Upconversion Luminescent Humidity Sensors Based on Lanthanide-Doped MOFs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Lanthanide-Doped MOFs (Denoted as Y/Yb/Er-MOF)
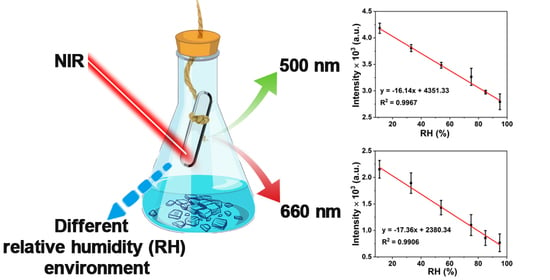
2.2. Y/Yb/Er-MOF Responses to Different RH Environments
3. Results and Discussion
3.1. Characterization of Structural Properties
3.2. Upconversion Luminescence of Y/Yb/Er-MOF
3.3. Response of Y/Yb/Er-MOF to Relative Humidity (RH)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cho, M.Y.; Kim, S.; Kim, I.S.; Kim, E.S.; Wang, Z.J.; Kim, N.Y.; Kim, S.W.; Oh, J.M. Perovskite-Induced Ultrasensitive and Highly Stable Humid-ity Sensor Systems Prepared by Aerosol Deposition at Room Temperature. Adv. Funct. Mater. 2019, 30, 1907449. [Google Scholar] [CrossRef]
- Lan, L.; Le, X.; Dong, H.; Xie, J.; Ying, Y.; Ping, J. One-step and large-scale fabrication of flexible and wearable humidity sensor based on laser-induced graphene for real-time tracking of plant transpiration at bio-interface. Biosens. Bioelectron. 2020, 165, 112360. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Chen, M.-Q.; Xia, F. Research Advances in Microfiber Humidity Sensors. Small 2018, 14, e1800524. [Google Scholar] [CrossRef] [PubMed]
- Torres Alonso, E.; Shin, D.W.; Rajan, G.; Neves, A.I.S.; Russo, S.; Craciun, M.F. Water-Based Solution Processing and Wafer-Scale Integration of All-Graphene Humidity Sensors. Adv. Sci. 2019, 6, 1802318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Zhao, L.; Xu, L.; Wang, Y.; Liu, K.; Wang, Y.; Chen, G.Y.; Liu, T.; Wang, Y. Review of Optical Humidity Sensors. Sensors 2021, 21, 8049. [Google Scholar] [CrossRef] [PubMed]
- Boudaden, J.; Steinmaßl, M.; Endres, H.-E.; Drost, A.; Eisele, I.; Kutter, C.; Müller-Buschbaum, P. Polyimide-Based Capacitive Humidity Sensor. Sensors 2018, 18, 1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionete, E.I.; Spiridon, S.-I.; Monea, B.-F.; Ebrasu-Ion, D.; Vaseashta, A. SWCNT-Pt-P2O5 -Based Sensor for Humidity Measurements. IEEE Sens. J. 2016, 16, 7593–7599. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Zhang, Y.; Zhang, Y.; Liu, K.; Zhang, J.; Yang, J.; Yuan, L. Spider silk-based humidity sensor. Opt. Lett. 2019, 44, 2907–2910. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, J.; Zhao, D.; Lian, X.; Cui, Y.; Yang, Y.; Qian, G. Ratiometric near infrared luminescent thermometer based on lan-thanide metal-organic frameworks. J. Solid State Chem. 2016, 241, 99–104. [Google Scholar] [CrossRef]
- Zhang, P.; Song, N.; Liu, S.; Li, Q.; Wang, Y.; Zhou, B. Tuning the photoluminescence of lanthanide metal–organic framework nanospheres through ligand-induced phase transition towards sensing. J. Mater. Chem. C 2021, 9, 6208–6216. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, D.; Li, Z.; Ma, X.; Wan, X.; Deng, Z.; Peng, X. A self-confinement synthesis of a POM-decorated MOF thin film for actively hydrolyzing ethyl acetate. Chem. Commun. 2020, 56, 13840–13843. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, H.; Xin, Y.; Zhang, J. Metal–organic frameworks: A universal strategy towards super-elastic hydrogels. Polym. Chem. 2019, 10, 2263–2272. [Google Scholar] [CrossRef]
