Preparation of Thermo-Sensitive Molecular Imprinted SERS Substrate with Robust Recyclability for Detection of Ofloxacin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Measurements
2.3. Preparation of Materials
2.3.1. Preparation of TiO2
2.3.2. Preparation of Ag Nanoparticles
2.3.3. Preparation of TiO2@Ag
2.3.4. Preparation of TM@TiO2@Ag and TN@TiO2@Ag
2.4. Raman Measurement
2.5. Photocatalytic Experiments
3. Results
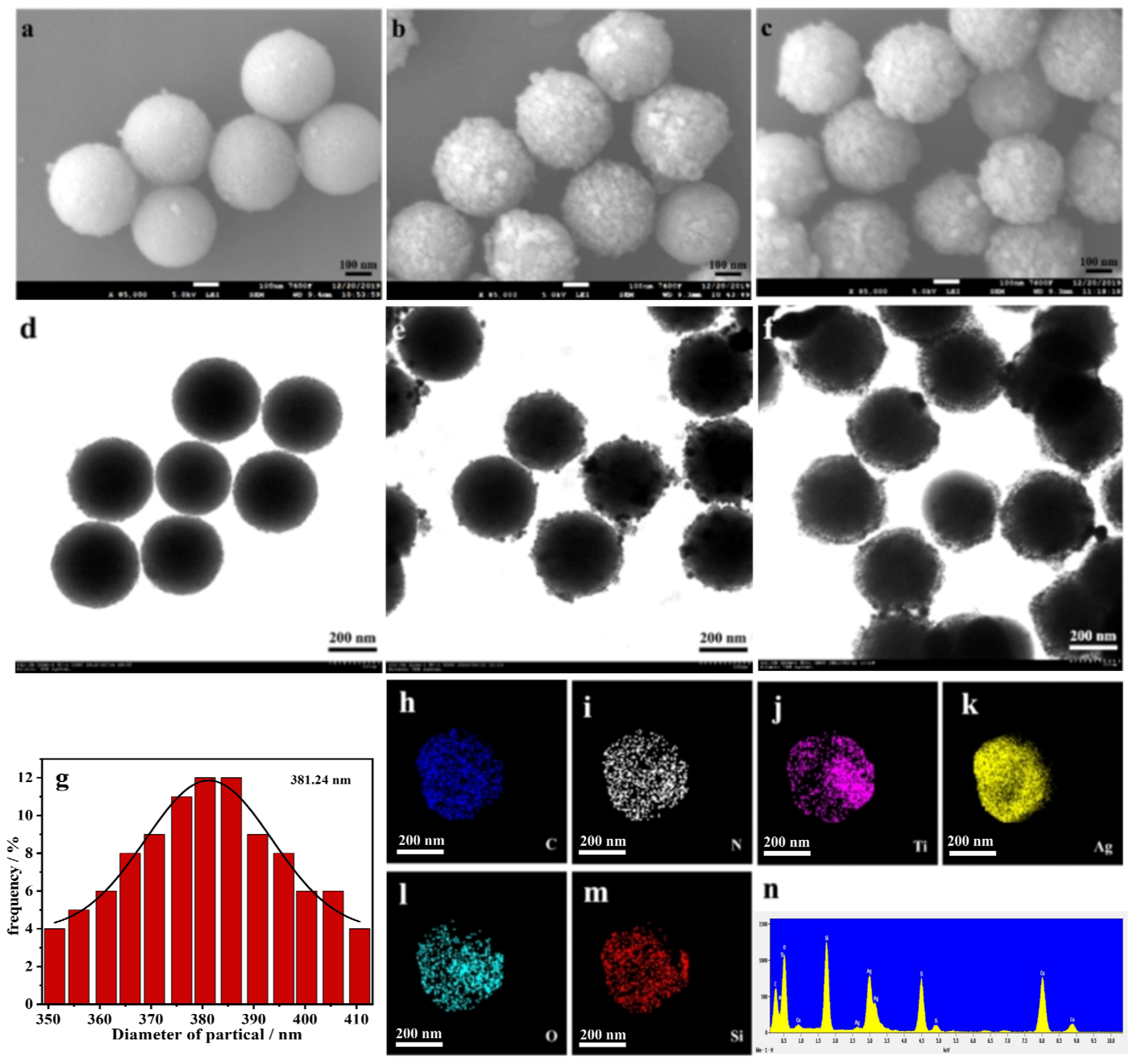
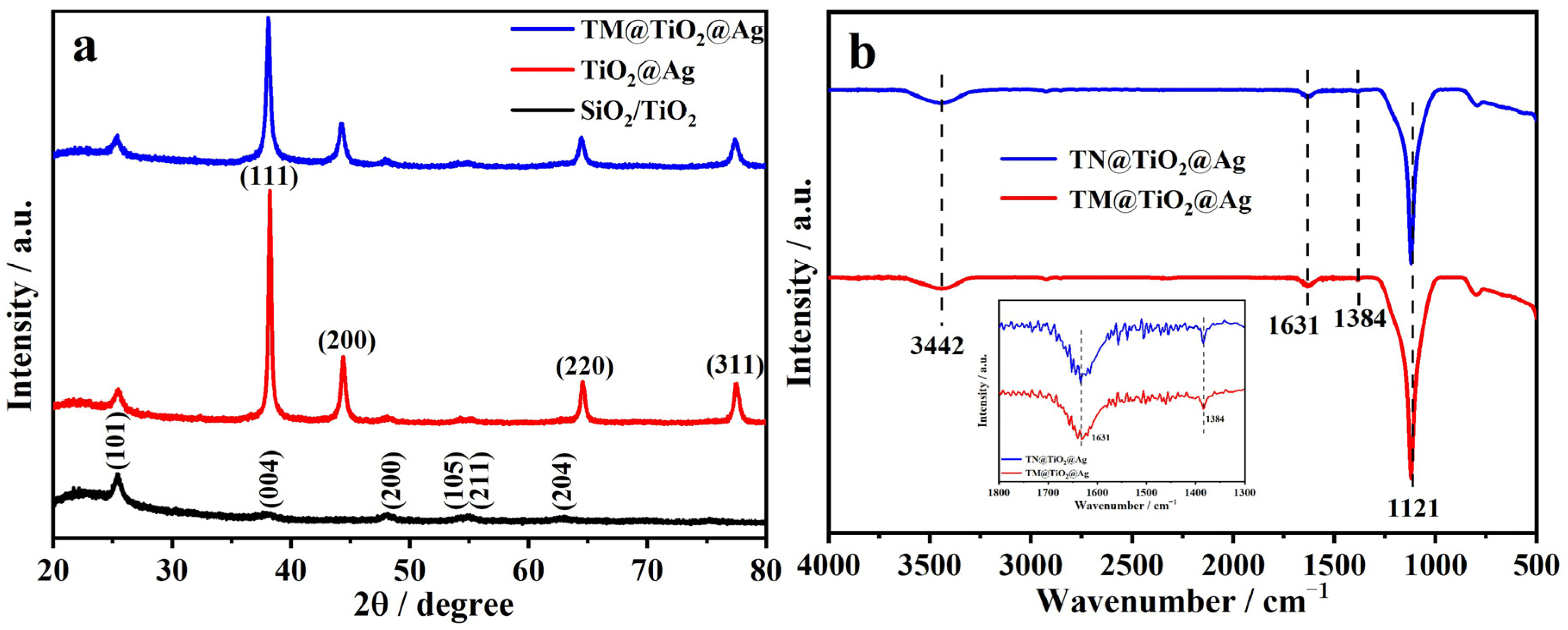
3.1. Characterization of TM@TiO2@Ag
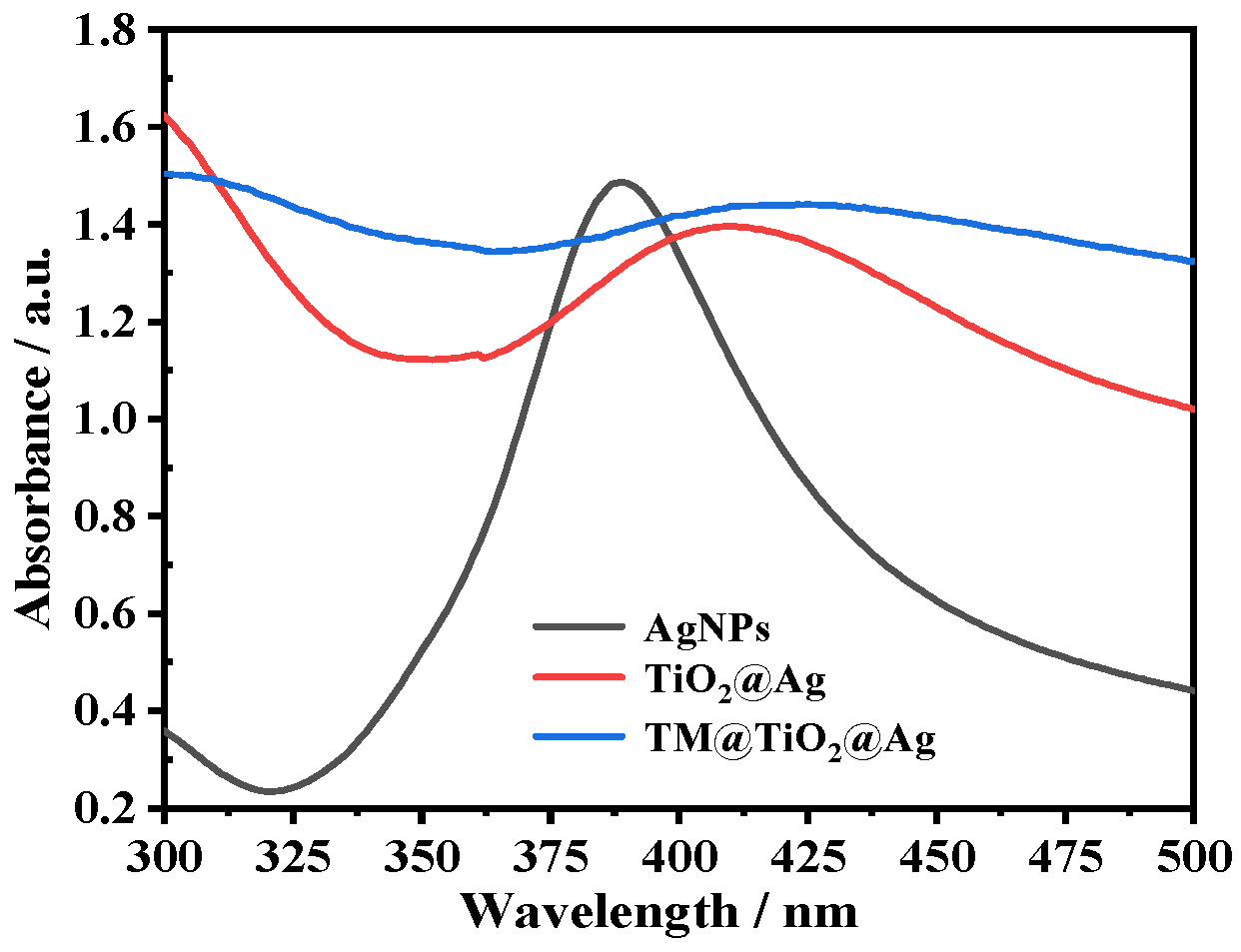
3.2. Optical Property of TM@TiO2@Ag
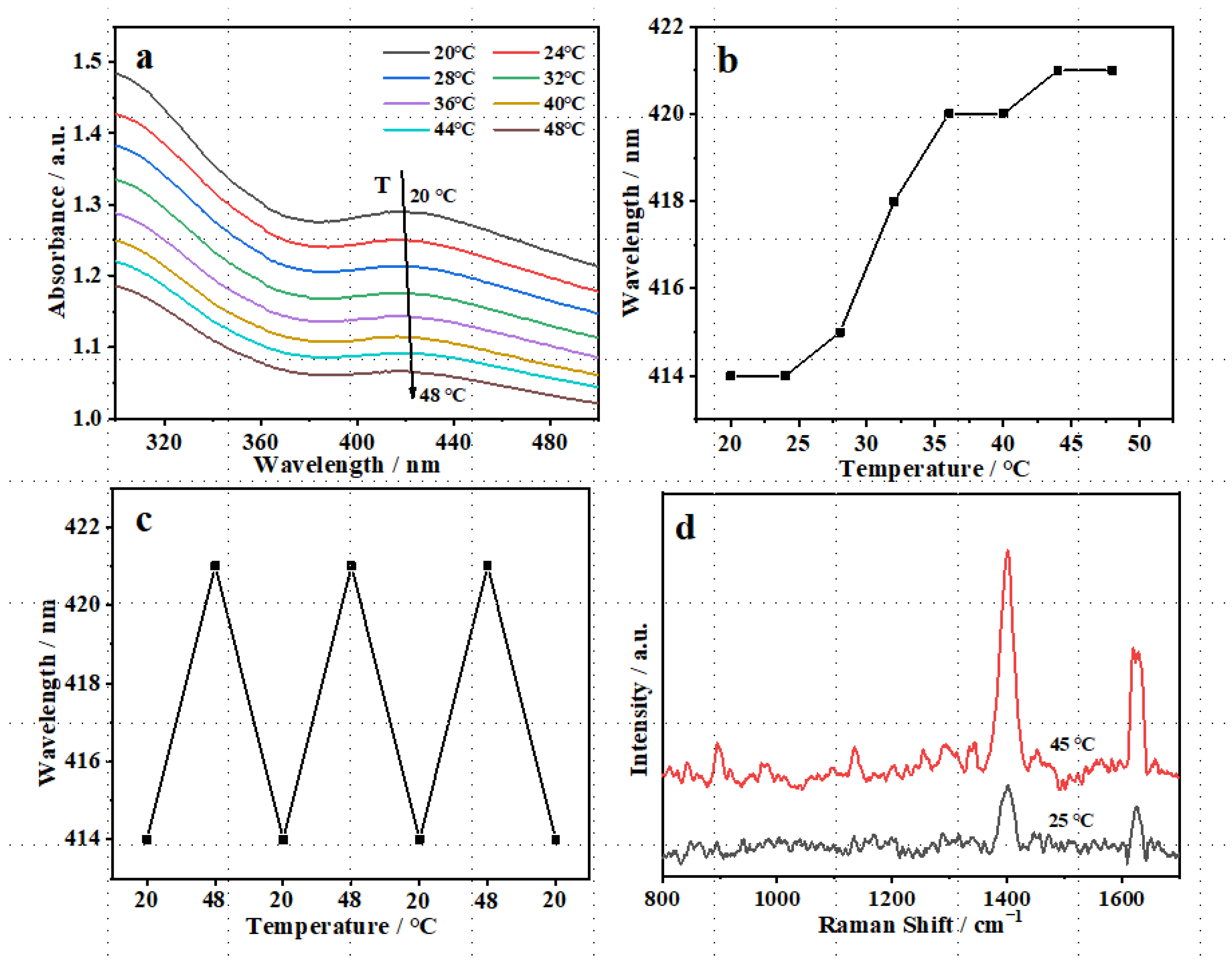
3.3. Thermo-Sensitivity of TM@TiO2@Ag
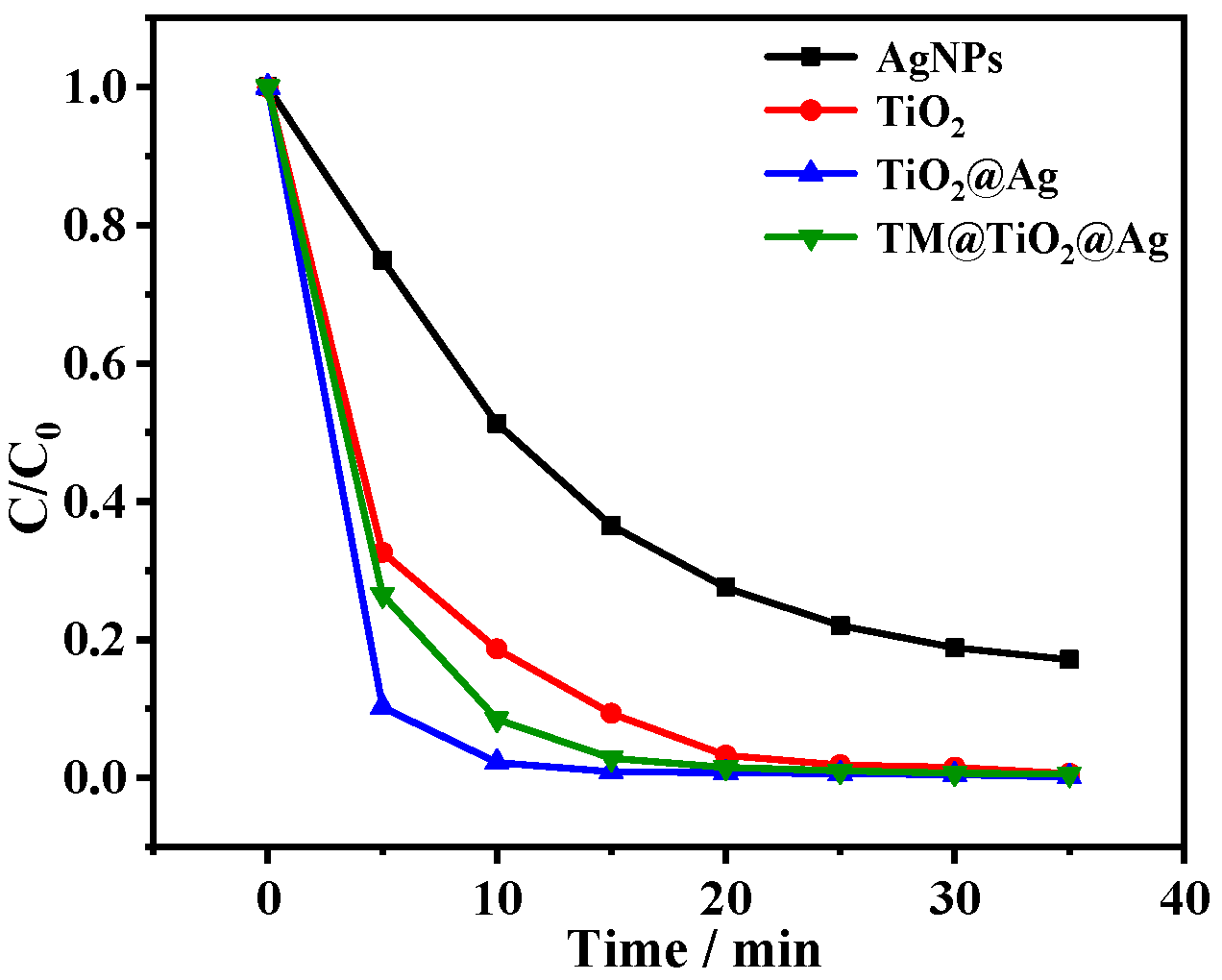
3.4. Photocatalytic Performance of TM@TiO2@Ag
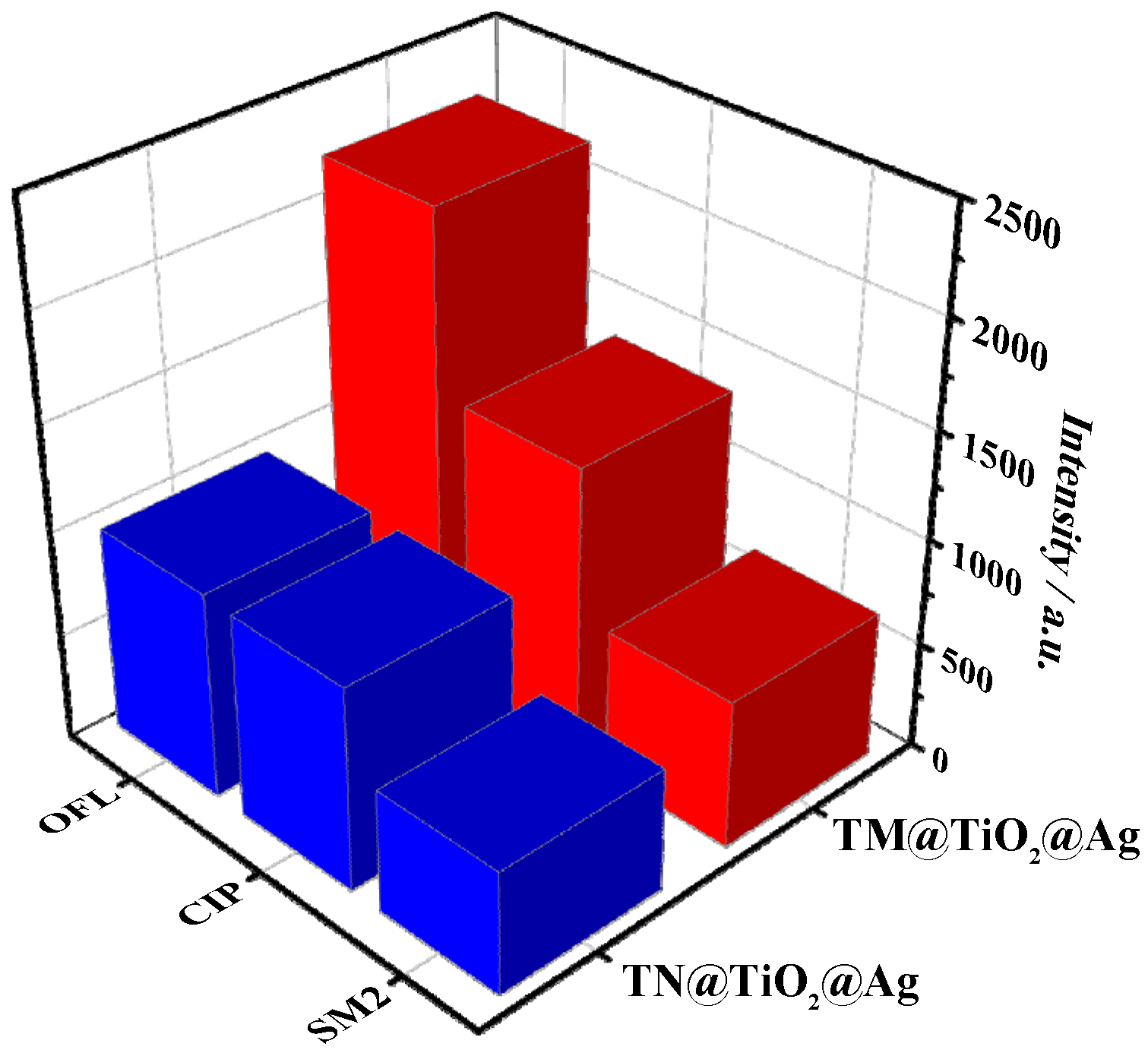
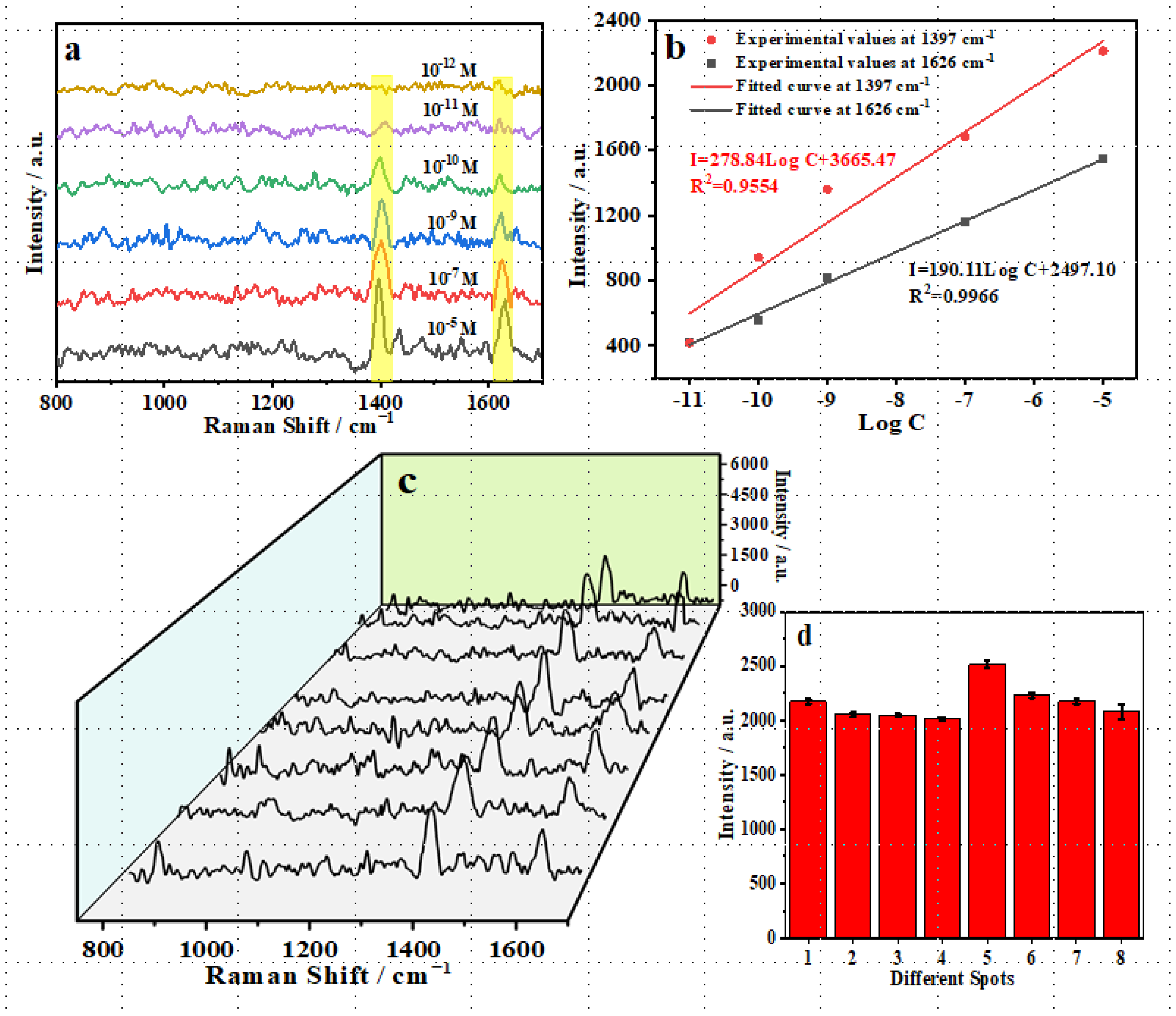
3.5. Selectivity, Sensitivity, and Stability of TM@TiO2@Ag in OFL SERS Detection
3.6. The Recyclability of TM@TiO2@Ag
3.7. Detection of OFL in Actual Sample
3.8. Comparison with Other Methods for OFL
3.9. Mechanism of SERS Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gai, S.; Zhang, J.; Fan, R.Q.; Xing, K.; Chen, W.; Zhu, K.; Zheng, X.B.; Wang, P.; Fang, X.K.; Yang, Y.L. Highly stable zinc-based metal-organic frameworks and corresponding flexible composites for removal and detection of antibiotics in water. ACS Appl. Mater. Interfaces 2020, 12, 8650–8662. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Iyer, P.K. Conjugated polyelectrolyte based sensitive detection and removal of antibiotics tetracycline from water. ACS Appl. Mater. Interfaces 2017, 9, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, S.; Li, Y.X.; Lu, D.; Zhang, J.; Sun, C.J. Application of aptamers in detection and chromatographic purification of antibiotics in different matrices. TrAC Trends Anal. Chem. 2017, 95, 1–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Zhang, D.N.