Green Synthesis of ZnO/BC Nanohybrid for Fast and Sensitive Detection of Bisphenol A in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
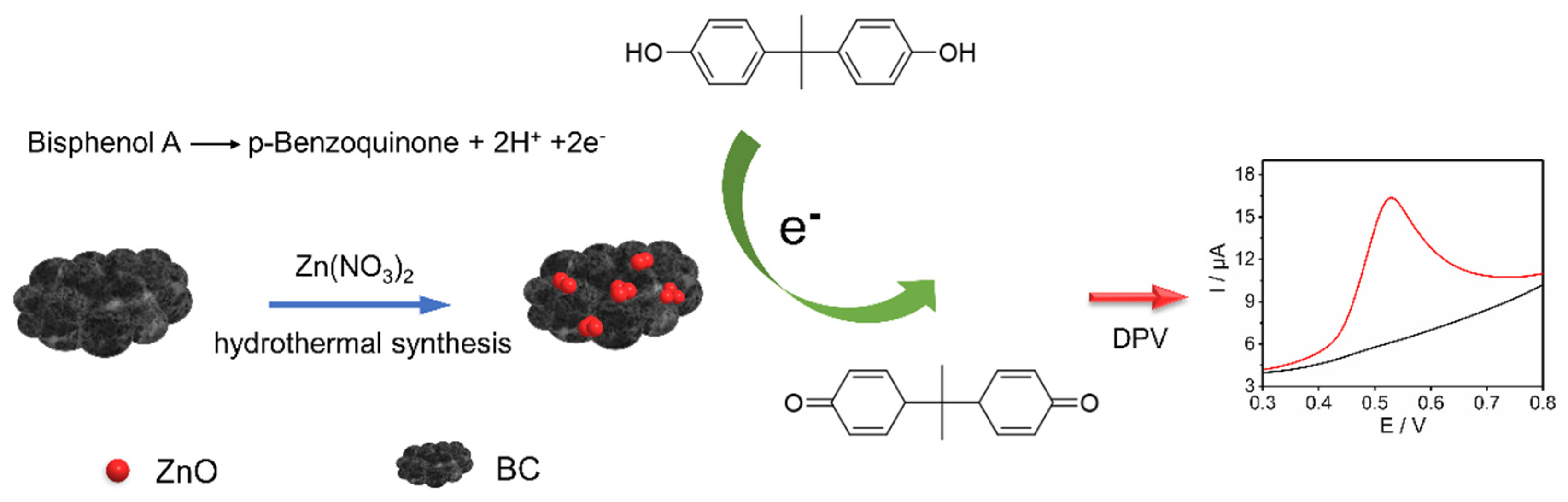
2.2. Preparation of ZnO/BC Nanohybrid
2.3. Preparation of ZnO/BC Electrochemical Sensor
2.4. Preparation of Actual Water Samples
