Engineering Raspberry-like Plasmonic Nanoclusters as Tags in Surface-Enhanced Raman Scattering-Based Immunoassays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Raspberry-like pAuNCs
2.3. Modification of Antibody on pAuCNs
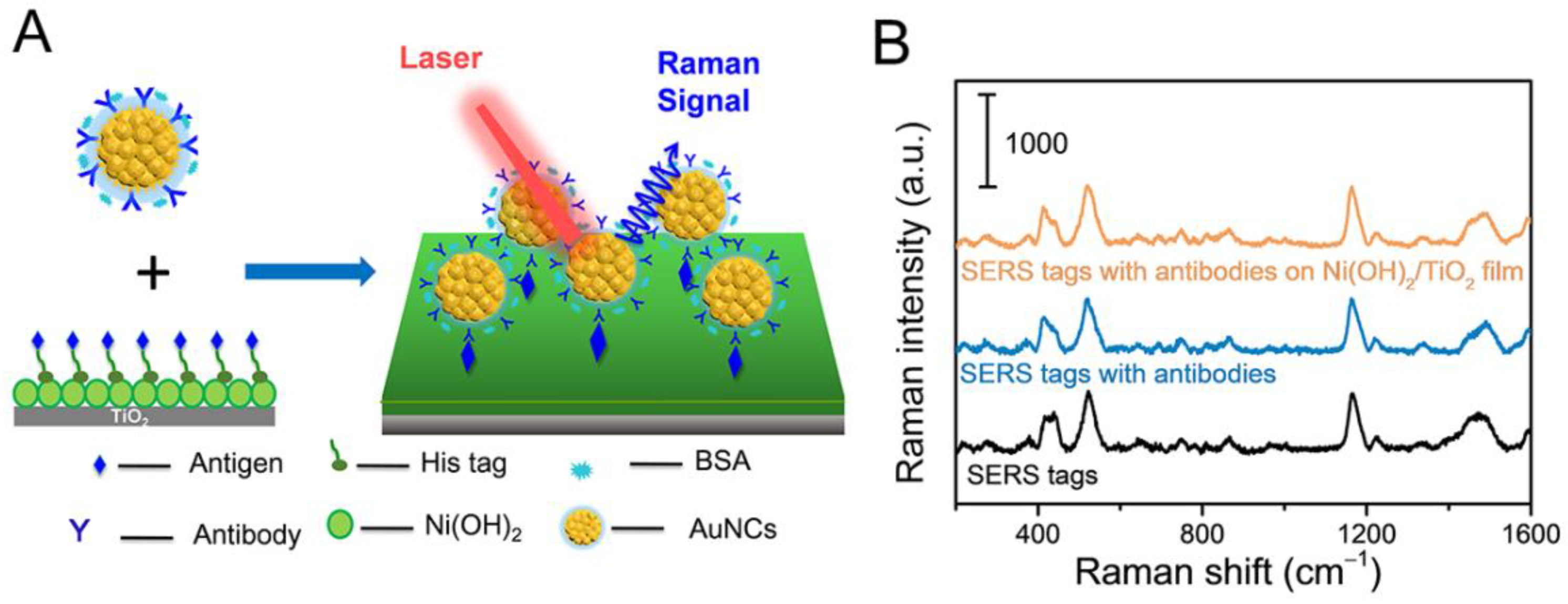
2.4. Synthesis of Substrate for Antigen Capture
2.5. Procedure of the SERS-Based Immunoassay
2.6. SERS Detection
2.7. Finite-Difference Time-Domain (FDTD) Simulation of Au Nanocluster
2.8. Instrumentation
3. Results and Discussion
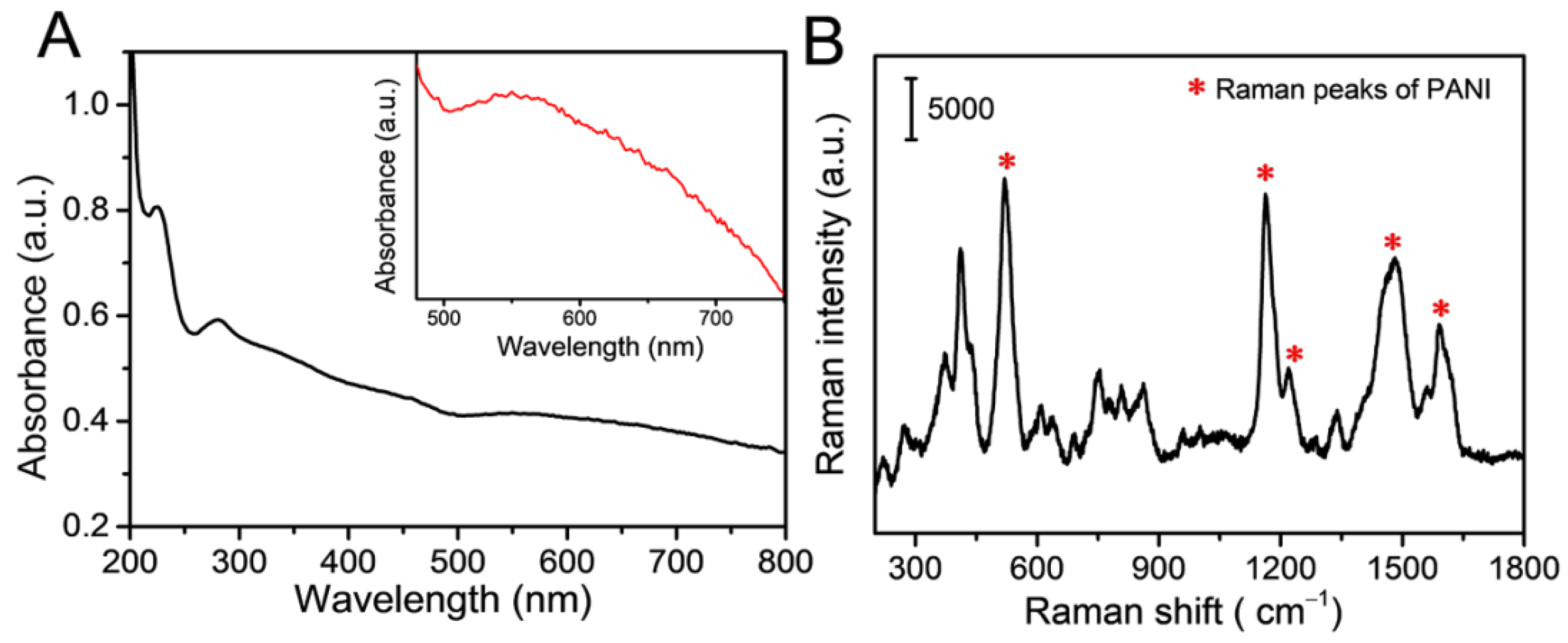
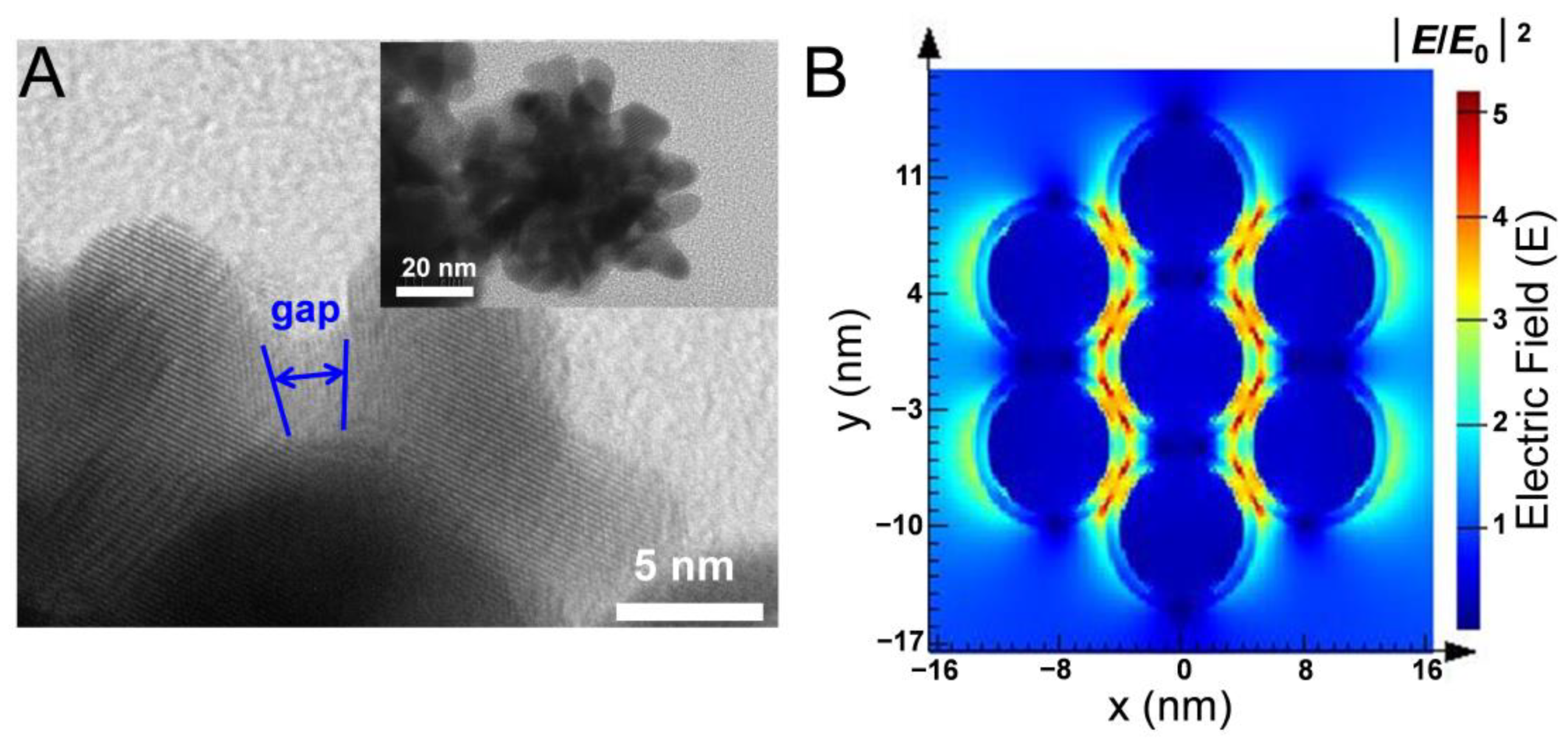
3.1. Fabrication and Characteristics of Raspberry-like pAuNCs
3.2. SERS Performance of the pAuNCs
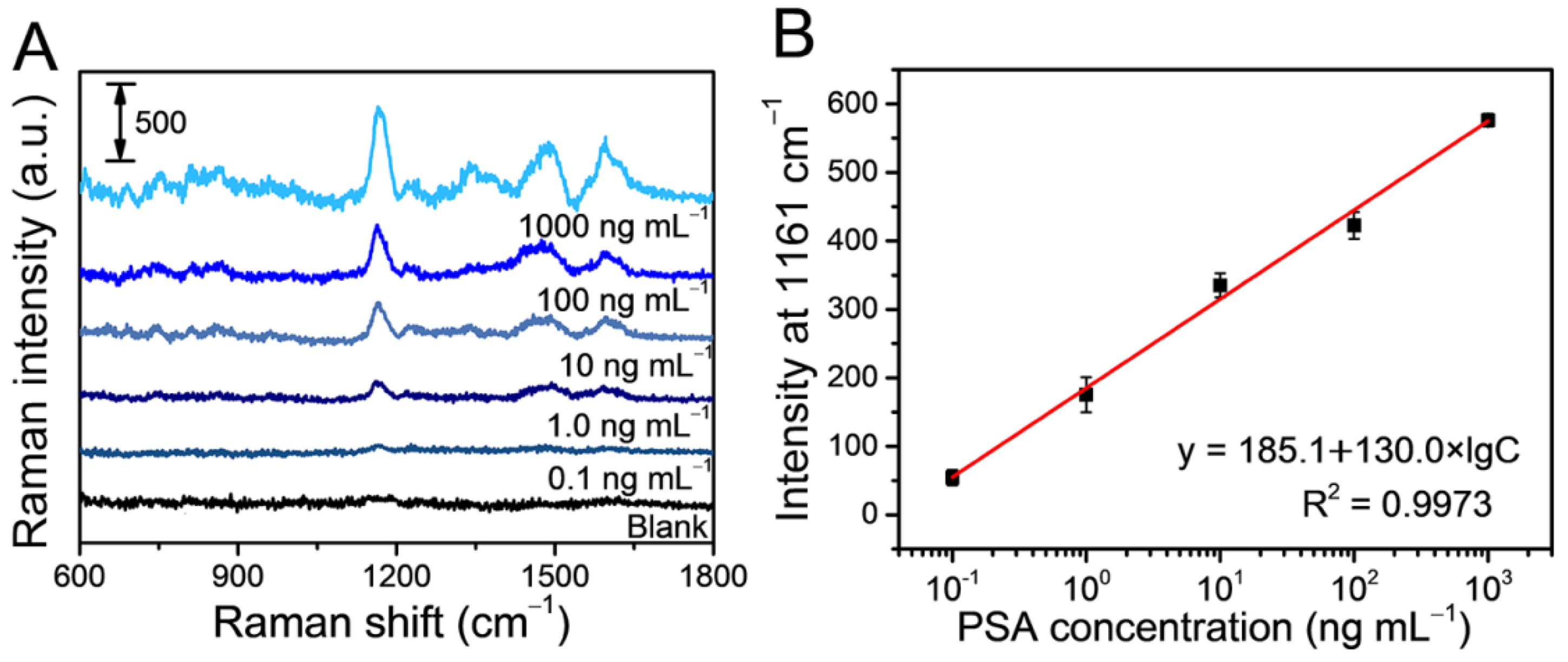
3.3. Evaluation of the SERS-Based Immunoassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, S.E.J.; Charron, G.; Cortés, E.; Kneipp, J.; Chapelle, M.L.; Langer, J.; Procházka, M.; Tran, V.; Schlücker, S. Towards reliable and quantitative surface-enhanced Raman scattering (SERS): From key parameters to good analytical practice. Angew. Chem. Int. Ed. 2020, 59, 5454–5462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Mousavi, M.Z.; Giovannini, G.; Zhao, Y.; Hubarevich, A.; Soler, M.A.; Rocchia, W.; Garoli, D.; Angelis, F.D. Multiplexed discrimination of single amino acid residues in polypeptides in a single SERS hot spot. Angew. Chem. Int. Ed. 2020, 59, 11423–11431. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Cong, S.; Zhao, Z. Defect engineering in semiconductor-based SERS. Chem. Sci. 2022, 13, 1210. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-inspired 3D SERS substrate for multiple mycotoxins detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zheng, B.; Li, J.; Zhou, Y.; Liu, H.; Ahmed, S.A.; Wang, K.; Xia, X. Three-dimensional metamaterial for plasmon-enhanced Raman scattering at any excitation wavelengths from the visible to near-infrared range. Anal. Chem. 2021, 93, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Oner, I.; Querebillo, C.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkuhler, S.; Ly, K.; Weidinger, I. High electromagnetic field enhancement of TiO2 nanotube electrodes. Angew. Chem. Int. Ed. 2018, 57, 7225–7229. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Jian, X.; Zhao, Y.; Dai, Z.; Xu, J.; Gao, Z.; Mei, Y.; Song, Y.-Y. Understanding of chiral site-dependent enantioselective identification on a plasmon-free semiconductor based SERS substrate. Chem. Sci. 2022, 13, 6550–6557. [Google Scholar] [CrossRef]
- Yang, L.; Feng, J.; Wang, J.; Gao, Z.; Xu, J.; Mei, Y.; Song, Y. Engineering large-scaled electrochromic semiconductor films as reproductive SERS substrates for operando investigation at the solid/liquid interfaces. Chin. Chem. Lett. 2022, 33, 5169–5173. [Google Scholar] [CrossRef]
- Camacho, S.A.; Sobral-Filho, R.G.; Aoki, P.H.B.; Constantino, C.J.L.; Brolo, A.G. Zika immunoassay based on surface-enhanced Raman scattering nanoprobes. ACS Sens. 2018, 3, 587–594. [Google Scholar] [CrossRef]
- Pham, X.; Hahm, E.; Kim, T.H.; Kim, H.-M.; Lee, S.H.; Lee, S.C.; Kang, H.; Lee, H.-Y.; Jeong, D.H.; Choi, H.S.; et al. Enzyme-amplified SERS immunoassay with Ag-Au bimetallic SERS hot spots. Nano Res. 2020, 13, 3338–3346. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. Gold nanoring core-shell satellites with abundant built-in hotspots and great analyte penetration: An immunoassay platform for the SERS/fluorescence-based detection of carcinoembryonic antigen. Chem. Eng. J. 2021, 409, 128173. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Q.; Ma, S.; Liu, H.; Dong, B.; Yang, J.; Liu, D. Prussian blue as a highly sensitive and background-free resonant Raman reporter. Anal. Chem. 2017, 89, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-D.; Zhang, R.; Chen, H.; Huang, Z.-P.; Ye, X.; Wang, H.; Deng, A.-M.; Kong, J.-L. An ultrasensitive polydopamine bi-functionalized SERS immunoassay for exosome-based diagnosis and classification of pancreatic cancer. Chem. Sci. 2018, 9, 5372–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, K.; Mei, Q.; Guo, X.; Xu, Y.; Yang, D.; Sánchez, B.J.; Sheng, B.; Liu, C.; Hu, Z.; Yu, G.; et al. Antimicrobial peptide based magnetic recognition elements and Au@Ag-GO SERS tags with stable internal standards: A three in one biosensor for isolation, discrimination and killing of multiple bacteria in whole blood. Chem. Sci. 2018, 9, 8781–8795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlebtsov, B.N.; Bratashov, D.N.; Byzova, N.A.; Dzantiev, B.B.; Khlebtsov, N.G. SERS-based lateral flow immunoassay of troponin I by using gap enhanced Raman tags. Nano Res. 2019, 12, 413–420. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Mei, R.; Yin, Y.; You, J.; Chen, L. Polystyrene encapsulated SERS tags as promising standard tools: Simple and universal in synthesis; highly sensitive and ultrastable for bioimaging. Anal. Chem. 2019, 91, 5270–5277. [Google Scholar] [CrossRef]
- Xie, L.; Lu, J.; Liu, T.; Chen, G.; Liu, G.; Ren, B.; Tian, Z. Key role of direct adsorption on SERS sensitivity: Synergistic effect among target, aggregating agent, and surface with Au or Ag colloid as surface-enhanced Raman spectroscopy substrate. J. Phys. Chem. Lett. 2020, 11, 1022–1029. [Google Scholar] [CrossRef]
