3D Printing Technologies in Biosensors Production: Recent Developments
Abstract
:1. Introduction
- Binder jetting (BJ): in this process a liquid bonding agent is selectively deposited to join powder materials.
- Directed energy deposition (DED): refers to an AM technique known by other names such as laser-engineered net shaping (LENS), direct metal deposition (DMD), electron beam additive manufacturing (EBAM), directed light fabrication, and 3D laser cladding, where thermal energy is used to melt the raw materials in layer-by-layer fashion.
- Material extrusion (ME): refers to a process in which the material is extruded through a nozzle or orifice.
- Material jetting (MJ): refers to the selective deposition of droplets of materials, including photopolymer and wax.
- Powder bed fusion (PBF): refers to an AM method in which regions of a powder bed are fused by thermal energy.
- Sheet lamination (SL): refers to an AM process in which sheets of material are bonded to create the final object.
- Vat photopolymerization (VP): refers to an AM process in which the object is created from a liquid photopolymer in a vat cured by light-activated polymerization.
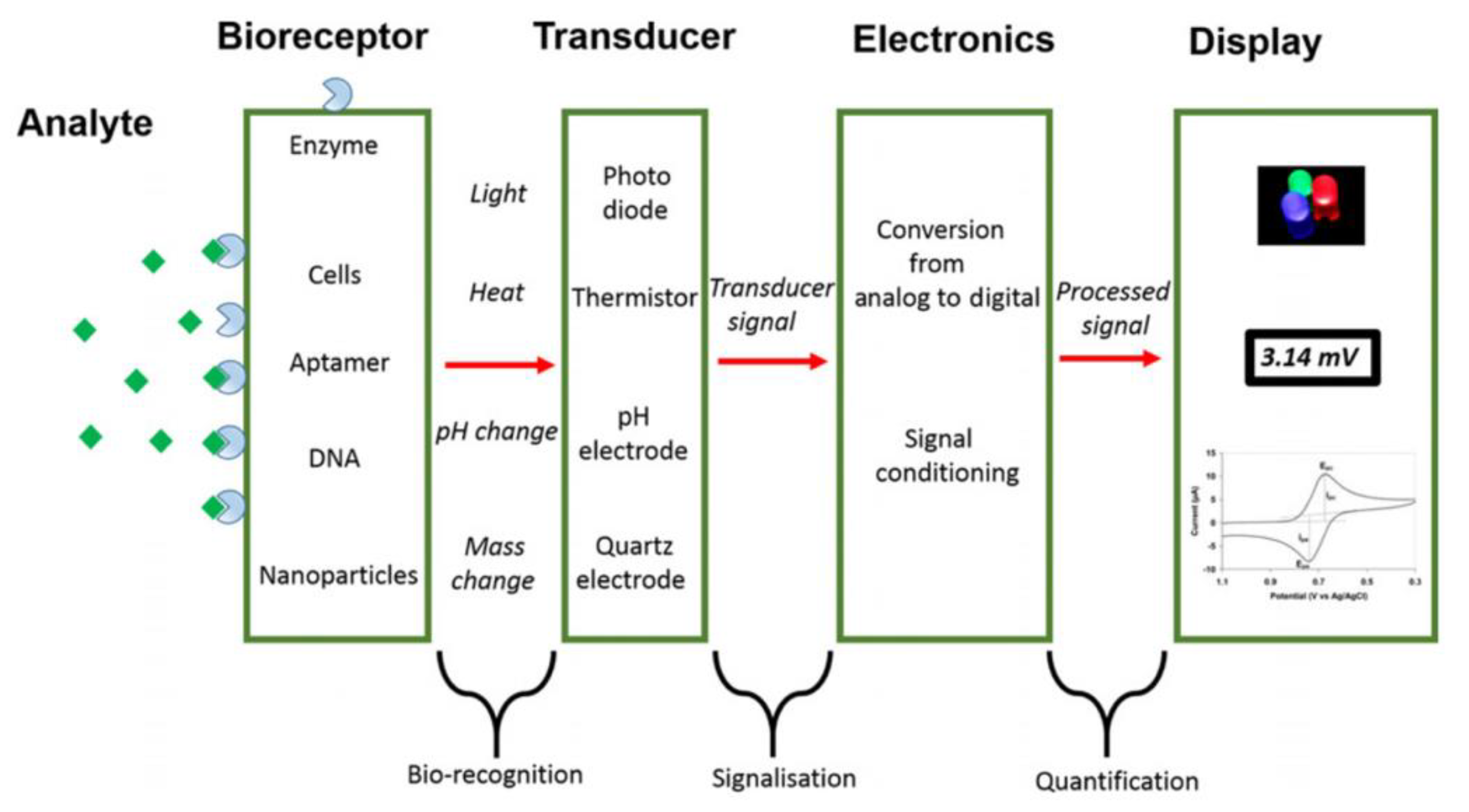
- Analyte: the species of interest to be identified during the analysis.
- Bioreceptor: the species that selectively recognizes the analyte. Naturally occurring or in vitro expressed molecules such enzymes, cells, aptamers, deoxyribonucleic acid (DNA), or antibodies can be used for the analyte bio-recognition process, generating a detectable signal in the form of light, heat, pH, charge, or mass change, etc.
- Transducer: a device capable of converting the analyte/receptor binding into measurable optical or electrical signals that are usually proportional to the amount or concentration of the analyte.
- Display: the system, such as the liquid crystal display of a computer or a direct printer, that generates the numeric or graphical results of the biosensor analysis.