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Galvan, D.D.; Yu, Q.M. Surface-enhanced Raman scattering for rapid detection and characterization of antibiotic-resistant bacteria. Adv. Healthc. Mater. 2018, 7, 1701335. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.Q.; Li, Y.Y.; Miao, X.X.; Zhang, Y.Q.; Yan, J.; Yu, T.Y.; Liu, J. Sensitive detection of antibiotics using aptamer conformation cooperated enzyme assisted SERS technology. Analyst 2019, 144, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.Y.; Yao, Y.; Ping, J.F.; Ying, Y.B. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.N.; Peng, D.P.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.X.; Shabbir, M.A.; Cheng, G.Y.; Yuan, Z.H. Current advances in immunoassays for the detection of antibiotics residues: A Review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Wang, W.E.; Sang, Q.Q.; Yang, M.; Du, J.; Yang, L.B.; Jiang, X.; Han, X.X.; Zhao, B. Detection of several quinolone antibiotic residues in water based on Ag-TiO2 SERS strategy. Sci. Total Environ. 2020, 702, 134956. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Huang, P.C.; Wu, F.Y. A Label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020, 304, 125377. [Google Scholar] [CrossRef]
- Pawar, M.K.; Tayade, K.C.; Sahoo, S.K.; Mahulikar, P.P.; Kuwar, A.S.; Chaudhari, B.L. Selective ciprofloxacin antibiotic detection by fluorescent siderophore pyoverdin. Biosens. Bioelectron. 2016, 81, 274–279. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, A.; Kumar, A.; Singh, M.B.; Rao, A.L.J. Spectrophotometric methods for the determination of fluoroquinolones: A Review. Crit. Rev. Anal. Chem. 2008, 38, 2–18. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.-M. Optical methods of antibiotic residues detections: A comprehensive review. Sens. Actuators B Chem. 2018, 269, 238–256. [Google Scholar] [CrossRef]