- Zhu, R.; Feng, H.; Li, Q.; Su, L.; Fu, Q.; Li, J.; Song, J.; Yang, H. Asymmetric core-shell gold nanoparticles and controllable assemblies for SERS ratiometric detection of microRNA. Angew. Chem. Int. Ed. 2021, 60, 12560–12568. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; Álvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stéphan, O.; Kociak, M.; Liz-Marzán, L.M.; de Abajo, G.F.J. Zeptomol detection through controlled ultrasensitive surface-enhanced Raman scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, W.; El-Sayed, M.A. On the universal scaling behavior of the distance decay of plasmon coupling in metal nanoparticle pairs: A plasmon ruler equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Yan, B.; Thubagere, A.; Premasiri, W.R.; Ziegler, L.D.; Negro, L.D.; Reinhard, B.M. Engineered SERS substrates with multiscale signal enhancement: Nanoparticle cluster arrays. ACS Nano 2009, 3, 1190–1202. [Google Scholar] [CrossRef]
- Pastorello, M.; Sigoli, F.A.; dos Santos, D.P.; Mazali, I.O. On the use of Au@Ag core-shell nanorods for SERS detection of Thiram diluted solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118113. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Dong, L.; Liu, R.; Lu, S.; He, Z.; Shan, W.; Geng, F.; Cai, Y.; Liu, J. Synthesis of highly-branched Au@AgPd core/shell nanoflowers for in situ SERS monitoring of catalytic reactions. Chin. Chem. Lett. 2020, 31, 2437–2441. [Google Scholar] [CrossRef]
- Gu, W.; Zhao, Y.; Zhuang, S.; Zha, J.; Dong, J.; You, Q.; Gan, Z.; Xia, N.; Li, J.; Deng, H.; et al. Unravelling the structure of a medium-sized metalloid gold nanocluster and its filming property. Angew. Chem. Int. Ed. 2021, 60, 11184–11189. [Google Scholar] [CrossRef] [PubMed]