2. 3D Printing and Biosensors Production
2.1. Material Extrusion and Biosensors
2.1.1. Material Extrusion for Biomedical Applications
2.1.2. Material Extrusion for Biophysical Studies, Electrochemical Measurements, and Enzyme-Based Ink Development
2.1.3. Material Extrusion for Mycotoxins Analysis of Food and Feed
2.1.4. Material Extrusion Applied to Environmental Safety Monitoring
2.2. Vat Photopolymerization and Biosensors
2.2.1. Vat Photopolymerization for High-Resolution 3D Printing of Biomedical Devices
2.2.2. Vat Photopolymerization and Food Safety Evaluation
2.2.3. Vat Photopolymerization as a Prominent tool for Biosensor Manufacturing Optimization
2.3. Material Jetting and Biosensors
2.3.1. Material Jetting 3D Printing for Healthcare Monitoring
2.3.2. Material Jetting for Environmental Toxicology: Freshwater Pollutants Detection
2.4. Other 3D Printing Technologies and Biosensors
3. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Rights and Permissions
References
- Charles, W.H. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4,575,330, 11 March 1986. [Google Scholar]
- Wang, Y.; Xu, Z.; Wu, D.; Bai, J. Current Status and Prospects of Polymer Powder 3D Printing Technologies. Materials 2020, 13, 2406. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.; Didier, C.M.; Sommerhage, F.; Rajaraman, S. Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells. Biosensors 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D Printing: Approaches and Bioapplications. Biosens. Bioelectron. 2021, 175, 112849. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- ASTM International. Standard Terminology for Additive Manufacturing Technologies: Designation F2792-12a; ASTM-Standards, F2792; ASTM International: West Conshohocken, CA, USA, 2012. [Google Scholar]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [Green Version]
- Braunger, M.L.; Fier, I.; Rodrigues, V.; Arratia, P.E.; Riul, A. Microfluidic Mixer with Automated Electrode Switching for Sensing Applications. Chemosensors 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for Chemists of Terms Used in Biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef] [Green Version]
- Fracchiolla, N.; Artuso, S.; Cortelezzi, A. Biosensors in Clinical Practice: Focus on Oncohematology. Sensors 2013, 13, 6423–6447. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.C.; Lyons, C. Electrode Systems For Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 2006, 102, 29–45. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and Sensibility. Chem. Soc. Rev. 2013, 42, 3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Ji, R.; Long, K.; Bu, T.; Chen, K.; Zhuang, S. A Review of 3D-Printed Sensors. Appl. Spectrosc. Rev. 2017, 52, 623–652. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. 3D-Printed Sensors: Current Progress and Future Challenges. Sens. Actuators A Phys. 2020, 305, 111916. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors-A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Kadimisetty, K.; Bhalerao, K.S.; Chen, T.; Rusling, J.F. 3D-Printed Immunosensor Arrays for Cancer Diagnostics. Sensors 2020, 20, 4514. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.C.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-Printed Graphene Direct Electron Transfer Enzyme Biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. Smartphone-Addressable 3D-Printed Electrochemical Ring for Nonenzymatic Self-Monitoring of Glucose in Human Sweat. Anal. Chem. 2021, 93, 3331–3336. [Google Scholar] [CrossRef]