- Peng, J.; Kong, D.Z.; Liu, L.Q.; Song, S.S.; Kuang, H.; Xu, C.L. Determination of quinoxaline antibiotics in fish feed by enzyme-linked immunosorbent assay using a monoclonal antibody. Anal. Methods 2015, 7, 5204–5209. [Google Scholar] [CrossRef]
- Shanin, I.A.; Zvereva, E.A.; Eremin, S.A.; Sviridov, O.V.; Zherdev, A.V.; Dzantiev, B.B. Development of an immunoenzyme assay to control the total content of antibiotics of the fluoroquinolone group in milk. Appl. Biochem. Microbiol. 2020, 55, 563–569. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhu, Y.; Ding, S.Y.; He, F.Y.; Beier, R.C.; Li, J.C.; Jiang, H.Y.; Feng, C.W.; Wan, Y.P.; Zhang, S.X.; et al. Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal. Chem. 2007, 79, 4471–4483. [Google Scholar] [CrossRef]
- Piatkowska, M.; Jedziniak, P.; Zmudzki, J. Multiresidue Method for the simultaneous determination of veterinary medicinal products, feed additives and illegal dyes in eggs using liquid chromatography-tandem mass spectrometry. Food Chem. 2016, 197, 571–580. [Google Scholar] [CrossRef]
- Yan, H.Y.; Wang, H.; Qin, X.Y.; Liu, B.M.; Du, J.J. Ultrasound-assisted dispersive liquid-liquid microextraction for determination of fluoroquinolones in pharmaceutical wastewater. J. Pharm. Biomed. Anal. 2011, 54, 53–57. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Shen, D.-X.; Ding, T.; Xu, J.-Z.; Jiang, Y.; Wu, B.; Lian, H.-Z. Simultaneous determination of 14 quinolones in royal jelly by liquid chromatography-tandem mass spectrometry using anion-exchange solid-phase extraction. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1415–1430. [Google Scholar] [CrossRef]