- Phung, V.-D.; Jung, W.-S.; Nguyen, T.-A.; Kim, J.-H.; Lee, S.-W. Reliable and quantitative SERS detection of dopamine levels in human blood plasma using a plasmonic Au/Ag nanocluster substrate. Nanoscale 2018, 10, 22493–22503. [Google Scholar] [CrossRef] [PubMed]
- Kuttner, C.; Höller, R.P.M.; Quintanilla, M.; Schnepf, M.J.; Dulle, M.; Fery, A.; Liz-Marzán, L.M. SERS and plasmonic heating efficiency from anisotropic core/satellite superstructures. Nanoscale 2019, 11, 17655–17663. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, X.; Zhang, Y. Photocatalytic properties of silver nanospherical arrays driven by surface plasmons. Chemosensors 2021, 9, 336. [Google Scholar] [CrossRef]
- Dang, H.; Park, S.; Wu, Y.; Choi, N.; Yang, J.; Lee, S.; Joo, S.; Chen, L.; Choo, J. Reproducible and sensitive plasmonic sensing platforms based on Au-nanoparticle-internalized nanodimpled substrates. Adv. Funct. Mater. 2021, 31, 2105703. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Li, J.; Tu, Z.; Gu, B.; Wang, S. Ultrasensitive and multiplex detection of four pathogenic bacteria on a bi-channel lateral flow immunoassay strip with three-dimensional membrane-like SERS nanostickers. Biosens. Bioelectron. 2022, 214, 114525. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and sensitive SERS platform with analyte enrichment and filtration function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef]
- Bertok, T.; Bertokova, A.; Hroncekova, S.; Chocholova, E.; Svecova, N.; Lorencova, L.; Kasak, P.; Tkac, J. Novel prostate cancer biomarkers: Aetiology, clinical performance and sensing applications. Chemosensors 2021, 9, 205. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, Z.; Guo, S.; Guo, D.; Wang, R. Coupling an artificial receptor with macrophage membrane for targeted and synergistic treatment of cholestasis. Supramol. Mater. 2022, 1, 100020. [Google Scholar]