- Wang, L.; Gao, W.; Ng, S.; Pumera, M. Chiral Protein-Covalent Organic Framework 3D-Printed Structures as Chiral Biosensors. Anal. Chem. 2021, 93, 5277–5283. [Google Scholar] [CrossRef]
- Cheng, H.; Yi, L.; Wu, J.; Li, G.; Zhao, G.; Xiao, Z.; Hu, B.; Zhao, L.; Tian, J. Drug Preconcentration and Direct Quantification in Biofluids Using 3D-Printed Paper Cartridge. Biosens. Bioelectron. 2021, 189, 113266. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, A.K.; Papadakis, G.; Parasyris, K.; Stavrinidis, A.; Gizeli, E. 3D-Printed Bioreactors for DNA Amplification: Application to Companion Diagnostics. Sens. Actuators B Chem. 2020, 319, 128161. [Google Scholar] [CrossRef]
- Mojena-Medina, D.; Hubl, M.; Bäuscher, M.; Jorcano, J.L.; Ngo, H.-D.; Acedo, P. Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors. Sensors 2020, 20, 5711. [Google Scholar] [CrossRef]
- Parate, K.; Rangnekar, S.V.; Jing, D.; Mendivelso-Perez, D.L.; Ding, S.; Secor, E.B.; Smith, E.A.; Hostetter, J.M.; Hersam, M.C.; Claussen, J.C. Aerosol-Jet-Printed Graphene Immunosensor for Label-Free Cytokine Monitoring in Serum. ACS Appl. Mater. Interfaces 2020, 12, 8592–8603. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Naranjo, J.E.; Perez-Gonzalez, V.H.; Mata-Gómez, M.A.; Aguilar, O. 3D-Printed Hybrid-Carbon-Based Electrodes for Electroanalytical Sensing Applications. Electrochem. Commun. 2021, 130, 107098. [Google Scholar] [CrossRef]
- Crevillen, A.G.; Mayorga-Martinez, C.C.; Zelenka, J.; Rimpelová, S.; Ruml, T.; Pumera, M. 3D-Printed Transmembrane Glycoprotein Cancer Biomarker Aptasensor. Appl. Mater. Today 2021, 24, 101153. [Google Scholar] [CrossRef]
- He, Z.; Huffman, J.; Curtin, K.; Garner, K.L.; Bowdridge, E.C.; Li, X.; Nurkiewicz, T.R.; Li, P. Composable Microfluidic Plates (CPlate): A Simple and Scalable Fluid Manipulation System for Multiplexed Enzyme-Linked Immunosorbent Assay (ELISA). Anal. Chem. 2021, 93, 1489–1497. [Google Scholar] [CrossRef]
- Samarentsis, A.G.; Pantazis, A.K.; Tsortos, A.; Friedt, J.-M.; Gizeli, E. Hybrid Sensor Device for Simultaneous Surface Plasmon Resonance and Surface Acoustic Wave Measurements. Sensors 2020, 20, 6177. [Google Scholar] [CrossRef]
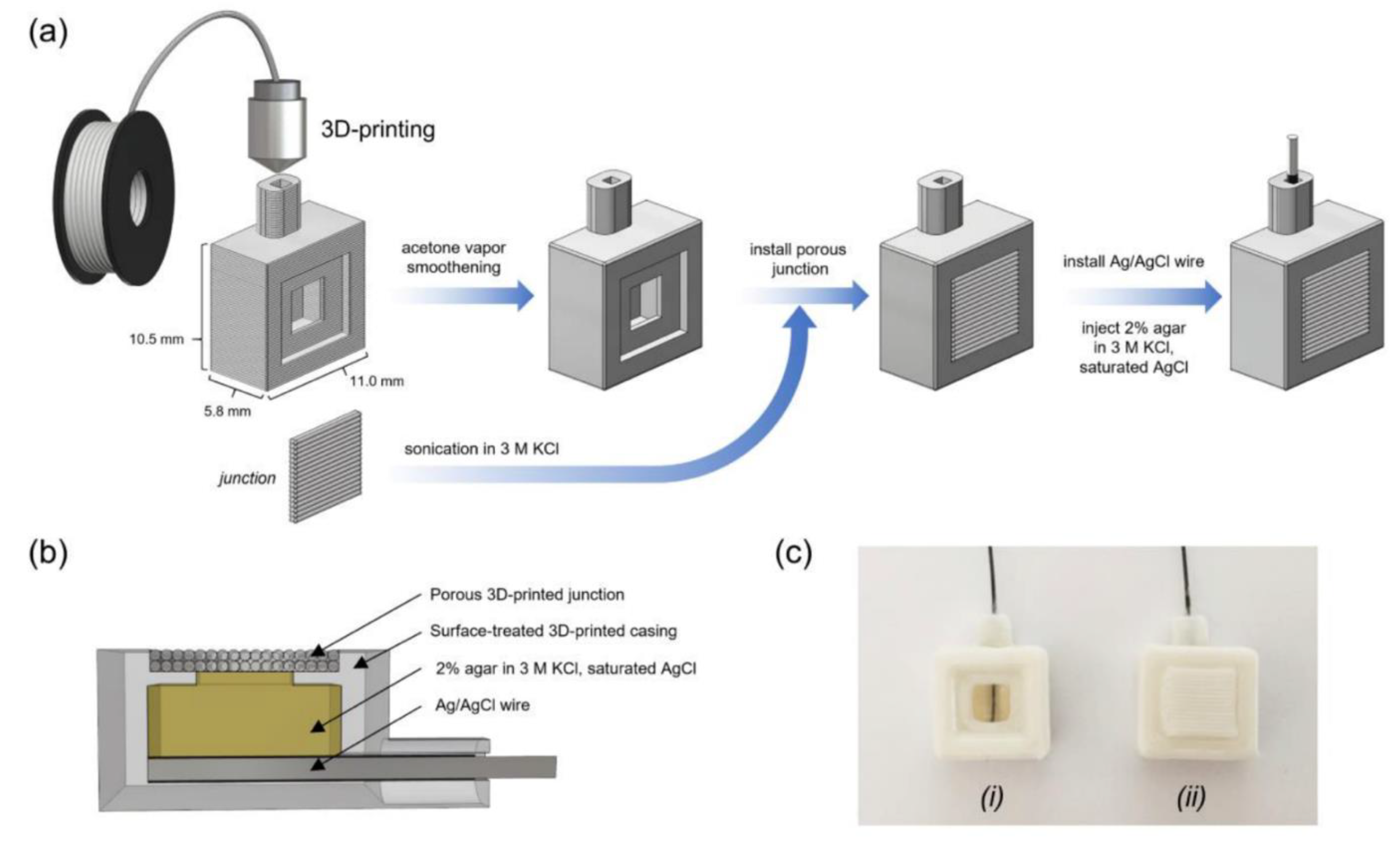
- Sibug-Torres, S.M.; Go, L.P.; Enriquez, E.P. Fabrication of a 3D-Printed Porous Junction for Ag|AgCl|gel-KCl Reference Electrode. Chemosensors 2020, 8, 130. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, D.; Guo, Q.; Xiao, J.; Zheng, M.; Yang, J. Study of the Enzyme Activity Change Due to Inkjet Printing for Biosensor Fabrication. ACS Biomater. Sci. Eng. 2021, 7, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Niaraki Asli, A.E.; Guo, J.; Lai, P.L.; Montazami, R.; Hashemi, N.N. High-Yield Production of Aqueous Graphene for Electrohydrodynamic Drop-on-Demand Printing of Biocompatible Conductive Patterns. Biosensors 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Sun, J.; Gao, Q.; Yang, X.; Ye, Y.; Ji, J.; Sun, X. 3D “Honeycomb” Cell/Carbon Nanofiber/Gelatin Methacryloyl (GelMA) Modified Screen-Printed Electrode for Electrochemical Assessment of the Combined Toxicity of Deoxynivalenol Family Mycotoxins. Bioelectrochemistry 2021, 139, 107743. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Wang, Y.; Kwon, E.Y.; Wang, L.; Cheng, N.; Niu, X.; Ding, S.; Van Wie, B.J.; Lin, Y.; Du, D. Nanomaterial-Enhanced 3D-Printed Sensor Platform for Simultaneous Detection of Atrazine and Acetochlor. Biosens. Bioelectron. 2021, 184, 113238. [Google Scholar] [CrossRef]
- Silva, V.A.O.P.; Fernandes-Junior, W.S.; Rocha, D.P.; Stefano, J.S.; Munoz, R.A.A.; Bonacin, J.A.; Janegitz, B.C. 3D-Printed Reduced Graphene Oxide/Polylactic Acid Electrodes: A New Prototyped Platform for Sensing and Biosensing Applications. Biosens. Bioelectron. 2020, 170, 112684. [Google Scholar] [CrossRef]
