An Automated, Self-Powered, and Integrated Analytical Platform for On-Line and In Situ Air Quality Monitoring
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Preparation of Standard Gaseous Solutions of O3 and NO2
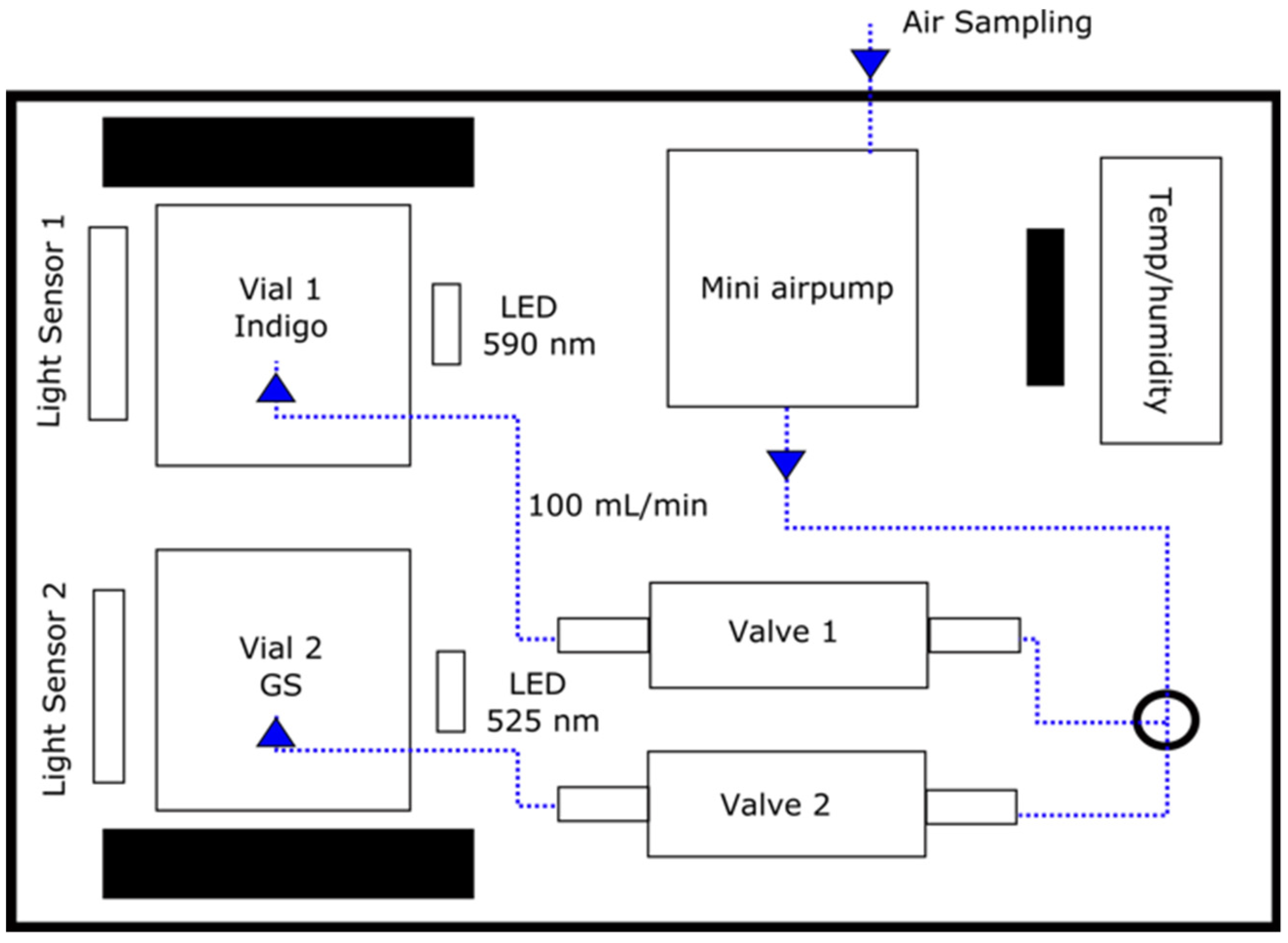
2.3. Analytical Platform for Gas Monitoring
2.4. Analysis Protocol
3. Results and Discussion
3.1. Choice of the Reagents and Evaluation of the Performance of the Detection Device
3.2. Ozone
3.3. Nitrogen Dioxide
3.4. Evaluation of Zero Air Bubbling in the Analytical Signal
3.5. Evaluation of Airflow and Sampling Time
3.6. Calibration with Standard Gaseous Solutions
3.7. Application of the Proposed Platform for Air Quality Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarasick, D.; Galbally, I.E.; Cooper, O.R.; Schultz, M.G.; Ancellet, G.; Leblanc, T.; Wallington, T.J.; Ziemke, J.; Liu, X.; Steinbacher, M.; et al. Tropospheric Ozone Assessment Report: Tropospheric Ozone from 1877 to 2016, Observed Levels, Trends and Uncertainties. Elementa 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Villena, G.; Bejan, I.; Kurtenbach, R.; Wiesen, P.; Kleffmann, J. Interferences of Commercial NO2 Instruments in the Urban Atmosphere and in a Smog Chamber. Atmos. Meas. Tech. 2012, 5, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Machado, C.M.D.; Cardoso, A.A.; Allen, A.G. Atmospheric Emission of Reactive Nitrogen during Biofuel Ethanol Production. Environ. Sci. Technol. 2008, 42, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Warmiński, K.; Bęś, A. Atmospheric Factors Affecting a Decrease in the Night-Time Concentrations of Tropospheric Ozone in a Low-Polluted Urban Area. Water Air Soil Pollut. 2018, 229, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passaretti Filho, J.; Da Silveira Petruci, J.F.; Cardoso, A.A. Development of a Simple Method for Determination of NO2 in Air Using Digital Scanner Images. Talanta 2015, 140, 73–80. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/naaqs/ozone-o3-air-quality-standards (accessed on 6 October 2022).