- He, S.; Chua, J.; Tan, E.K.M.; Kah, J.C.Y. Optimizing the SERS enhancement of a facile gold nanostar immobilized paper-based SERS substrate. RSC Adv. 2017, 7, 16264–16272. [Google Scholar] [CrossRef]
- Li, J.J.; Yan, H.; Tan, X.C.; Lu, Z.C.; Han, H.Y. Cauliflower-inspired 3D SERS substrate for multiple mycotoxins detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Puebla, R.A.; Contreras-Caceres, R.; Pastoriza-Santos, I.; Perez-Juste, J.; Liz-Marzan, L.M. Au@pNIPAM colloids as molecular traps for surface-enhanced, spectroscopic, ultra-sensitive analysis. Angew. Chem. 2009, 48, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Qian, Y.; Gao, Q.; Dong, J.; Qian, W.P. In situ controllable preparation of gold nanorods in thermo-responsive hydrogels and their application in surface enhanced Raman scattering. J. Mater. Chem. 2010, 20, 8711–8716. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Meng, G.W.; Zheng, P.; Cushing, S.; Wu, N.Q.; Huang, Q.; Zhu, C.H.; Zhang, Z.; Wang, Z.W. A surface-enhanced Raman scattering sensor integrated with battery-controlled fluidic device for capture and detection of trace small molecules. Sci. Rep. 2015, 5, 12865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.Y.; Ma, X.Y.; Xue, M.Y.; Lian, H.-Z. Application of thermoresponsive hydrogel/gold nanorods composites in the detection of diquat. Talanta 2017, 174, 192–197. [Google Scholar] [CrossRef]
- Hu, B.X.; Sun, D.W.; Wei, Q.Y. A dynamically optical and highly stable pNIPAM@ Au NRs nanohybrid substrate for sensitive SERS detection of malachite green in fish fillet. Talanta 2020, 218, 121188. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, L.; Yan, H.; Lu, Z.C.; Yin, W.M.; Han, H.Y. Enzyme induced molecularly imprinted polymer on SERS substrate for ultrasensitive detection of patulin. Anal. Chim. Acta 2020, 1101, 111–119. [Google Scholar] [CrossRef]