- João, A.F.; Squissato, A.L.; Richter, E.M.; Muñoz, R.A.A. Additive-Manufactured Sensors for Biofuel Analysis: Copper Determination in Bioethanol Using a 3D-Printed Carbon Black/Polylactic Electrode. Anal. Bioanal. Chem. 2020, 412, 2755–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupan, O.; Krüger, H.; Siebert, L.; Ababii, N.; Kohlmann, N.; Buzdugan, A.; Bodduluri, M.T.; Magariu, N.; Terasa, M.-I.; Strunskus, T.; et al. Additive Manufacturing as a Means of Gas Sensor Development for Battery Health Monitoring. Chemosensors 2021, 9, 252. [Google Scholar] [CrossRef]
- Lehman, S.E.; McCracken, J.M.; Miller, L.A.; Jayalath, S.; Nuzzo, R.G. Biocompliant Composite Au/PHEMA Plasmonic Scaffolds for 3D Cell Culture and Noninvasive Sensing of Cellular Metabolites. Adv. Healthc. Mater. 2021, 10, 2001040. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Lu, J.; Zhou, Y.; Gong, J. Dual-Modal Visual/Photoelectrochemical All-in-One Bioassay for Rapid Detection of AFP Using 3D Printed Microreactor Device. Biosens. Bioelectron. 2020, 158, 112158. [Google Scholar] [CrossRef]
- Cao, L.; Han, G.-C.; Xiao, H.; Chen, Z.; Fang, C. A Novel 3D Paper-Based Microfluidic Electrochemical Glucose Biosensor Based on RGO-TEPA/PB Sensitive Film. Anal. Chim. Acta 2020, 1096, 34–43. [Google Scholar] [CrossRef]
- Mao, D.; Li, W.; Zhang, F.; Yang, S.; Isak, A.N.; Song, Y.; Guo, Y.; Cao, S.; Zhang, R.; Feng, C.; et al. Nanocomposite of Peroxidase-Like Cucurbit[6]Uril with Enzyme-Encapsulated ZIF-8 and Application for Colorimetric Biosensing. ACS Appl. Mater. Interfaces 2021, 13, 39719–39729. [Google Scholar] [CrossRef] [PubMed]
- Dubbin, K.; Dong, Z.; Park, D.M.; Alvarado, J.; Su, J.; Wasson, E.; Robertson, C.; Jackson, J.; Bose, A.; Moya, M.L.; et al. Projection Microstereolithographic Microbial Bioprinting for Engineered Biofilms. Nano Lett. 2021, 21, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Chen, T.; Ozkaya, G.U.; Choudhary, D.; Molinolo, A.A.; Gutkind, J.S.; Rusling, J.F. Detecting Cancer Metastasis and Accompanying Protein Biomarkers at Single Cell Levels Using a 3D-Printed Microfluidic Immunoarray. Biosens. Bioelectron. 2021, 171, 112681. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Abafogi, A.T.; Ponnuvelu, D.V.; Song, I.; Ko, K.; Park, S. Enhanced Luminescent Detection of Circulating Tumor Cells by a 3D Printed Immunomagnetic Concentrator. Biosensors 2021, 11, 278. [Google Scholar] [CrossRef]
- Sharafeldin, M.; James, T.; Davis, J.J. Open Circuit Potential as a Tool for the Assessment of Binding Kinetics and Reagentless Protein Quantitation. Anal. Chem. 2021, 93, 14748–14754. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Qi, W.; Wang, S.; Wang, M.; Lin, J. Optical Biosensor for Rapid Detection of Salmonella Typhimurium Based on Porous Gold@Platinum Nanocatalysts and a 3D Fluidic Chip. ACS Sens. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Calabria, D.; Guardigli, M.; Severi, P.; Trozzi, I.; Pace, A.; Cinti, S.; Zangheri, M.; Mirasoli, M. A Smartphone-Based Chemosensor to Evaluate Antioxidants in Agri-Food Matrices by In Situ AuNP Formation. Sensors 2021, 21, 5432. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Gharia, A.; Niknejad, A.; Stojanović, V.; Anwar, M. Microfluidic Packaging Integration with Electronic-Photonic Biosensors Using 3D Printed Transfer Molding. Biosensors 2020, 10, 177. [Google Scholar] [CrossRef]