- Bell, M.; Ellis, H. Comparison of the 1-Hr and 8-Hr National Ambient Air Quality Standards for Ozone Using Models. J. Air Waste Manag. Assoc. 2003, 53, 1531–1540. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.epa.gov/naaqs/nitrogen-dioxide-no2-primary-air-quality-standards (accessed on 1 August 2022).
- Available online: https://www.who.int/news/item/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 1 August 2022).
- Aleixandre, M.; Gerboles, M. Review of Small Commercial Sensors for Indicative Monitoring of Ambient Gas. Chem. Eng. Trans. 2012, 30, 169–174. [Google Scholar] [CrossRef]
- Thompson, J.E. Crowd-Sourced Air Quality Studies: A Review of the Literature & Portable Sensors. Trends Environ. Anal. Chem. 2016, 11, 23–34. [Google Scholar] [CrossRef]
- Castell, N.; Dauge, F.R.; Schneider, P.; Vogt, M.; Lerner, U.; Fishbain, B.; Broday, D.; Bartonova, A. Can Commercial Low-Cost Sensor Platforms Contribute to Air Quality Monitoring and Exposure Estimates? Environ. Int. 2017, 99, 293–302. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- da Silveira Petruci, J.F.; Barreto, D.N.; Dias, M.A.; Felix, E.P.; Cardoso, A.A. Analytical Methods Applied for Ozone Gas Detection: A Review. TrAC—Trends Anal. Chem. 2022, 149, 116552. [Google Scholar] [CrossRef]
- Toma, C.; Alexandru, A.; Popa, M.; Zamfiroiu, A. IoT Solution for Smart Cities’ Pollution Monitoring and the Security Challenges. Sensors 2019, 19, 3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.; Han, S.; Zhang, X.; Yu, J.; Xiang, X.; Yang, J.; Qiao, L.; Zu, X.; Chen, Y.; Li, S. Ultrahigh oxygen evolution reaction activity in Au doped co-based nanosheets. RSC Adv. 2022, 12, 6205. [Google Scholar] [CrossRef] [PubMed]
- WangJian, J.; Changcang, C.; Ben, Q.; Wenting, N.; Yuchen, Z.; Yongqing, H.; Yongliang, F.; Tang, Y. Highly porous Fe2O3-SiO2 layer for acoustic wave based H2S sensing: Mass loading or elastic loading effects? Sens. Actuators B 2022, 367, 132160. [Google Scholar]
- Cerrato-Alvarez, M.; Miró-Rodríguez, C.; Pinilla-Gil, E. A Passive Sampling–Voltammetric Detection Approach Based on Screen-Printed Electrodes Modified with Indigotrisulfonate for the Determination of Ozone in Ambient Air. Sens. Actuators B Chem. 2018, 273, 735–741. [Google Scholar] [CrossRef]
- Lozano, A.; Usero, J.; Vanderlinden, E.; Raez, J.; Contreras, J.; Navarrete, B. Air Quality Monitoring Network Design to Control Nitrogen Dioxide and Ozone, Applied in Malaga, Spain. Microchem. J. 2009, 93, 164–172. [Google Scholar] [CrossRef]
- Garcia, G.; Allen, A.G.; Cardoso, A.A. Development of a Sensitive Passive Sampler Using Indigotrisulfonate for the Determination of Tropospheric Ozone. J. Environ. Monit. 2010, 12, 1325–1329. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Frutos-Puerto, S.; Miró-Rodríguez, C.; Pinilla-Gil, E. Measurement of Tropospheric Ozone by Digital Image Analysis of Indigotrisulfonate-Impregnated Passive Sampling Pads Using a Smartphone Camera. Microchem. J. 2020, 154, 104535. [Google Scholar] [CrossRef]
- Filho, J.P.; Costa, M.A.M.; Cardoso, A.A. A Micro-Impinger Sampling Device for Determination of Atmospheric Nitrogen Dioxide. Aerosol Air Qual. Res. 2019, 19, 2597–2603. [Google Scholar] [CrossRef]
