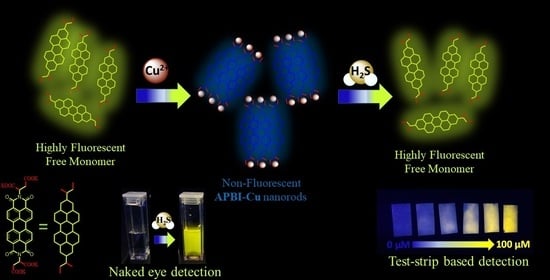
A Dual Fluorometric and Colorimetric Sulfide Sensor Based on Coordinating Self-Assembled Nanorods: Applicable for Monitoring Meat Spoilage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentations
2.2. Synthesis of APBI-Cu Nanorods
2.3. Fluorescence Sensing Experiment in Water
2.4. Detection of Sulfide in Chicken Sample
3. Results and Discussions
3.1. Design and Synthesis of APBI-Cu Nanorods
3.2. Detection of Sulfide in Water
3.3. Detection of Sulfide in Water and Meat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Chan, A.; Ali, S.; Saha, A.; Haushalter, K.J.; Lam, W.-L.M.; Glasheen, M.; Parker, J.; Brenner, M.; Mahon, S.B. Hydrogen sulfide—Mechanisms of toxicity and development of an antidote. Sci. Rep. 2016, 6, 20831. [Google Scholar] [CrossRef]
- Ren, M.; Xu, Q.; Bai, Y.; Wang, S.; Kong, F. Construction of a dual-response fluorescent probe for copper (II) ions and hydrogen sulfide (H2S) detection in cells and its application in exploring the increased copper-dependent cytotoxicity in present of H2S. Spectrochim. Acta Part A 2021, 249, 119299. [Google Scholar] [CrossRef]
- Chu, L.; Dong, Z.; Xu, X.; Cochran, D.L.; Ebersole, J.L. Role of glutathione metabolism of Treponema denticola in bacterial growth and virulence expression. Infect. Immun. 2002, 70, 1113–1120. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; De La Cruz, L.K.; Roy Choudhury, M.; Anifowose, A.; Wang, B. Toward hydrogen sulfide based therapeutics: Critical drug delivery and developability issues. Med. Res. Rev. 2018, 38, 57–100. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Panagaki, T.; Randi, E.B.; Augsburger, F.; Szabo, C. Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 18769–18771. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Yang, Y.; Wang, H. The Role of H2S Regulating NLRP3 Inflammasome in Diabetes. Int. J. Mol. Sci. 2022, 23, 4818. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 2005, 42, 539–548. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen sulfide: Advances in understanding human toxicity. Int. J. Toxicol. 2010, 29, 569–581. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Hao, M.; Yu, L.L.; Li, Y. A novel selective detection method for sulfide in food systems based on the GMP-Cu nanozyme with laccase activity. Talanta 2021, 235, 122775. [Google Scholar] [CrossRef]
- Singh, S.; Shin, Y.; Lee, Y.S. Antimicrobial seafood packaging: A review. J. Food Sci. Technol. 2016, 53, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Wang, J.; Zhang, Z.; Du, X.; Zhang, J.; Wang, J. Near-infrared fluorescent probe with a large Stokes shift for detection of hydrogen sulfide in food spoilage, living cells, and zebrafish. J. Agric. Food Chem. 2022, 70, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Lynch, M.J.; Crane, B.R. Design, validation, and application of an enzyme-coupled hydrogen sulfide detection assay. Biochemistry 2018, 58, 474–483. [Google Scholar] [CrossRef]
- Jarosz, A.P.; Yep, T.; Mutus, B. Microplate-based colorimetric detection of free hydrogen sulfide. Anal. Chem. 2013, 85, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Qazi, F.; Hussain, Z.; Idrees, M.U.; Soomro, S.; Soomro, S. Recent trends in electrochemical detection of NH3, H2S and NOx gases. Int. J. Electrochem. Sci. 2017, 12, 1711–1733. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Cui, L.; Zheng, F.; Song, Q. Electroactive Au@ Ag nanoparticles driven electrochemical sensor for endogenous H2S detection. Biosens. Bioelectron. 2018, 117, 53–59. [Google Scholar] [CrossRef]