- Jordan, R.S.; Frye, J.; Hernandez, V.; Prado, I.; Giglio, A.; Abbasizadeh, N.; Flores-Martinez, M.; Shirzad, K.; Xu, B.; Hill, I.M.; et al. 3D Printed Architected Conducting Polymer Hydrogels. J. Mater. Chem. B 2021, 9, 7258–7270. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D Printed Microfluidic Devices for Circulating Tumor Cells (CTCs) Isolation. Biosens. Bioelectron. 2020, 150, 111900. [Google Scholar] [CrossRef]
- Terutsuki, D.; Mitsuno, H.; Kanzaki, R. 3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging. Sensors 2020, 20, 5779. [Google Scholar] [CrossRef] [PubMed]
- Arshavsky-Graham, S.; Enders, A.; Ackerman, S.; Bahnemann, J.; Segal, E. 3D-Printed Microfluidics Integrated with Optical Nanostructured Porous Aptasensors for Protein Detection. Microchim. Acta 2021, 188, 67. [Google Scholar] [CrossRef] [PubMed]
- Siller, I.G.; Preuss, J.-A.; Urmann, K.; Hoffmann, M.R.; Scheper, T.; Bahnemann, J. 3D-Printed Flow Cells for Aptamer-Based Impedimetric Detection of E. Coli Crooks Strain. Sensors 2020, 20, 4421. [Google Scholar] [CrossRef] [PubMed]
- Agostino, V.; Massaglia, G.; Gerosa, M.; Sacco, A.; Saracco, G.; Margaria, V.; Quaglio, M. Environmental Electroactive Consortia as Reusable Biosensing Element for Freshwater Toxicity Monitoring. New Biotechnol. 2020, 55, 36–45. [Google Scholar] [CrossRef]
- Achille, C.; Parra-Cabrera, C.; Dochy, R.; Ordutowski, H.; Piovesan, A.; Piron, P.; Van Looy, L.; Kushwaha, S.; Reynaerts, D.; Verboven, P.; et al. 3D Printing of Monolithic Capillarity-Driven Microfluidic Devices for Diagnostics. Adv. Mater. 2021, 33, 2008712. [Google Scholar] [CrossRef]
- Ho, D.H.; Hong, P.; Han, J.T.; Kim, S.; Kwon, S.J.; Cho, J.H. 3D-Printed Sugar Scaffold for High-Precision and Highly Sensitive Active and Passive Wearable Sensors. Adv. Sci. 2020, 7, 1902521. [Google Scholar] [CrossRef] [Green Version]
- Kanitthamniyom, P.; Zhou, A.; Feng, S.; Liu, A.; Vasoo, S.; Zhang, Y. A 3D-Printed Modular Magnetic Digital Microfluidic Architecture for on-Demand Bioanalysis. Microsyst. Nanoeng. 2020, 6, 48. [Google Scholar] [CrossRef]
- Aggas, J.R.; Abasi, S.; Phipps, J.F.; Podstawczyk, D.A.; Guiseppi-Elie, A. Microfabricated and 3-D Printed Electroconductive Hydrogels of PEDOT:PSS and Their Application in Bioelectronics. Biosens. Bioelectron. 2020, 168, 112568. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D Printed Medicines: A New Branch of Digital Healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef]
- Ferretti, P.; Santi, G.M.; Leon-Cardenas, C.; Freddi, M.; Donnici, G.; Frizziero, L.; Liverani, A. Molds with Advanced Materials for Carbon Fiber Manufacturing with 3D Printing Technology. Polymers 2021, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Napadensky, E. Inkjet 3D Printing. In The Chemistry of Inkjet Inks; World Scientific: Singapore, 2009; p. 255. ISBN 978-981-281-821-828. [Google Scholar]
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet Printing of Proteins. Soft Matter 2009, 5, 4866. [Google Scholar] [CrossRef]
- Secor, E.B. Principles of Aerosol Jet Printing. Flex. Print. Electron. 2018, 3, 035002. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A Review of 3D Printing Techniques for Environmental Applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Xu, S.; Zhang, H.; Tan, Y.; Ma, C.; Song, R.; Jiang, L.; Yi, C. A 3D Printed Smartphone Optosensing Platform for Point-of-Need Food Safety Inspection. Anal. Chim. Acta 2017, 966, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.-T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153. [Google Scholar] [CrossRef]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Zimetti, F.; Marchi, C.; Bernini, F.; Bettini, R.; Elviri, L. Biocompatible 3D Printed Chitosan-Based Scaffolds Containing α-Tocopherol Showing Antioxidant and Antimicrobial Activity. Appl. Sci. 2021, 11, 7253. [Google Scholar] [CrossRef]