- Li, H.J.; Wang, X.N.; Wang, Z.R.; Jiang, J.Q.; Wei, M.B.; Zheng, J.H.; Yan, Y.S.; Li, C.X. Thermo-responsive molecularly imprinted sensor based on the surface-enhanced Raman scattering for selective detection of R6G in the water. Dalton Trans. 2017, 46, 11282–11290. [Google Scholar] [CrossRef]
- Li, H.B.; Li, J.; Zhu, Y.Y.; Xie, W.Y.; Shao, R.; Yao, X.X.; Gao, A.Q.; Yin, Y.D. Cd2+-doped amorphous TiO2 hollow spheres for robust and ultrasensitive photoelectrochemical sensing of hydrogen sulfide. Anal. Chem. 2018, 90, 5496–5502. [Google Scholar] [CrossRef]
- Phadtare, S.; Kumar, A.; Vinod, V.P.; Dash, C.; Palaskar, D.V.; Rao, M.; Shukla, P.G.; Sivaram, S.; Sastry, M. Direct assembly of gold nanoparticle “shells” on polyurethane microsphere “cores” and their application as enzyme immobilization templates. Chem. Mater. 2003, 15, 1944–1949. [Google Scholar] [CrossRef]
- Feng, Q.A.; Tang, D.Y.; Lv, H.T.; Zhang, W.L.; Li, W.B. Surface-initiated ATRP to modify ZnO nanoparticles with poly(N-isopropylacrylamide): Temperature-controlled switching of photocatalysis. J. Alloys Compd. 2017, 691, 185–194. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Wu, T.; Liu, J.X.; Wang, Y.P. Application of a thermo-sensitive imprinted SERS substrate to the rapid trace detection of ofloxacin. Anal. Methods 2020, 39, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Q.; Sun, D.W.; Pu, H.B.; Wei, Q.Y. Surface-enhanced Raman scattering of core-shell Au@ Ag nanoparticles aggregates for rapid detection of difenoconazole in grapes. Talanta 2019, 191, 449–456. [Google Scholar] [CrossRef] [PubMed]
