zhou, Z.; Wang, J. Self-Powered Photoelectrochemical Aptasensor for Oxytetracycline Cathodic Detection Based on a Dual Z-Scheme WO3/g-C3N4/MnO2 Photoanode. Anal. Chem. 2021, 93, 9129–9138. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Dai, H.; Zheng, H.; Hong, Z.; Lin, Y. Dual-readout immunosensor constructed based on brilliant photoelectrochemical and photothermal effect of polymer dots for sensitive detection of sialic acid. Biosens. Bioelectron. 2019, 142, 111567. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Qin, Y.; Li, J.; Hu, L.; Gu, W.; Zhu, C. Proton-Regulated Catalytic Activity of Nanozymes for Dual-Modal Bioassay of Urease Activity. Anal. Chem. 2021, 93, 9897–9903. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhao, Y.; Gao, X.; Li, H.; Jie, G. Versatile Electrochemiluminescence and Photoelectrochemical Detection of Glutathione Using Mn2+ Substitute Target by DNA-Walker-Induced Allosteric Switch and Signal Amplification. Anal. Chem. 2019, 91, 14117–14124. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhao, X.; Na, N.; Ouyang, J. Integrating Near-Infrared Visual Fluorescence with a Photoelectrochemical Sensing System for Dual Readout Detection of Biomolecules. Anal. Chem. 2021, 93, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Kong, Q.; Ge, S.; Yu, J. Time-resolution addressable photoelectrochemical strategy based on hollow-channel paper analytical devices. Biosens. Bioelectron. 2018, 120, 64–70. [Google Scholar] [CrossRef]
- Gai, P.; Kong, X.; Zhang, S.; Song, P.; Li, F. Photo-driven self-powered biosensor for ultrasensitive microRNA detection via DNA conformation-controlled co-sensitization behavior. Chem. Commun. 2020, 56, 7116–7119. [Google Scholar] [CrossRef]
- Yu, Z.; Gong, H.; Li, Y.; Xu, J.; Zhang, J.; Zeng, Y.; Liu, X.; Tang, D. Chemiluminescence-Derived Self-Powered Photoelectrochemical Immunoassay for Detecting a Low-Abundance Disease-Related Protein. Anal. Chem. 2021, 93, 13389–13397. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-J.; Chen, F.-Z.; Hu, J.; Zhou, H.; Chen, G.; Yu, X.-D.; Ban, R.; Lin, P.; Zhao, W.-W. Regulating Light-Sensitive Gate of Organic Photoelectrochemical Transistor toward Sensitive Biodetection at Zero Gate Bias. Small Struct. 2021, 2, 2100087. [Google Scholar] [CrossRef]
- Ruan, Y.; Chen, F.; Xu, Y.; Zhang, T.; Yu, S.; Zhao, W.; Jiang, D.; Chen, H.; Xu, J. An Integrated Photoelectrochemical Nanotool for Intracellular Drug Delivery and Evaluation of Treatment Effect. Angew. Chem. Int. Ed. 2021, 60, 25762–25765. [Google Scholar] [CrossRef]





| Nanostructure | Feature | Material | Target | Ref. |
|---|---|---|---|---|
| 0D | ||||
| QDs | Easily regulated bandgaps, excellent surface modification property and similar size to many biomolecules | CdTe QDs | CA125 | [16] |
| ZnS@Ag2S QDs | Hg2+ | [17] | ||
| DNA TET-CdTe QDs-MB | miRNA-141 | [18] | ||
| TPP doped Pdots | Telomerase activity | [23] | ||
| Carbon-based NPs | Low cytotoxicity, resistance to photobleaching, and fascinating biocompatibility | N-doped C-dots | CEA | [38] |