- Govardhan, K.; Grace, A.N. Metal/metal oxide doped semiconductor based metal oxide gas sensors—A review. Sens. Lett. 2016, 14, 741–750. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Lumbreras, M.; Schoonman, J.; Siadat, M. Electrosprayed metal oxide semiconductor films for sensitive and selective detection of hydrogen sulfide. Sensors 2009, 9, 9122–9132. [Google Scholar] [CrossRef]
- Du, J.; Wang, J.; Huang, W.; Deng, Y.; He, Y. Visible light-activatable oxidase mimic of 9-mesityl-10-methylacridinium ion for colorimetric detection of biothiols and logic operations. Anal. Chem. 2018, 90, 9959–9965. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Gomes, P.C.F.L.; da Petruci, S.J.F.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry–A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Singh, A.; Jose, D.A. Colorimetric sensor for the detection of H2S and its application in molecular half-subtractor. Anal. Chim. Acta 2018, 1040, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sahoo, P. A colorimetric sensor for hydrogen sulfide: Detection from biogas and quantitative estimation in water. Sens. Actuators B 2019, 291, 287–292. [Google Scholar] [CrossRef]
- Cho, S.H.; Suh, J.M.; Eom, T.H.; Kim, T.; Jang, H.W. Colorimetric sensors for toxic and hazardous gas detection: A review. Electron. Mater. Lett. 2021, 17, 1–17. [Google Scholar] [CrossRef]
- Kang, S.; Oh, J.; Han, M.S. A colorimetric sensor for hydrogen sulfide detection using direct inhibition of active site in G-quadruplex DNAzyme. Dyes Pigm. 2017, 139, 187–192. [Google Scholar] [CrossRef]
- Sen, A.; Albarella, J.D.; Carey, J.R.; Kim, P.; McNamara III, W.B. Low-cost colorimetric sensor for the quantitative detection of gaseous hydrogen sulfide. Sens. Actuators B 2008, 134, 234–237. [Google Scholar] [CrossRef]
- Alyan, A.K.; Hanafi, R.S.; Gad, M.Z. Point-of-care testing and optimization of sample treatment for fluorometric determination of hydrogen sulphide in plasma of cardiovascular patients. J. Adv. Res. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Chemchem, M.; Chemchem, A.; Aydıner, B.; Seferoğlu, Z. Recent advances in colorimetric and fluorometric sensing of neurotransmitters by organic scaffolds. Eur. J. Med. Chem. 2022, 244, 114820. [Google Scholar] [CrossRef]
- Yu, X.; Gong, Y.; Ji, H.; Cheng, C.; Lv, C.; Zhang, Y.; Zang, L.; Zhao, J.; Che, Y. Rapid Assessment of Meat Freshness by the Differential Sensing of Organic Sulfides Emitted during Spoilage. ACS Sens. 2022, 7, 1395–1402. [Google Scholar] [CrossRef]
- Hou, F.; Cheng, J.; Xi, P.; Chen, F.; Huang, L.; Xie, G.; Shi, Y.; Liu, H.; Bai, D.; Zeng, Z. Recognition of copper and hydrogen sulfide in vitro using a fluorescein derivative indicator. Dalton Trans. 2012, 41, 5799–5804. [Google Scholar] [CrossRef]
- Wu, S.; Ma, X.; Wang, Y.; Zhou, J.; Li, X.; Wang, X. A novel fluorescent BODIPY-based probe for detection of Cu2+ and H2S based on displacement approach. Spectrochim. Acta Part A 2021, 249, 119330. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn (II) complexes as probes for detecting hydrogen sulfide and its anion: Bioimaging applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Zhao, M.; Lu, F.; Zheng, L. Formation of supermolecular chiral gels from L-aspartic acid-based perylenebisimides and benzene dicarboxylic acids. New J. Chem. 2017, 41, 7643–7649. [Google Scholar] [CrossRef]