- An, T.C.; Chen, J.Y.; Nie, X.; Li, G.Y.; Zhang, H.M.; Liu, X.L.; Zhao, H.J. Synthesis of carbon nanotube-anatase TiO2 sub-micrometer-sized sphere composite photocatalyst for synergistic degradation of gaseous styrene. ACS Appl. Mater. Interfaces 2012, 4, 5988–5996. [Google Scholar] [CrossRef]
- Chen, D.H.; Chen, C.J. Formation and characterization of Au-Ag bimetallic nanoparticles in water-in-oil microemulsions. J. Mater. Chem. 2002, 12, 1557–1562. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, D.Y.; Lv, H.T.; Zhang, W.L.; Li, W.B. Temperature-responsive zinc oxide nanorods arrays grafted with poly(N-isopropylacrylamide) via SI-ATRP. RSC Adv. 2015, 5, 62024–62032. [Google Scholar] [CrossRef]
- Li, G.Y.; Yu, N.N.; Gao, Y.R.; Tao, Q.; Liu, X.Y. Polymeric hollow spheres assembled from ALG-g-PNIPAM and β-cyclodextrin for controlled drug release. Int. J. Biol. Macromol. 2016, 82, 381–386. [Google Scholar] [CrossRef]
- Sun, R.; Hughes, S. Fractional extraction and physico-chemical characterization of hemicelluloses and cellulose from sugar beet pulp. Carbohydr. Polym. 1998, 36, 293–299. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Ma, C.C.; Wang, W.; Zhang, M.T.; Hao, X.P.; Chen, S.G. Fabrication of Cu2O-Ag nanocomposites with enhanced durability and bactericidal activity. J. Colloid Interface Sci. 2019, 557, 156–167. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Weng, X.L.; Qian, W.P. Preparation and thermoresponsive properties of AuNPs/PNIPAM composite particle. Acta Phys.-Chim. Sin. 2008, 24, 2159–2164. [Google Scholar]
- Zhu, Y.R.; Lu, Y.G.; Shi, L.Y.; Yang, Y.L. β-Cyclodextrin functionalized N,Zn codoped carbon dots for specific fluorescence detection of fluoroquinolones in milk samples. Microchem. J. 2020, 153, 104517. [Google Scholar] [CrossRef]