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D Printed Chitosan Scaffolds: A New TiO2 Support for the Photocatalytic Degradation of Amoxicillin in Water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef]
- Zanotti, I.; Marando, S.; Remaggi, G.; Bergonzi, C.; Bernini, F.; Bettini, R.; Elviri, L. The Adaptation of Lipid Profile of Human Fibroblasts to Alginate 2D Films and 3D Printed Scaffolds. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129734. [Google Scholar] [CrossRef] [PubMed]
- Guo; Niaraki Asli; Williams; Lai; Wang; Montazami; Hashemi Viability of Neural Cells on 3D Printed Graphene Bioelectronics. Biosensors 2019, 9, 112. [CrossRef] [PubMed] [Green Version]
- Mazumder, P.M.; Sasmal, D. Mycotoxins—Limits and Regulations. Anc. Sci. Life 2001, 20, 1–19. [Google Scholar]
- Zhao, Z.; Tian, X.; Song, X. Engineering Materials with Light: Recent Progress in Digital Light Processing Based 3D Printing. J. Mater. Chem. C 2020, 8, 13896–13917. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Kazemi Oskuee, R.; Eivazzadeh-Keihan, R.; Rezayi, M.; Baradaran, B.; Maleki, A.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Development of Biosensors for Detection of Alpha-Fetoprotein: As a Major Biomarker for Hepatocellular Carcinoma. TrAC Trends Anal. Chem. 2020, 130, 115961. [Google Scholar] [CrossRef]
- European Food Safety Authority. European Centre for Disease Prevention and Control The European Union One Health 2019 Zoonoses Report. EFS2 J. 2021, 19, e05926. [Google Scholar] [CrossRef]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-Y.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y.; Kim, J.-G. Dimensional Accuracy of Dental Models for Three-Unit Prostheses Fabricated by Various 3D Printing Technologies. Materials 2021, 14, 1550. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in Powder Bed Fusion 3D Printing in Drug Delivery and Healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]

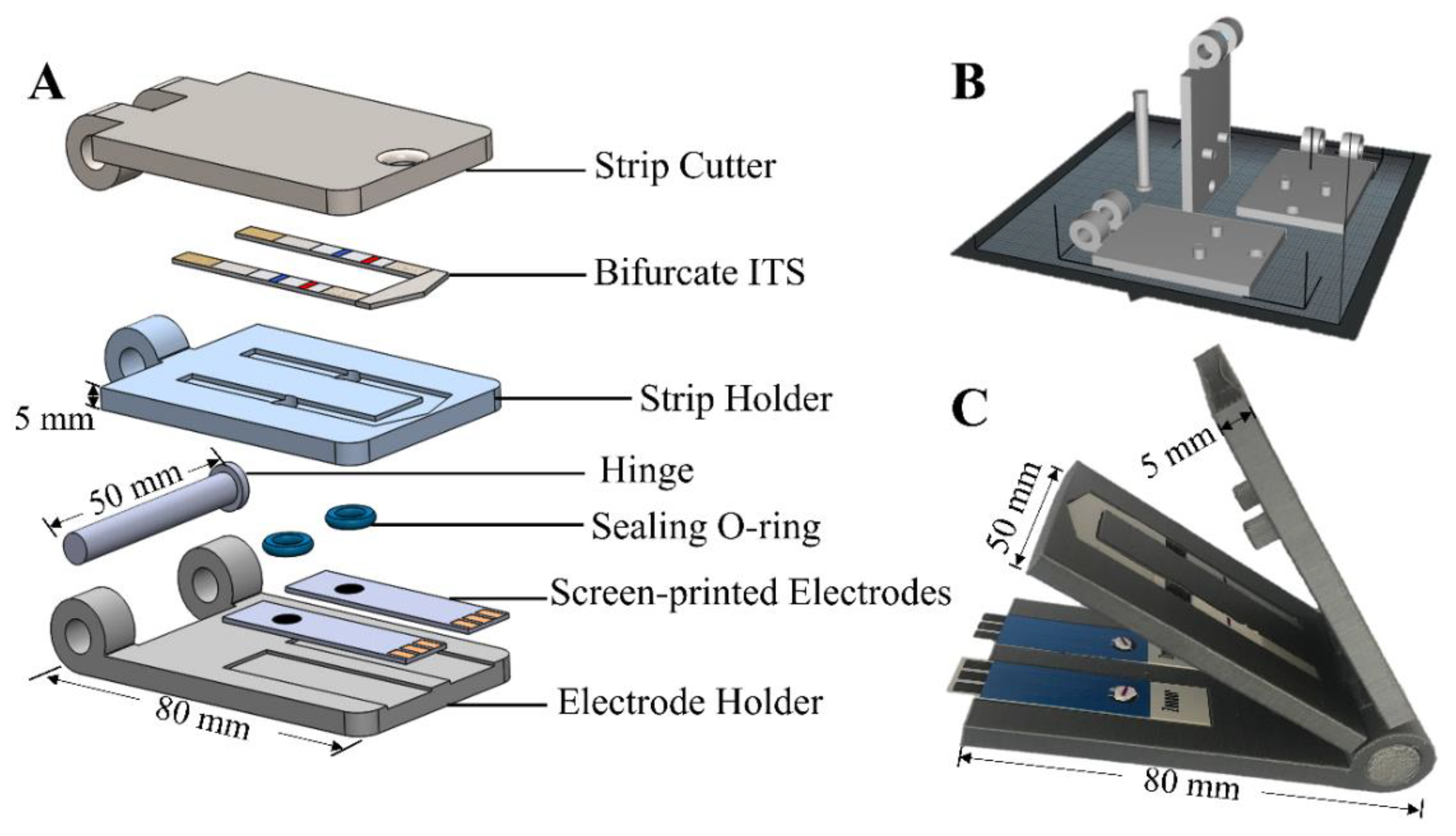
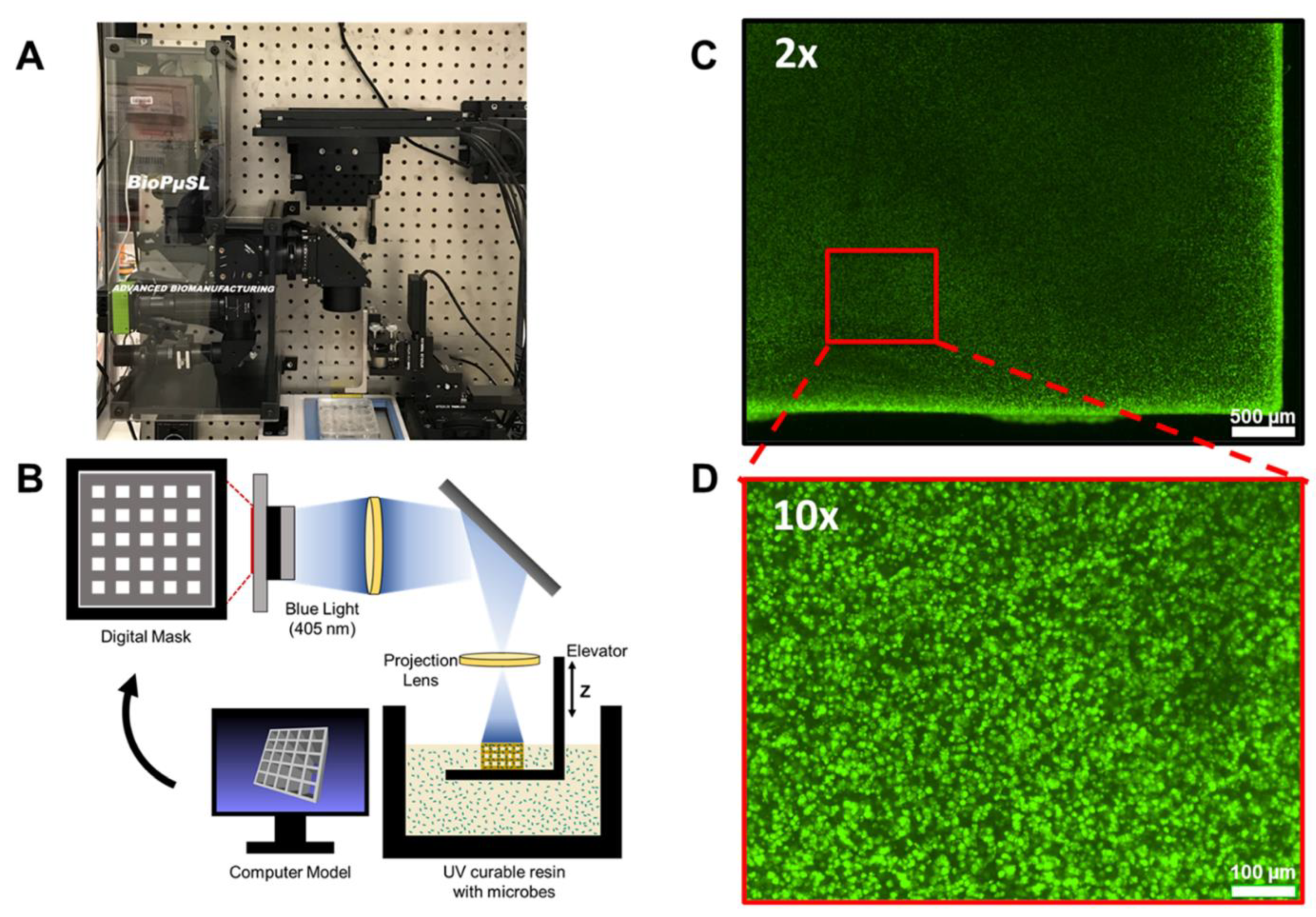
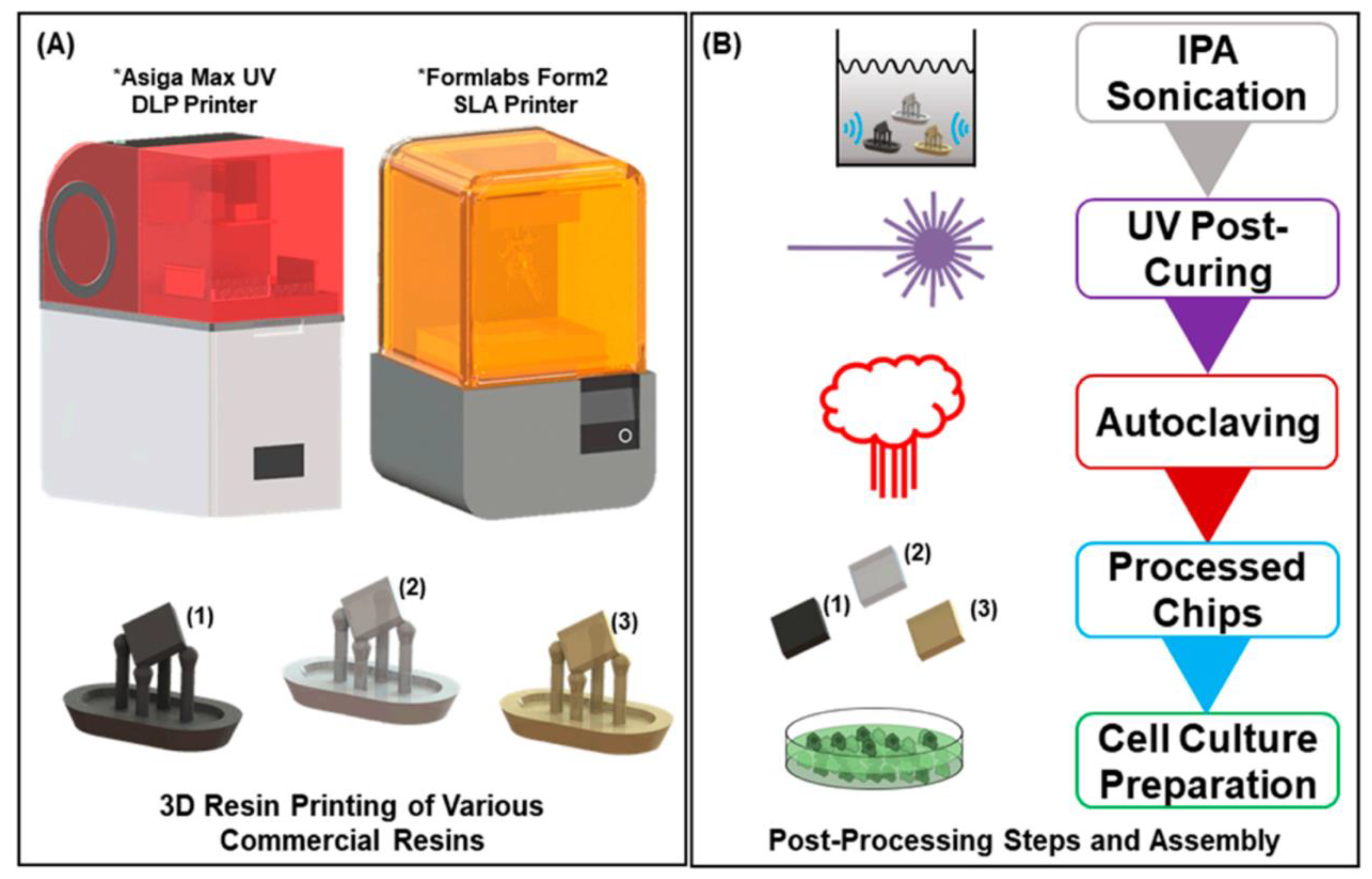
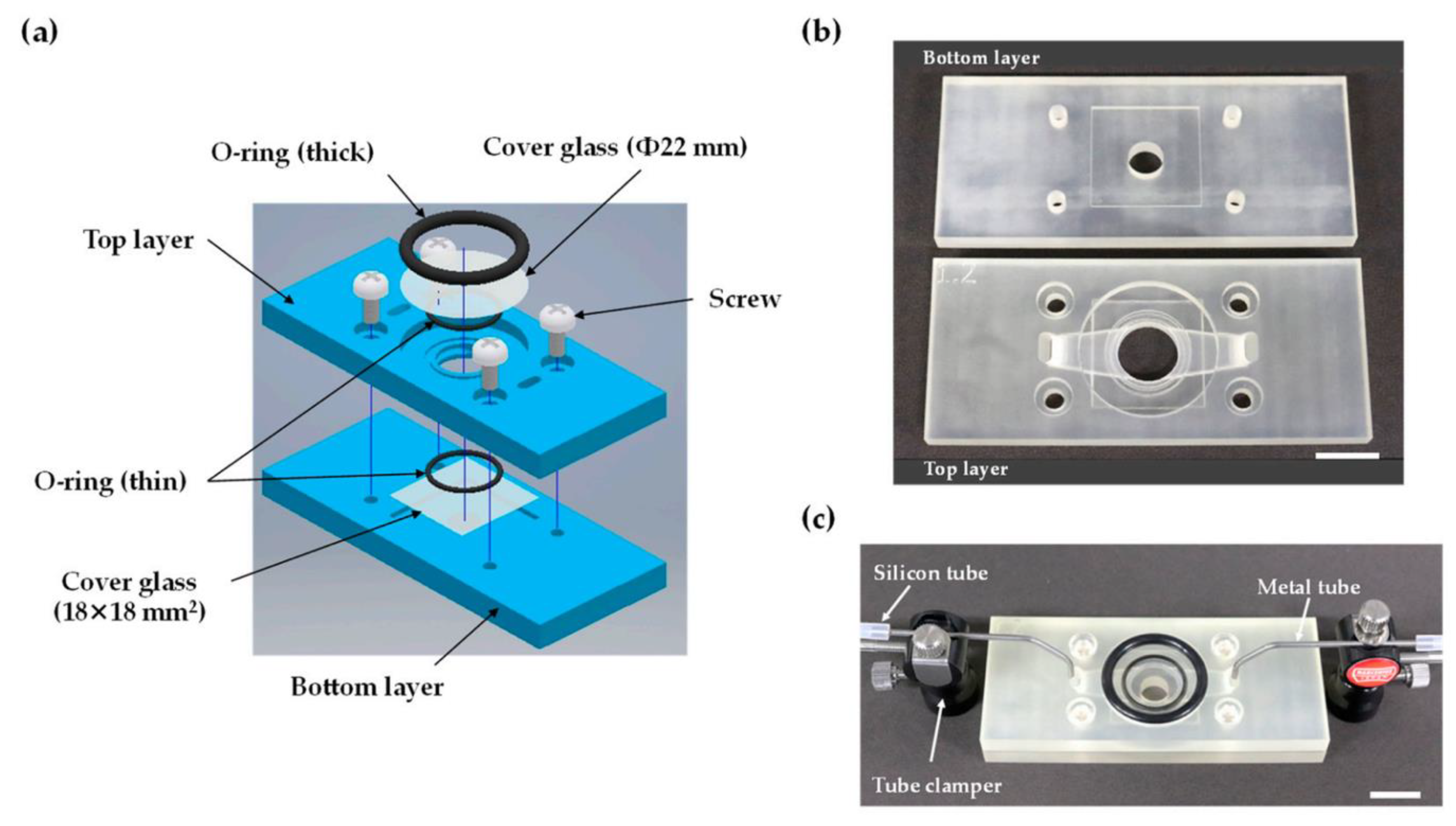
| 3D Printing Technologies | Field of Application | Analytical Purposes | Limit of Detection | Biological Sample | 3D-Printed Materials | Ref. |
|---|---|---|---|---|---|---|
| Material Extrusion: FDM | Biomedical | Hydrogen peroxide detection | 11.1 µM | N/A | Graphene/PLA | [20] |
| Material Extrusion: FDM | Biomedical: wearable sensors | Glucose determination in human sweat | 1.2 µmol/L | Human sweat | Carbon PLA/TPU | [21] |
| Material Extrusion: FDM | Biochemical: chiral sensors | Tryptophan enantiomers resolution and quantification | N/A | N/A | PLA | [22] |
| Material Extrusion: FDM | Biomedical: diagnosis | Anticancer drugs direct quantification | 5 × 10−8 M in serum | Human biological fluids | PLA | [23] |
| Material Extrusion: FDM | Biomedical: point-of-care (POC) diagnostics | DNA amplification (LAMP) | N/A | Human saliva | PP | [24] |
| Material Extrusion: Inkjet printing | Biomedical | Epithelial cell cultures monitoring | 4.36 cell-index unit/cells × cm−2 | Epithelial cells | AgNPs/SU-8 | [25] |
| Material Extrusion: AJP | Biomedical | Cytokine monitoring in bovine serum | IFN-γ: 25 pg/mL; IL-10: 46 pg/mL | Bovine serum | Graphene-nitrocellulose | [26] |
| Material Extrusion: AJP | Biomedical: diagnosis | SARS-CoV-2 antigens detection | S1 protein: 2.8 × 10−15 M; RBD: 16.9 × 10−15 M | Human biological fluids | AuNPs-PDMS | [27] |