| GOD@Con-A | N-glycan expression on the surface of MCF-7 cells | [44] | ||
| ZnO NDs@g-C3N4 QDs | CCRF-CEM cell | [46] | ||
| Noble-metal NPs | Easy to synthesize in different sizes, excellent stability and biocompatibility | Au NCs-Ag@SiO2 | Alkaline phosphatase activity | [50] |
| Au@Ag NPs-TiO2 NRs | DNA | [52] | ||
| Ag@Au asymmetric core–satellite | PSA | [57] | ||
| 1D | ||||
| Metal-oxide NWs | Strong light absorption property, low toxicity, and high chemical stability and biocompatibility | TiO2 NWs | Glucose | [70] |
| TiO2 nanoneedles @MoO3 | RAW264.7 cells | [71] | ||
| ZnO NRs-CdTe QDs | DNA sequence of HIV-1 | [72] | ||
| Fe2O3 NRs-Au NPs | lysozyme | [73] | ||
| Metal-chalcogenide NRs | Narrow bandgaps, significantly increased binding sites, and decreased radial transport distance | β-CD@CdS NRs | DNA sequence of HIV | [77] |
| Pt SAs/CdS NRs | PSA | [80] | ||
| Bi2S3 NRs | PNK | [83] | ||
| Carbon NTs | Excellent photogenerated electron-transfer property | TiO2 NPs@CNTs | MC-LR | [85] |
| MIL-68(In)-NH2/MWCNT/CdS NPs | Tc | [86] | ||
| Au NPs/CNTs | CEA | [87] | ||
| 2D | ||||
| Carbon-based graphene-like nanostructures | Outstanding electron conductivity and high specific surface area | Bi2MoO6/BNG | Lincomycin | [91] |
| PCN-224/rGO | p-ASA | [93] | ||
| AC60-Gr-GO | AFP | [95] | ||
| CoO/Au NPs/g-C3N4 | MC-LR | [101] | ||
| SnO2/SnS2/mpg-C3N4 | N-terminalpro-brain natriuretic peptide | [103] | ||
| TiO2/g-C3N4/CdS | T4 polynucleotide kinase | [104] | ||
| PAF-130/g-C3N4 | α-synuclein | [105] | ||
| TMDs | High specific surface area and short photogenerated charge-carrier transfer distance | AAO-MoS2 film | miRNA-155 | [106] |
| MoS2 flakes-ZnO NRs | PG | [107] | ||
| ZnPc NPs-MoS2 Nanosheets | Edifenphos | [109] | ||
| Au NPs-C3N4-WS2 nanosheets | 5fC | [114] | ||
| WS2-MoS2 nanosheets | 5fC | [115] | ||
| 3D | ||||
| Metal chalcogenides | Mimicking flower-like morphologies and excellent target-capture ability | Peony-like MoS2 Nanosheet Au NPs | Glucose | [121] |
| Ternary compound semiconductors | Low cost, high chemical stability, and great visible light-harvesting property | La-CdS-ZnIn2S4-Au@ZnO | NT-proBNP | [122] |
| Bi NPs-ZnSnO3 microspheres | NE | [123] | ||
| Carbon-based architectures | Abundant active sites, high light-harvesting property, and fast charge-carrier transfer speed | Coral-like g-C3N4 | MNZ | [125] |
| Flower-like Au-3D-rGO | Thrombin | [126] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhu, Y.-C.; Zhao, W.-W. Recent Advances of Nanostructured Materials for Photoelectrochemical Bioanalysis. Chemosensors 2022, 10, 14. https://doi.org/10.3390/chemosensors10010014
Zhang L, Zhu Y-C, Zhao W-W. Recent Advances of Nanostructured Materials for Photoelectrochemical Bioanalysis. Chemosensors. 2022; 10(1):14. https://doi.org/10.3390/chemosensors10010014
Chicago/Turabian StyleZhang, Ling, Yuan-Cheng Zhu, and Wei-Wei Zhao. 2022. "Recent Advances of Nanostructured Materials for Photoelectrochemical Bioanalysis" Chemosensors 10, no. 1: 14. https://doi.org/10.3390/chemosensors10010014