- Mirzajani, R.; Pourreza, N.; Burromandpiroze, J. Fabrication of magnetic Fe3O4@nSiO2@mSiO2-NH2 core-shell mesoporous nanocomposite and its application for highly efficient ultrasound assisted dispersive SPE-spectrofluorimetric detection of ofloxacin in urine and plasma samples. Ultrason. Sonochemistry 2018, 40, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.T.; Wang, L.M.; Shen, G.Q.; Zhang, D.W.; Xie, J.L.; Mamut, A.; Huang, W.W.; Zhou, S.S. Colorimetric determination of ofloxacin using unmodified aptamers and the aggregation of gold nanoparticles. Microchim. Acta 2018, 185, 355. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kim, D.H. Synthesis of Bi2S3/Bi2WO6 hierarchical microstructures for enhanced visible light driven photocatalytic degradation and photoelectrochemical sensing of ofloxacin. Chem. Eng. J. 2018, 354, 692–705. [Google Scholar] [CrossRef]
- Feng, L.M.; Xue, Q.; Liu, F.; Cao, Q.P.; Feng, J.J.; Yang, L.; Zhang, F.L. Voltammetric determination of ofloxacin by using a laser-modified carbon glassy electrode. Microchim. Acta 2020, 187, 86. [Google Scholar] [CrossRef]
- El-Zahry, M.R.; Lendl, B. Structure elucidation and degradation kinetic study of ofloxacin using surface enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; O’Donoghue, M.B.; Huang, Y.F.; Kang, H.Z.; Phillips, J.A.; Chen, X.L.; Estevez, M.C.; Yang, C.Y.J.; Tan, W.H. A surface energy transfer nanoruler for measuring binding site distances on live cell surfaces. J. Am. Chem. Soc. 2010, 132, 16559–16570. [Google Scholar] [CrossRef]


| Sample | Spiked/10−12 M | Found/10−12 M | Recovery/% | RSD/% |
|---|---|---|---|---|
| Drinking water | 100 | 107.5 | 107.5 | 12.5 |
| 1000 | 955.3 | 95.5 | 8.2 | |
| 10,000 | 8819 | 88.2 | 9.6 |
| Material | Detection Method | Selectivity | Linear Range | LOD/nM | Regeneration Method | Ref. |