| Material Extrusion: FDM | Point-of-care diagnostics | Dopamine detection | 1.45 µg/mL | N/A | CNT/CB/PLA | [28] |
| Material Extrusion: FDM | Biomedical: epithelial cancer biomarkers detection | Mucin 1 quantification | 80 nM | Breast cancer cells | Nanocarbon-PLA | [29] |
| Material Extrusion: DLP | Biomedical: multiplexed protein biomarker ELISA | IL-6, CRP, CEA, PSA | IL-6: 1.75 pg/mL; CRP: 26 pg/mL; CEA: 7.5 pg/mL; PSA: 62 pg/mL | Rat Plasma | PEDGA | [30] |
| Material Extrusion: FDM | Biochemical and Biophysical | Protein absorption | N/A | N/A | PLA | [31] |
| Material Extrusion: FDM | General: miniaturized electrochemical sensor systems | Cyclic voltammetry of redox couple standard and potentiometric pH measurements | N/A | N/A | ABS | [32] |
| Material Extrusion: inkjet printing-drop-on-demand printer | Biosensors manufacturing optimization | Catalytic activity and conformational changes evaluation in enzymes | N/A | N/A | PP | [33] |
| Material Extrusion: inkjet printing-drop-on-demand printer | Biocompatible conductive ink fabrication for neuronal sensing | Graphene patterns-based conductive inks | N/A | N/A | Graphene-PI | [34] |
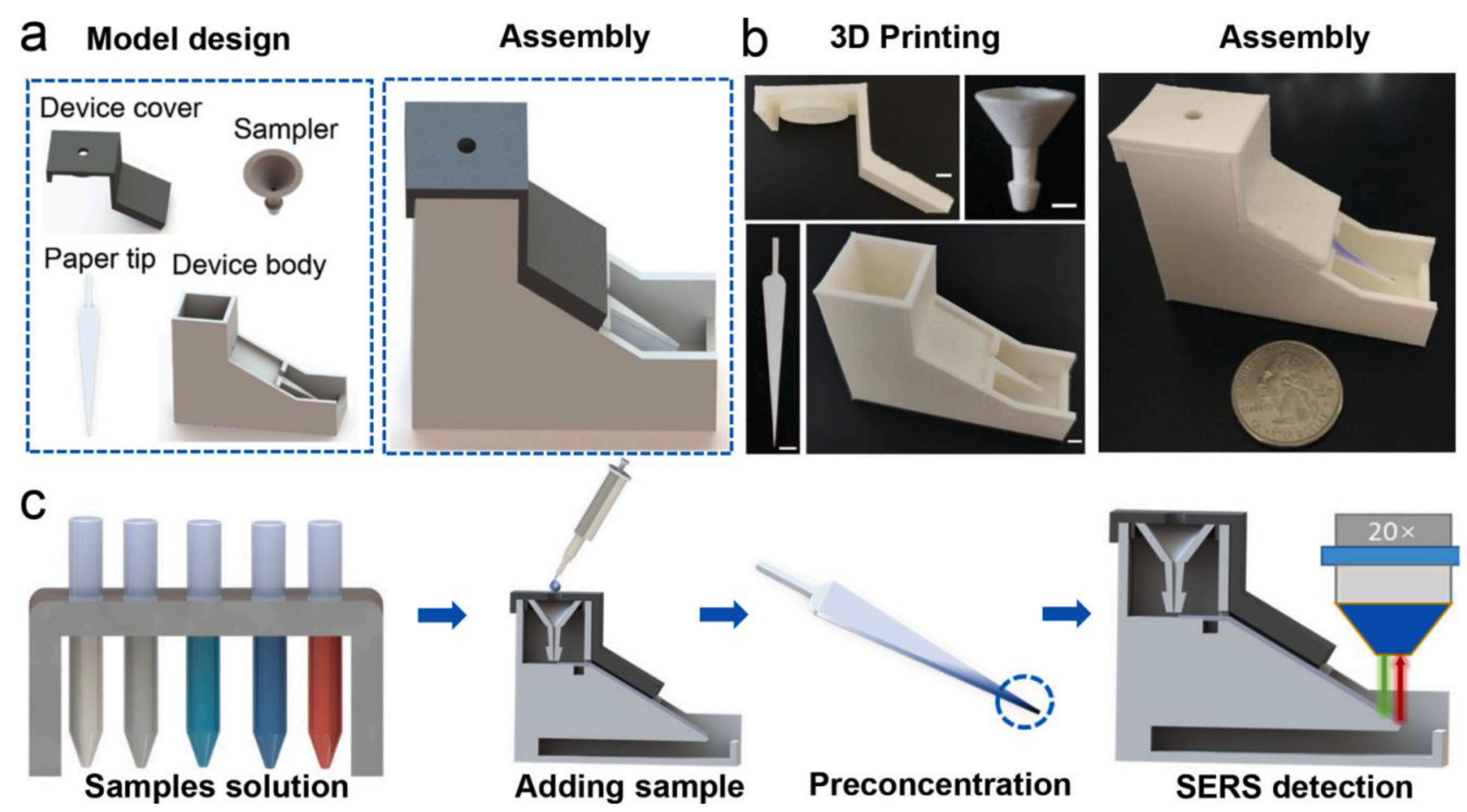
| Material Extrusion | Food and feed quality | Mycotoxins quantification | DON: 0.07; 3-ADON: 0.10; 15-ADON: 0.06 μg/mL | Food and feed | Gelatin-Methacryloyl | [35] |
| Material Extrusion: FDM | Environmental: water pollution monitoring | Herbicides (atrazine and acetochlor) detection | Atrazine: 0.24 ppb; acetochlor: 3.2 ppb | Water | PLA | [36] |
| Material Extrusion: FDM | Environmental (water pollution monitoring) and Biomedical | Serotonin quantification in synthetic urine and catechol determination in water | Serotonin: 0.032 μmol/L; catechol: 0.26 μmol/L | Synthetic biological fluids and water | Graphene oxide-PLA | [37] |
| Material Extrusion: FDM | Quality control: biofuels | Copper determination in bioethanol | 0.097 μg/L | Biofuels | Carbon black-PLA | [38] |
| Material Extrusion: inkjet printing-direct ink writing | General: battery safety | Gas detection in lithium-ion batteries | N/A | Li-ion batteries | CuMPs-polyethylene oxide | [39] |
| Vat Photopolymerization | Biomedical: living biosensor | In situ monitoring of cellular metabolites | N/A | Cells | Au.pHEMA | [40] |
| Vat Photopolymerization | Biomedical: point-of-care (POC) diagnostics | Tumor markers (alpha-fetoprotein) detection | 0.01 ng/mL | Human blood | N/A | [41] |
| Vat Photopolymerization | Biomedical: diabetics diagnosis | Glucose determination in human sweat and blood | 25 μM | Human sweat and blood | rGO-TEPA/PB | [42] |
| Vat Photopolymerization | Biomedical | Glucose and cholesterol quantification in human blood | Glucose: 1.2 μM; cholesterol: 2.3 μM | Human blood | White resin | [43] |
| Vat Photopolymerization: SLA | Biomedical: living biosensor | Stereolithographic printing of engineered microbial in biosensor for in situ monitoring of uranium in groundwater | 2.5 μM | Groundwater | PEGDa | [44] |
| Vat Photopolymerization: SLA | Biomedical | Metastatic cancer biomarkers quantification | DSG3: 0.10 fg/mL; VEGF-A, VEGF-C, β-Tub: 0.20 fg/mL | Human biological fluids | Chitosan | [45] |
| Vat Photopolymerization: SLA | Biomedical: biocompatible biosensor | Biocompatibility evaluation of commercial resins towards rat cardiomyocytes | N/A | Rat cardiomyocytes | Commercial resins | [3] |
| Vat Photopolymerization: digital light processing | Biomedical: cancer diagnosis | Circulating tumor cells (CTCs) detection in human blood | 10 cells/mL | Human blood | PU | [46] |