- Wang, C.; Tian, L.; Zhu, W.; Wang, S.; Wang, P.; Liang, Y.; Zhang, W.; Zhao, H.; Li, G. Dye@bio-MOF-1 Composite as a Dual-Emitting Platform for Enhanced Detection of a Wide Range of Explosive Molecules. ACS Appl. Mater. Interfaces 2017, 9, 20076–20085. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent multifunctional lanthanides-based metal–organic frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Liu, Q.; Shi, M.; Feng, W.; Li, F. Lanthanide-Doped Nanoparticles with Upconversion and Downshifting Near-Infrared Luminescence for Bioimaging. Inorg. Chem. 2019, 58, 9351–9357. [Google Scholar] [CrossRef]
- Sun, G.; Xie, Y.; Sun, L.; Zhang, H. Lanthanide upconversion and downshifting luminescence for biomolecules detection. Nanoscale Horizons 2021, 6, 766–780. [Google Scholar] [CrossRef]
- You, W.; Tu, D.; Zheng, W.; Shang, X.; Song, X.; Zhou, S.; Liu, Y.; Li, R.; Chen, X. Large-scale synthesis of uniform lanthanide-doped NaREF4 up-conversion/downshifting nanoprobes for bioapplications. Nanoscale 2018, 10, 11477–11484. [Google Scholar] [CrossRef]
- Liu, J.; Rijckaert, H.; Zeng, M.; Haustraete, K.; Laforce, B.; Vincze, L.; Van Driessche, I.; Kaczmarek, A.M.; Van Deun, R. Simultaneously Excited Downshifting/Upconversion Luminescence from Lanthanide-Doped Core/Shell Fluoride Nanoparticles for Multimode Anticounterfeiting. Adv. Funct. Mater. 2018, 28, 1707365. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, T.; Chung, M.N.; Sun, X.; Xiao, Y.; Qiao, X.; Wang, F. Yb3+-sensitized upconversion and downshifting luminescence in Nd3+ ions through energy migration. Dalton Trans. 2018, 47, 8581–8584. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermome-try. Adv. Opt. Mater. 2018, 7, 1801239. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Q.; Sang, X.; Hu, H.; Li, S.; Zhang, D.; Liu, C.; Wang, Q.; Zhang, B.; Wang, W.; et al. Modulated Luminescence of Lanthanide Materials by Local Surface Plasmon Resonance Effect. Nanomater. 2021, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zang, L.; Guo, C. Influence of lanthanide ion energy levels on luminescence of corresponding metalloporphyrins. Phys. Chem. Chem. Phys. 2017, 19, 7728–7732. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Jing, P.; Yan, N.; Hilbers, M.; Zhang, H.; Rothenberg, G.; Tanase, S. Dual-mode humidity detection using a lanthanide-based metal–organic framework: Towards multifunctional humidity sensors. Chem. Commun. 2017, 53, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Stangl, J.M.; Dietrich, D.; Sedykh, A.E.; Janiak, C.; Müller-Buschbaum, K. Luminescent MOF polymer mixed matrix membranes for humidity sensing in real status analysis. J. Mater. Chem. C 2018, 6, 9248–9257. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Guo, Y.; Lv, X.; Dou, X. 2D TiO2 nanosheets for ultrasensitive humidity sensing application benefited by abundant surface oxygen vacancy defects. Sens. Actuators B Chem. 2018, 262, 350–358. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, H.Y.; Wang, W.C.; Li, K.; Wang, X.C.; Li, X.J. Capacitive humidity sensing properties of ZnO cauliflowers grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2013, 177, 740–744. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cheng, X.; Feng, C.; Chen, L.; Zhou, J.; Ruan, S. A novel humidity sensor based on NaTaO3 nanocrystalline. Sens. Actuators B Chem. 2012, 174, 485–489. [Google Scholar] [CrossRef]