- Qiao, N.; Zheng, J. Nonenzymatic glucose sensor based on glassy carbon electrode modified with a nanocomposite composed of nickel hydroxide and graphene. Microchim. Acta 2012, 177, 103–109. [Google Scholar] [CrossRef]
- Sarma, T.K.; Chowdhury, D.; Paul, A.; Chattopadhyay, A. Synthesis of Au nanoparticle–conductive polyaniline composite using H2O2 as oxidising as well as reducing agent. Chem. Commun. 2002, 10, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jiang, M.; Hu, Y.; Wu, L.; Zhao, K.; Xue, X.; Zheng, X. A new chemiluminescence method for the determination of 8-hydroxyguanine based on L-histidine bound nickel nanoparticles. Chem. Commun. 2020, 56, 6535–6538. [Google Scholar] [CrossRef]
- Qi, X.; Su, G.; Bo, G.; Cao, L.; Liu, W. Synthesis of NiO and NiO/TiO2 films with electrochromic and photocatalytic activities. Surf. Coat. Technol. 2015, 272, 79–85. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Huang, S.; Gao, Z.; Song, Y. Engineering Raspberry-like Plasmonic Nanoclusters as Tags in Surface-Enhanced Raman Scattering-Based Immunoassays. Chemosensors 2022, 10, 442. https://doi.org/10.3390/chemosensors10110442
Xu J, Huang S, Gao Z, Song Y. Engineering Raspberry-like Plasmonic Nanoclusters as Tags in Surface-Enhanced Raman Scattering-Based Immunoassays. Chemosensors. 2022; 10(11):442. https://doi.org/10.3390/chemosensors10110442
Chicago/Turabian StyleXu, Jingwen, Shizhen Huang, Zhida Gao, and Yanyan Song. 2022. "Engineering Raspberry-like Plasmonic Nanoclusters as Tags in Surface-Enhanced Raman Scattering-Based Immunoassays" Chemosensors 10, no. 11: 442. https://doi.org/10.3390/chemosensors10110442