| Vat Photopolymerization: digital light processing | Biomedical: biomarker detection in complex matrices | C-reactive protein as model biomarker | 1 ng/mL | Fetal bovine serum | Plastic material (Not specified) | [47] |
| Vat Photopolymerization | Quality control: foods | Salmonella typhimurium detection in food | 17 CFU/mL | Food | ABS | [48] |
| Vat Photopolymerization: SLA | Quality control: agri-food matrices | Antioxidant capacity (TAC) in food extracts and beverages | Gallic acid equivalent: 30 μM | Food and beverages | N/A | [49] |
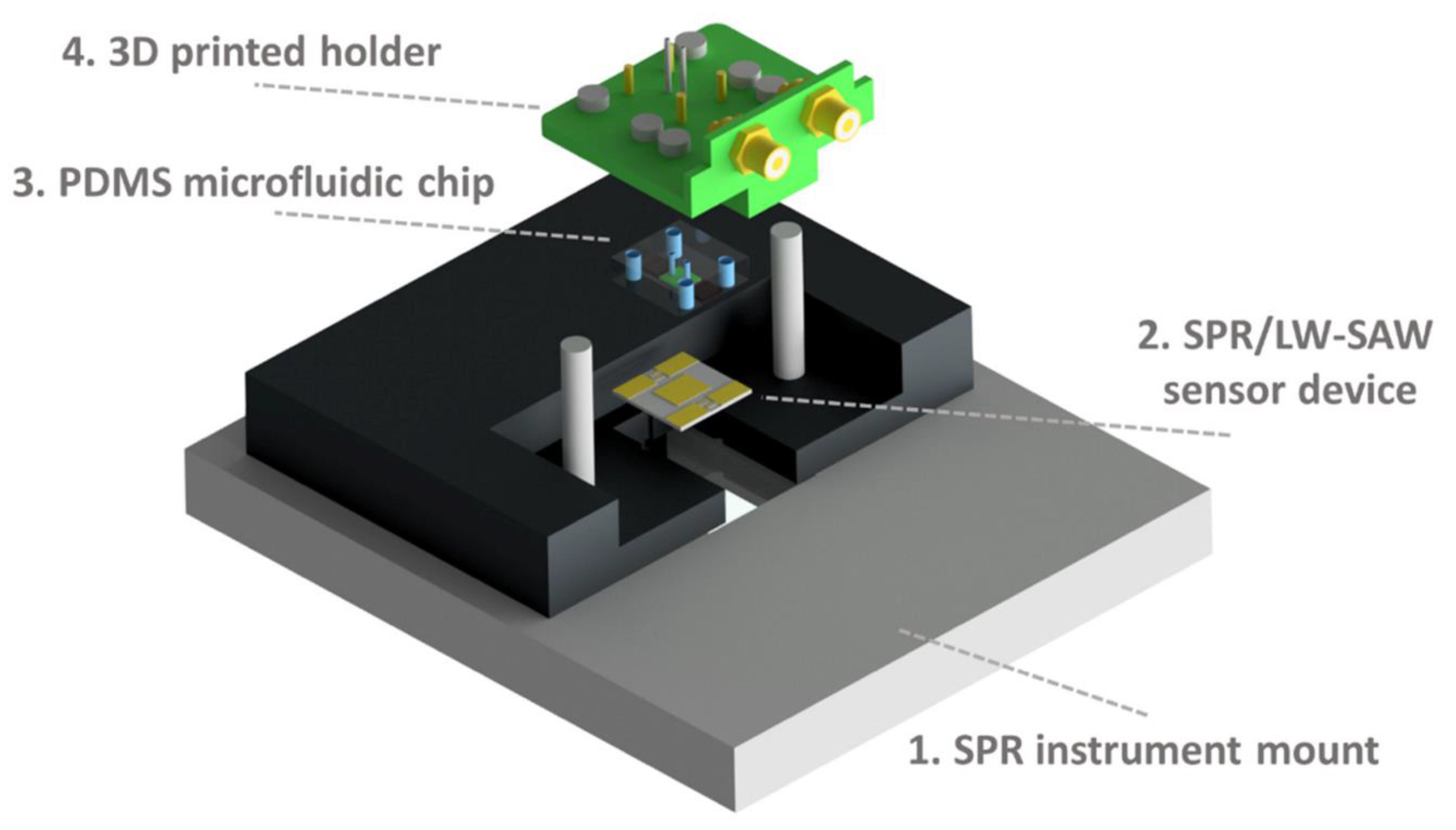
| Vat Photopolymerization: SLA | Biophotonic technologies | 3D-printed transfer molding for photonic biosensor optimization | N/A | N/A | PAMPSA-PAAm | [50] |
| Vat Photopolymerization: SLA | Wearable and implantable bioelectronics, robotics, energy storage, and cell cultures | Logic of architecture design applied to conductive hydrogel manufacturing | N/A | N/A | N/A | [51] |
| Material Jetting: MultiJet technology | Biomedical: early diagnostics | Cancer metastasis monitoring | 106 cells/mL | Human biological fluids | VisiJet M3 crystal | [52] |
| Material Jetting: MultiJet technology | Biomedical: portable-living biosensor | Cell-based biosensor for volatile compounds detection | 1-octen-3-ol: 1 µM | N/A | VisiJet M2R-CL | [53] |
| Material Jetting: MultiJet technology | Biomedical | Proteins detection | 0.04 µM | Human biological fluids | VisiJet M2R-CL | [54] |
| Material Jetting: MultiJet technology | Biomedical: aptamer-based impedimetric biosensor | Escherichia coli label-free detection | 105 cells/mL | Fecal material | PMMA | [55] |
| Material Jetting: fluid dynamic modelling | Environmental: water pollution evaluation | Freshwater toxicity monitoring | Ni(II), Cr(III): < 2 mg/L | Freshwater | N/A | [56] |
| Binder Jetting | Biomedical: allergy diagnosis | Immunoglobulin E detection | 0.2 µg/mL | Human blood | PMMA | [57] |
| Powder Bed Fusion | Biomedical: point-of-care (POC) diagnostics-wearable biosensor | Real-time monitoring of electrical body signals | N/A | N/A | Sugar grains | [58] |
| 3D-printed modular magnetic digital microfluidic: SLA + FDM | Biomedical: point-of-care (POC) diagnostics | Biomarkers sensing, pathogen identification, antibiotic resistance determination, glucose and protein quantification | HBsAg: 61.6 ng/mL; CRP: 59.8 ng/mL; BSA: 54.6 µg/mL; glucose: 0.47 mg/dL | Human biological fluids | Clear resins/ABS | [59] |
| Inkjet printing + Microlithography | Bioelectronics | Quantitative comparison between microlithography and 3D printing in hydrogels manufacturing for biosensing and tissue engineering | N/A | N/A | PEDOT.PSS/p(HEMA-co-EGMA) | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remaggi, G.; Zaccarelli, A.; Elviri, L. 3D Printing Technologies in Biosensors Production: Recent Developments. Chemosensors 2022, 10, 65. https://doi.org/10.3390/chemosensors10020065
Remaggi G, Zaccarelli A, Elviri L. 3D Printing Technologies in Biosensors Production: Recent Developments. Chemosensors. 2022; 10(2):65. https://doi.org/10.3390/chemosensors10020065
Chicago/Turabian StyleRemaggi, Giulia, Alessandro Zaccarelli, and Lisa Elviri. 2022. "3D Printing Technologies in Biosensors Production: Recent Developments" Chemosensors 10, no. 2: 65. https://doi.org/10.3390/chemosensors10020065
APA StyleRemaggi, G., Zaccarelli, A., & Elviri, L. (2022). 3D Printing Technologies in Biosensors Production: Recent Developments. Chemosensors, 10(2), 65. https://doi.org/10.3390/chemosensors10020065