- Luo, J.; Xu, H.; Liu, Y.; Zhao, Y.; Daemen, L.L.; Brown, C.; Timofeeva, T.V.; Ma, S.; Zhou, H.-C. Hydrogen Adsorption in a Highly Stable Porous Rare-Earth Metal-Organic Framework: Sorption Properties and Neutron Diffraction Studies. J. Am. Chem. Soc. 2008, 130, 9626–9627. [Google Scholar] [CrossRef]
- Nakazawa, E.; Shionoya, S. Cooperative Luminescence in YbPO4. Phys. Rev. Lett. 1970, 25, 1710–1712. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, P.; Weng, F.; Lin, H.; Wang, Y. Optical spectroscopy of Eu3+ and Tb3+ doped glass ceramics containing LiYbF4 nanocrystals. Appl. Phys. Lett. 2009, 94, 041909. [Google Scholar] [CrossRef]
- de la Rosa, E.; Solis, D.; Díaz-Torres, L.A.; Salas, P.; Angeles-Chavez, C.; Meza, O. Blue-green upconversion emission in ZrO2:Yb3+ nanocrystals. J. Appl. Phys. 2008, 104, 103508. [Google Scholar] [CrossRef]
- Qin, W.-P.; Liu, Z.; Sin, C.-N.; Wu, C.; Qin, G.-S.; Chen, Z.; Zheng, K.-Z. Multi-ion cooperative processes in Yb3+ clusters. Light. Sci. Appl. 2014, 3, e193. [Google Scholar] [CrossRef]
- Nakazawa, E. Cooperative optical transitions of Yb3+−Yb3+ and Gd3+−Yb3+ ion pairs in YbPO4 hosts. J. Lumin. 1976, 12–13, 675–680. [Google Scholar] [CrossRef]
- Knighton, R.C.; Soro, L.K.; Francés-Soriano, L.; Rodríguez-Rodríguez, A.; Pilet, G.; Lenertz, M.; Platas-Iglesias, C.; Hildebrandt, N.; Charbonnière, L.J. Cooperative Luminescence and Cooperative Sensitisation Upconversion of Lanthanide Complexes in Solution. Angew. Chem. Int. Ed. 2021, 61, e202113114. [Google Scholar] [CrossRef]
- Hehlen, M.P.; Kuditcher, A.; Rand, S.C.; Lüthi, S.R. Site-Selective, Intrinsically Bistable Luminescence of Yb3+ Ion Pairs in CsCdBr3. Phys. Rev. Lett. 1999, 82, 3050–3053. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, G.; Chen, J.; Xie, Y.; Jiang, H.; Sun, L. Upconversion Luminescent Humidity Sensors Based on Lanthanide-Doped MOFs. Chemosensors 2022, 10, 66. https://doi.org/10.3390/chemosensors10020066
Wang Z, Sun G, Chen J, Xie Y, Jiang H, Sun L. Upconversion Luminescent Humidity Sensors Based on Lanthanide-Doped MOFs. Chemosensors. 2022; 10(2):66. https://doi.org/10.3390/chemosensors10020066
Chicago/Turabian StyleWang, Zhuo, Guotao Sun, Jiabo Chen, Yao Xie, Hong Jiang, and Lining Sun. 2022. "Upconversion Luminescent Humidity Sensors Based on Lanthanide-Doped MOFs" Chemosensors 10, no. 2: 66. https://doi.org/10.3390/chemosensors10020066
APA StyleWang, Z., Sun, G., Chen, J., Xie, Y., Jiang, H., & Sun, L. (2022). Upconversion Luminescent Humidity Sensors Based on Lanthanide-Doped MOFs. Chemosensors, 10(2), 66. https://doi.org/10.3390/chemosensors10020066