2.5. Electrochemical Measurements
3. Results and Discussion
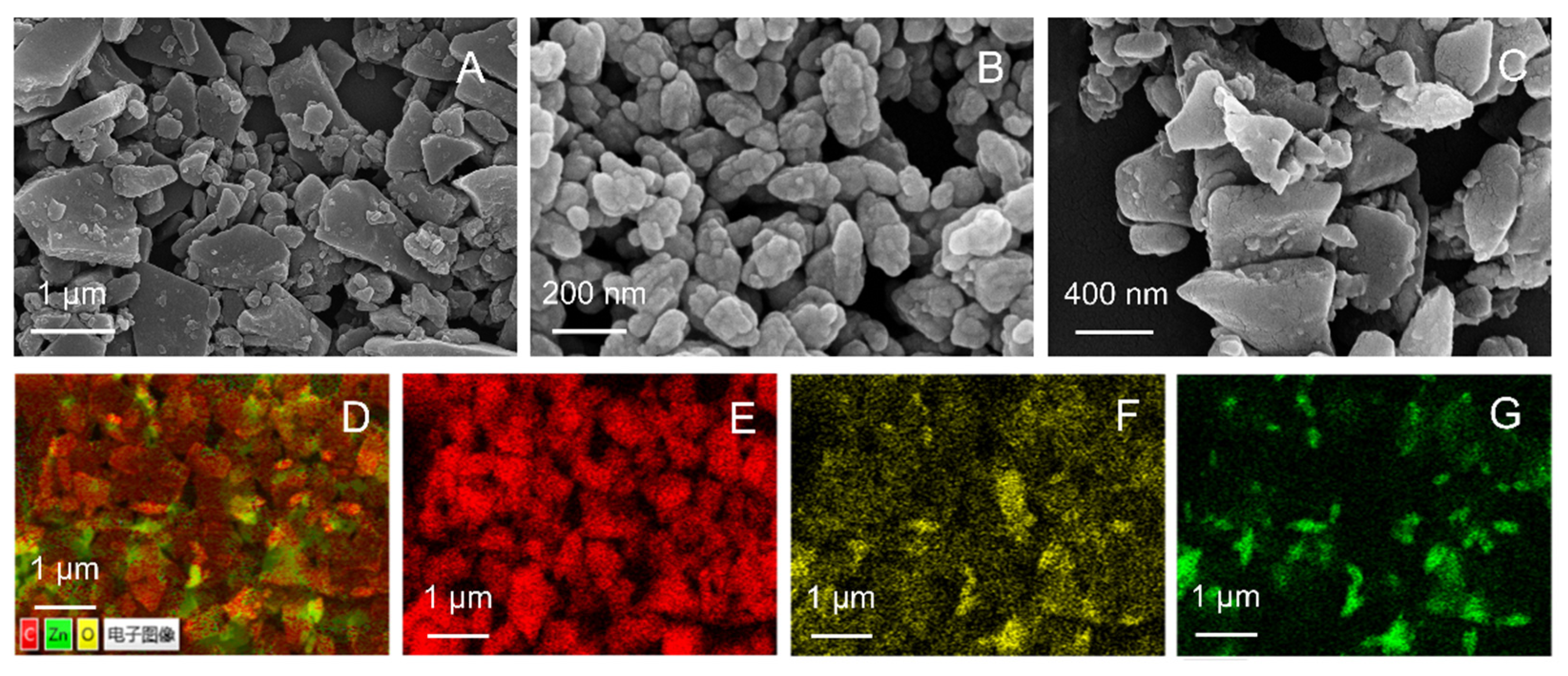
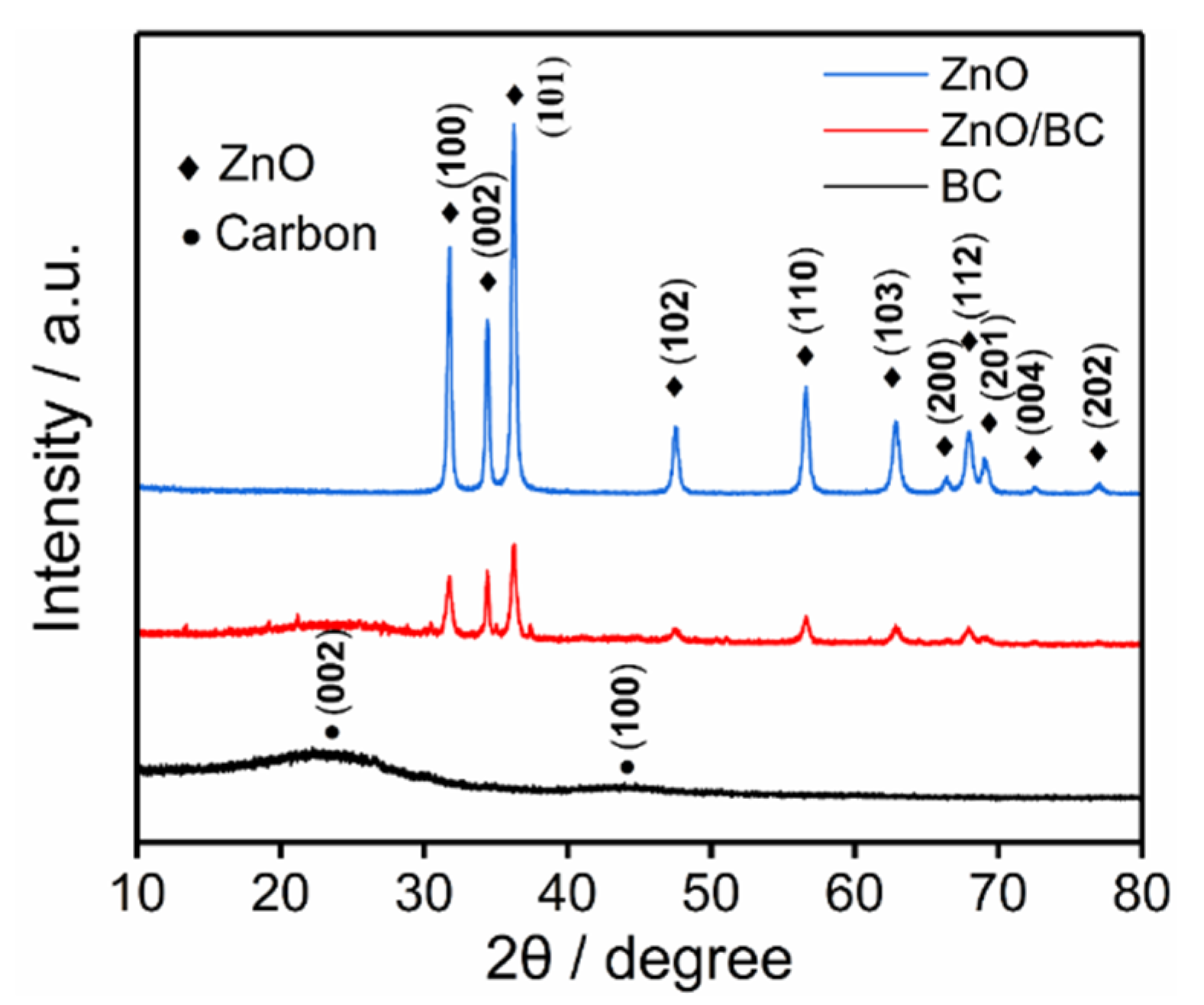