|---|---|---|---|---|---|---|
| β-Cyclodextrin functionalized N,Zn codoped carbon dots | Spectrofluorimetric | N.A. * | 0.075–3.75 μM | 50 | N.A. * | [41] |
| Fe3O4@nSiO2@mSiO2–NH2 | Spectrofluorimetric | Good | 1.0–500.0 µg/L | 0.58 | Elution with nitric acid | [42] |
| Aptamers and AuNPs | Colorimetric | Good | 20–400 nM | 3.38 | N.A. * | [43] |
| Bi2S3/Bi2WO6 | Photoelectrochemical | N.A. * | 1–100 μM | 906 | N.A. * | [44] |
| Laser-modified glassy carbon electrodes | Electrochemical | Good | 0.25–200 μM | 75 | N.A. * | [45] |
| Ag NPs | SERS | N.A. * | 100–500 ng/mL | 0.65 | N.A. * | [46] |
| TM@TiO2@Ag | SERS | Good | 10−5–10 μM | 0.011 | Photocatalytic degradation | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Wu, T.; He, X.; Wang, Y.; Lian, H.-z. Preparation of Thermo-Sensitive Molecular Imprinted SERS Substrate with Robust Recyclability for Detection of Ofloxacin. Chemosensors 2022, 10, 437. https://doi.org/10.3390/chemosensors10110437
Jiang C, Wu T, He X, Wang Y, Lian H-z. Preparation of Thermo-Sensitive Molecular Imprinted SERS Substrate with Robust Recyclability for Detection of Ofloxacin. Chemosensors. 2022; 10(11):437. https://doi.org/10.3390/chemosensors10110437
Chicago/Turabian StyleJiang, Caiyun, Ting Wu, Xin He, Yuping Wang, and Hong-zhen Lian. 2022. "Preparation of Thermo-Sensitive Molecular Imprinted SERS Substrate with Robust Recyclability for Detection of Ofloxacin" Chemosensors 10, no. 11: 437. https://doi.org/10.3390/chemosensors10110437
APA StyleJiang, C., Wu, T., He, X., Wang, Y., & Lian, H.-z. (2022). Preparation of Thermo-Sensitive Molecular Imprinted SERS Substrate with Robust Recyclability for Detection of Ofloxacin. Chemosensors, 10(11), 437. https://doi.org/10.3390/chemosensors10110437