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.-T.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Wang, Y.; Carrazzone, R.J.; Matson, J.B. A persulfide donor responsive to reactive oxygen species: Insights into reactivity and therapeutic potential. Angew. Chem. Int. Ed. 2018, 130, 6432–6436. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Chi, Y.; Yu, H.; Li, Y.; Jiang, T.; Wei, X.; Shi, J. Self-assembly, optical and electrical properties of perylene diimide dyes bearing unsymmetrical substituents at bay position. Sci. Rep. 2018, 8, 8208. [Google Scholar] [CrossRef]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Datar, A.; Balakrishnan, K.; Zang, L. One-dimensional self-assembly of a water soluble perylene diimide molecule by pH triggered hydrogelation. Chem. Commun. 2013, 49, 6894–6896. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S.; Singha, N.; Das, D. Self-Assembly Assisted Tandem Sensing of Pd2+ and CN− by a Perylenediimide-Peptide Conjugate. ChemistrySelect 2017, 2, 10061–10066. [Google Scholar] [CrossRef]
- Muthuraj, B.; Chowdhury, S.R.; Mukherjee, S.; Patra, C.R.; Iyer, P.K. Aggregation deaggregation influenced selective and sensitive detection of Cu 2+ and ATP by histidine functionalized water-soluble fluorescent perylene diimide under physiological conditions and in living cells. RSC Adv. 2015, 5, 28211–28218. [Google Scholar] [CrossRef]
- Feng, X.; An, Y.; Yao, Z.; Li, C.; Shi, G. A turn-on fluorescent sensor for pyrophosphate based on the disassembly of Cu2+-mediated perylene diimide aggregates. ACS Appl. Mater. Interfaces 2012, 4, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Datar, A.; Naddo, T.; Huang, J.; Oitker, R.; Yen, M.; Zhao, J.; Zang, L. Effect of side-chain substituents on self-assembly of perylene diimide molecules: Morphology control. J. Am. Chem. Soc. 2006, 128, 7390–7398. [Google Scholar] [CrossRef]
- Dalapati, R.; Balaji, S.; Trivedi, V.; Khamari, L.; Biswas, S. A dinitro-functionalized Zr (IV)-based metal-organic framework as colorimetric and fluorogenic probe for highly selective detection of hydrogen sulphide. Sens. Actuators B 2017, 245, 1039–1049. [Google Scholar] [CrossRef]
- Sheals, J.; Persson, P.; Hedman, B. IR and EXAFS spectroscopic studies of glyphosate protonation and copper (II) complexes of glyphosate in aqueous solution. Inorg. Chem. 2001, 40, 4302–4309. [Google Scholar] [CrossRef] [PubMed]
- Karaliota, A.; Kretsi, O.; Tzougraki, C. Synthesis and characterization of a binuclear coumarin-3-carboxylate copper (II) complex. J. Inorg. Biochem. 2001, 84, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, W.; Wang, L.; Zhu, X.; Zhang, Y.; Qu, P.; Liu, L.; Zhou, B.; Liu, Y.-N.; Xu, M. A retrievable, water-soluble and biocompatible fluorescent probe for recognition of Cu (II) and sulfide based on a peptide receptor. Talanta 2015, 143, 307–314. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.-J.; Fan, H.-L.; Shangguan, J.; Wang, H.; Mi, J. Removal of sulfur compounds by a copper-based metal organic framework under ambient conditions. Energy Fuels 2015, 29, 298–304. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Hussein, H.; Mohammed, M.S. Bio-removal of Pb, Cu, and Ni from solutions as nano-carbonates using a plant-derived urease enzyme–urea mixture. Environ. Sci. Pollut. Res. 2020, 27, 30741–30754. [Google Scholar] [CrossRef]
- Han, X.; Gu, C.; Ding, Y.; Yu, J.; Li, K.; Zhao, D.; Chen, B. Stable Eu3+/Cu2+-functionalized supramolecular Zinc (II) complexes as fluorescent probes for turn-on and ratiometric detection of hydrogen sulfide. ACS Appl. Mater. Interfaces 2021, 13, 20371–20379. [Google Scholar] [CrossRef]
- Kaushik, R.; Sakla, R.; Ghosh, A.; Selvan, G.T.; Selvakumar, P.M.; Jose, D.A. Selective detection of H2S by copper complex embedded in vesicles through metal indicator displacement approach. ACS Sens. 2018, 3, 1142–1148. [Google Scholar] [CrossRef]