- Castell, N.; Schneider, P.; Grossberndt, S.; Fredriksen, M.F.; Sousa Santos, G.; Vogt, M.; Bartonova, A. Localized Real-Time Information on Outdoor Air Quality at Kindergartens in Oslo, Norway Using Low-Cost Sensor Nodes. Environ. Res. 2018, 165, 410–419. [Google Scholar] [CrossRef]
- Cadeado, A.; Machado, C.; Oliveira, G.; e Silva, D.; Muñoz, R.; Silva, S. Internet of Things as a Tool for Sustainable Analytical Chemistry: A Review. J. Braz. Chem. Soc. 2022, 33, 681–692. [Google Scholar] [CrossRef]
- Cesar Souza Machado, C.; da Silveira Petruci, J.F.; Silva, S.G. An IoT Optical Sensor for Photometric Determination of Oxalate in Infusions. Microchem. J. 2021, 168, 106466. [Google Scholar] [CrossRef]
- Bui, D.A.; Hauser, P.C. Analytical Devices Based on Light-Emitting Diodes–A Review of the State-of-the-Art. Anal. Chim. Acta 2015, 853, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Petruci, J.F.d.S.; Batista, A.D. Novel Approaches for Colorimetric Measurements in Analytical Chemistry—A Review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Felix, E.P.; Cardoso, A.A. Colorimetric Determination of Ambient Ozone Using Indigo Blue Droplet. J. Braz. Chem. Soc. 2006, 17, 296–301. [Google Scholar] [CrossRef]
- Maruo, Y.Y.; Kunioka, T.; Akaoka, K.; Nakamura, J. Development and Evaluation of Ozone Detection Paper. Sens. Actuators B Chem. 2009, 135, 575–580. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic Paper-Based Analytical Device for the Determination of Nitrite and Nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Frutos-Puerto, S.; Arroyo, P.; Miró-Rodríguez, C.; Pinilla-Gil, E. A portable, low-cost, smartphone assisted methodology for on-site measurement of NO2 levels in ambient air by selective chemical reactivity and digital image analysis. Sens. Actuators B Chem. 2021, 338, 129867. [Google Scholar] [CrossRef]
- Caballero, S.; Esclapez, R.; Galind, N.; Mantilla, E.; Cresp, J. Use of a passive sampling network for the determination of urban NO2 spatiotemporal variations. Atmos. Environ. 2012, 63, 148–155. [Google Scholar] [CrossRef]
- Hossain, M.; Saffell, J.; Baron, R. Differentiating NO2 and O3 at low cost air quality amperometric gas sensors. ACS Sens. 2016, 1, 1291–1294. [Google Scholar] [CrossRef]
- Cavellin, L.D.; Weichenthal, S.; Tack, R.; Ragetti, M.S.; Smargiassi, A.; Hatzopoulou, M. Investigating the Use of Portable Air Pollution Sensors to Capture the Spatial Variability Of Traffic-Related Air Pollution. Environ. Sci. Technol. 2016, 50, 313–320. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Indigo | Nitrite (via GS) |
|---|---|---|
| Linear range | 5 to 100 µM | 1 to 100 µM |
| Correlation coefficient | 0.9997 | 0.9939 |
| Calibration equation | A = 0.0142 [ITS] + 0.0094 | A = 0.0114 [NO2−] + 0.1438 |
| Limit of Detection (3*SD/Slope) | 0.6 µM | 0.5 µM |
| Limit of Quantification (10*SD/Slope) | 1.9 µM | 1.6 µM |
| Ozone | Nitrogen Dioxide | |
|---|---|---|
| Linear Range | 40 to 130 ppbv | 3 to 200 ppbv |
| Equation | ABS = 0.006 [O3] − 0.0151 | ABS = 0.025 [NO2] + 0.0092 |
| R2 | 0.991 | 0.994 |
| Limit of Detection | 5 ppbv | 1 ppbv |
| Repeatability (RSD) | 2.7% (70 ppbv) | 2.2% (50 ppbv) |
| Sampling time | 60 min | |
| Sampling airflow | 100 mL min−1 | |
| Reagent volume | 800 µL | |
| Air Pollutants | Sampling System and Time | Detection System | Remarks | LOD (ppbv) | Ref. |
|---|---|---|---|---|---|
| NO2 | Filter paper/60 min | Smartphone (Digital image) | Off-line acquisition of the analytical signal. Low automatization | 3 | [31] |