- Lou, X.; Mu, H.; Gong, R.; Fu, E.; Qin, J.; Li, Z. Displacement method to develop highly sensitive and selective dual chemosensor towards sulfide anion. Analyst 2011, 136, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yin, J.; Yoon, J. A multi-responsive cyanine-based colorimetric chemosensor containing dipicolylamine moieties for the detection of Zn (II) and Cu (II) ions. Sens. Actuators B 2016, 230, 40–45. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Zhang, J.; Cheng, N.; Zhang, L.; Zhang, Z.; Yao, Z. Rapid detection of hydrogen sulfide in vegetables and monosodium glutamate based on perylene supramolecular aggregates using an indicator displacement assays strategy. Spectrochim. Acta Part A 2022, 276, 121223. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Lee, L.-Y.; Wang, Y.-L.; Chen, Y.-J.; Chen, C.-Y.; Wang, Y.-M. A water soluble and fast response fluorescent turn-on copper complex probe for H2S detection in zebra fish. Talanta 2016, 147, 445–452. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, R.; Song, Y.; Xing, K.; Wang, P.; Yang, Y. Dual-Emitting Eu (III)–Cu (II) heterometallic–organic framework: Simultaneous, selective, and sensitive detection of hydrogen sulfide and ascorbic acid in a wide range. ACS Appl. Mater. Interfaces 2018, 10, 32698–32706. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a source and target of H2S to improve its trophism and function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef]
- Yang, Q.; He, G.-W. Imbalance of homocysteine and H2S: Significance, mechanisms, and therapeutic promise in vascular injury. Oxid. Med. Cell. Longevity 2019, 2019, 11. [Google Scholar] [CrossRef]
- Yu, Y.; Li, G.; Wu, D.; Zheng, F.; Zhang, X.; Liu, J.; Hu, N.; Wang, H.; Wu, Y. Determination of hydrogen sulfide in wines based on chemical-derivatization-triggered aggregation-induced emission by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2019, 68, 876–883. [Google Scholar] [CrossRef]
- Yoshinari, N.; Kuwamura, N.; Kojima, T.; Konno, T. Development of coordination chemistry with thiol-containing amino acids. Coord. Chem. Rev. 2023, 474, 214857. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Hasanzadeh, M.; Seidi, F.; Pashazadeh-Panahi, P. An innovative colorimetric platform for the low-cost and selective identification of Cu (II), Fe (III), and Hg (II) using GQDs-DPA supported amino acids by microfluidic paper-based (µPADs) device: Multicolor plasmonic patterns. J. Environ. Chem. Eng. 2021, 9, 106197. [Google Scholar] [CrossRef]
- Pang, X.; Gao, L.; Feng, H.; Li, X.; Kong, J.; Li, L. A peptide-based multifunctional fluorescent probe for Cu 2+, Hg 2+ and biothiols. New J. Chem. 2018, 42, 15770–15777. [Google Scholar] [CrossRef]
- Mansur, A.R.; Seo, D.-H.; Song, E.-J.; Song, N.-E.; Hwang, S.H.; Yoo, M.; Nam, T.G. Identifying potential spoilage markers in beef stored in chilled air or vacuum packaging by HS-SPME-GC-TOF/MS coupled with multivariate analysis. LWT 2019, 112, 108256. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Jose, D.A. Recent progress in hydrogen sulphide (H2S) sensors by metal displacement approach. Coord. Chem. Rev. 2017, 347, 141–157. [Google Scholar] [CrossRef]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2021, 27, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.A.; Sharma, N.; Sakla, R.; Kaushik, R.; Gadiyaram, S. Fluorescent nanoprobes for the sensing of gasotransmitters hydrogen sulfide (H2S), nitric oxide (NO) and carbon monoxide (CO). Methods 2019, 168, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M. Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim. Acta Part A 2009, 71, 1901–1906. [Google Scholar] [CrossRef]
- Etaiw, S.E.d.H.; El-bendary, M.M. Crystal structure, characterization and catalytic activities of Cu (II) coordination complexes with 8-hydroxyquinoline and pyrazine-2-carboxylic acid. Appl. Organomet. Chem. 2018, 32, e4213. [Google Scholar] [CrossRef]
- Lloyd, D. Hydrogen sulfide: Clandestine microbial messenger? Trends Microbiol. 2006, 14, 456–462. [Google Scholar] [CrossRef]
- Li, Y.; Alaimo, C.P.; Kim, M.; Kado, N.Y.; Peppers, J.; Xue, J.; Wan, C.; Green, P.G.; Zhang, R.; Jenkins, B.M. Composition and toxicity of biogas produced from different feedstocks in California. Environ. Sci. Technol. 2019, 53, 11569–11579. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Sun, X.; Wei, W.; Mu, X.; Ahmad, W.; Hassan, M.M.; Ouyang, Q.; Chen, Q. Fabricating a nano-bionic sensor for rapid detection of H2S during pork spoilage using Ru NPs modulated catalytic hydrogenation conversion. Meat Sci. 2021, 177, 108507. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Y.; Luo, F.; Lin, C.; Wang, J.; Qiu, B.; Lin, Z. Multicolor hydrogen sulfide sensor for meat freshness assessment based on Cu2+-modified boron nitride nanosheets-supported subnanometer gold nanoparticles. Food Chem. 2022, 381, 132278. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Zhou, P.; Dong, C.; Ma, J. Sequential recognition of zinc ion and hydrogen sulfide by a new quinoline derivative with logic gate behavior. RSC Adv. 2014, 4, 18270–18277. [Google Scholar] [CrossRef]
- Gao, L.-L.; Wang, B.-B.; Chen, X.; Wang, Y.; Wu, W.-N.; Zhao, X.-L.; Yan, L.-L.; Fan, Y.-C.; Xu, Z.-H. Hydrazone derivative bearing coumarin for the relay detection of Cu2+ and H2S in an almost neat aqueous solution and bioimaging in lysosomes. Spectrochim. Acta Part A 2021, 255, 119693. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y. Responsive lanthanide coordination polymer for hydrogen sulfide. Anal. Chem. 2013, 85, 11020–11025. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Lv, K.; Zhong, W.; Wang, H.; Wu, Z.; Yi, P.; Jiang, J. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper (II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Zheng, C.-L.; Ding, S.-N. Label-free detection of sulfide ions based on fluorescence quenching of unmodified core–shell Au@ Ag nanoclusters. RSC Adv. 2014, 4, 9825–9829. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Abdollahi, H. Metal-ion-mediated fluorescent carbon dots for indirect detection of sulfide ions. Sens. Actuators B 2016, 230, 289–297. [Google Scholar] [CrossRef]
- Hai, Z.; Bao, Y.; Miao, Q.; Yi, X.; Liang, G. Pyridine–biquinoline–metal complexes for sensing pyrophosphate and hydrogen sulfide in aqueous buffer and in cells. Anal. Chem. 2015, 87, 2678–2684. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Zhou, P.; Dong, C.; Ma, J. An “off–on–off” fluorescent probe for the sequential detection of Zn 2+ and hydrogen sulfide in aqueous solution. New J. Chem. 2014, 38, 1802–1808. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalapati, R.; Hunter, M.; Zang, L. A Dual Fluorometric and Colorimetric Sulfide Sensor Based on Coordinating Self-Assembled Nanorods: Applicable for Monitoring Meat Spoilage. Chemosensors 2022, 10, 500. https://doi.org/10.3390/chemosensors10120500
Dalapati R, Hunter M, Zang L. A Dual Fluorometric and Colorimetric Sulfide Sensor Based on Coordinating Self-Assembled Nanorods: Applicable for Monitoring Meat Spoilage. Chemosensors. 2022; 10(12):500. https://doi.org/10.3390/chemosensors10120500
Chicago/Turabian StyleDalapati, Rana, Matthew Hunter, and Ling Zang. 2022. "A Dual Fluorometric and Colorimetric Sulfide Sensor Based on Coordinating Self-Assembled Nanorods: Applicable for Monitoring Meat Spoilage" Chemosensors 10, no. 12: 500. https://doi.org/10.3390/chemosensors10120500
APA StyleDalapati, R., Hunter, M., & Zang, L. (2022). A Dual Fluorometric and Colorimetric Sulfide Sensor Based on Coordinating Self-Assembled Nanorods: Applicable for Monitoring Meat Spoilage. Chemosensors, 10(12), 500. https://doi.org/10.3390/chemosensors10120500