| NO2 | Micro-impinger bubbler/60 min | Conventional spectrophotometer | Reagent solution must be transferred to the detection system. Off-line procedure | 7 | [22] |
| O3 | Cellulose pads/24 h | Smartphone (digital image) | 24 h of sampling time (passive sampling). Low automatization | 2 | [21] |
| O3 | Screen-printed electrodes/5 h | Portable potentiostat | Passive sampling extends the sampling time. Measurement performed in the lab | 0.8 | [18] |
| NO2 | Glass fiber filter/1 week | Ion chromatography | Not suitable to detect short time (i.e., 1 h) variations in NO2 concentration. High cost instrumentation | Not available | [32] |
| NO2 and O3 | Glass fiber filter/2 weeks | Conventional spectrophotometer and Ion chromatography | Not suitable to detect short time (i.e., 1 h) variations in NO2 and O3 concentrations. High cost instrumentation and off-line procedure | Not available | [19] |
| NO2 and O3 | Electrochemical sensor with MnO2 microparticles | Amperometry | Affected by humidity and validated with high concentration of the pollutants | Not available | [33] |
| NO2 and O3 | Aeroqual sensors (MOX)/20 min | Conductivity | Tendency to overestimate NO2 and O3 concentrations. | Not available | [34] |
| NO2 and O3 | Micro-impinger bubbler/60 min | Digital light sensors/Arduino | All analytical steps are performed in the same platform. Automatic data transmission and storage | 1 and 5 |
| Hour | O3 Platform (ppbv) | O3 Air Quality Station (ppbv) | NO2 Platform (ppbv) | NO2 Air Quality Station (ppbv) | Temp (°C) and RH (%) |
|---|---|---|---|---|---|
| 2–3 p.m. | 69 | 54 | <LOQ | 2 | 27/30 |
| 3–4 p.m. | 71 | 56 | 4 | 3 | 27/27 |
| 4–5 p.m. | 53 | 55 | 6 | 4 | 27/27 |
| 5–6 p.m. | 52 | 49 | 12 | 8 | 27/27 |
| 6–7 p.m. | <LOQ * | 28 | 26 | 20 | 26/30 |
| 7–8 p.m. | <LOQ | 26 | 26 | 23 | 24/34 |
| 8–9 p.m. | <LOQ | 24 | 28 | 25 | 23/37 |
| Hour | O3 (ppbv) | NO2 (ppbv) | Temp (°C) and RH (%) |
|---|---|---|---|
| 8–9 a.m. | <LOQ | 14 | 17/59 |
| 9–10 a.m. | <LOQ | 2 | 17/59 |
| 10–11a.m. | <LOQ | 3 | 18/55 |
| 11–12 a.m. | <LOQ | 5 | 20/52 |
| 12–1 p.m. | 47 | <LOQ | 22/46 |
| 1–2 p.m. | 41 | <LOQ | 24/44 |
| 2–3 p.m. | 40 | <LOQ | 25/38 |
| 3–4 p.m. | 42 | <LOQ | 26/31 |
| 4–5 p.m. | <LOQ | 6 | 26/31 |
| 5–6 p.m. | <LOQ | 22 | 26/34 |
| 6–7 p.m. | <LOQ | 33 | 26/34 |
| 7–8 p.m. | <LOQ | 59 | 26/31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Sousa, D.; Leal, V.G.; dos Reis, G.T.; da Silva, S.G.; Cardoso, A.A.; da Silveira Petruci, J.F. An Automated, Self-Powered, and Integrated Analytical Platform for On-Line and In Situ Air Quality Monitoring. Chemosensors 2022, 10, 454. https://doi.org/10.3390/chemosensors10110454
da Silva Sousa D, Leal VG, dos Reis GT, da Silva SG, Cardoso AA, da Silveira Petruci JF. An Automated, Self-Powered, and Integrated Analytical Platform for On-Line and In Situ Air Quality Monitoring. Chemosensors. 2022; 10(11):454. https://doi.org/10.3390/chemosensors10110454
Chicago/Turabian Styleda Silva Sousa, Danielle, Vanderli Garcia Leal, Gustavo Trindade dos Reis, Sidnei Gonçalves da Silva, Arnaldo Alves Cardoso, and João Flávio da Silveira Petruci. 2022. "An Automated, Self-Powered, and Integrated Analytical Platform for On-Line and In Situ Air Quality Monitoring" Chemosensors 10, no. 11: 454. https://doi.org/10.3390/chemosensors10110454