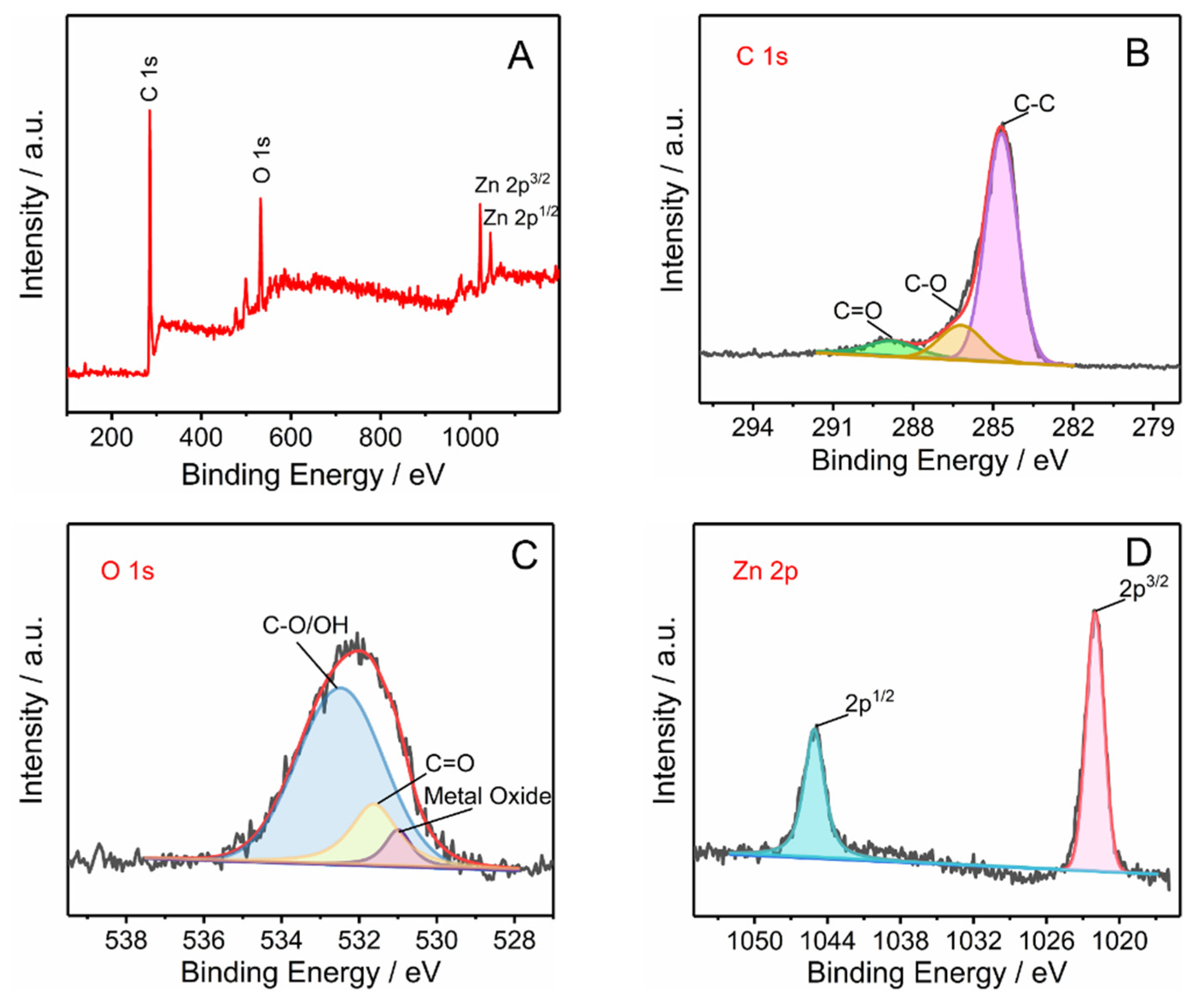
3.1. Characterization
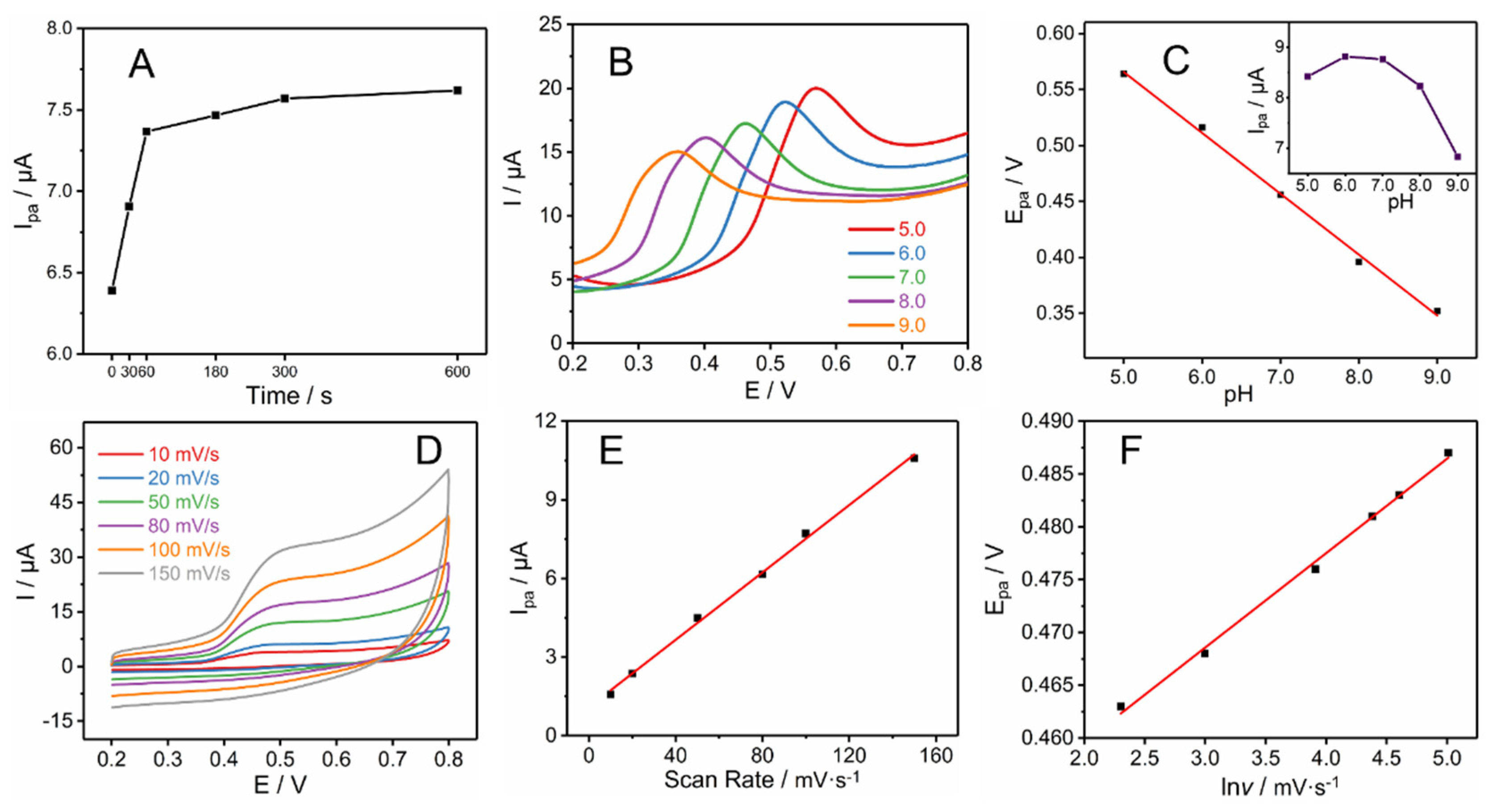
3.2. Experimental Parameter Optimization
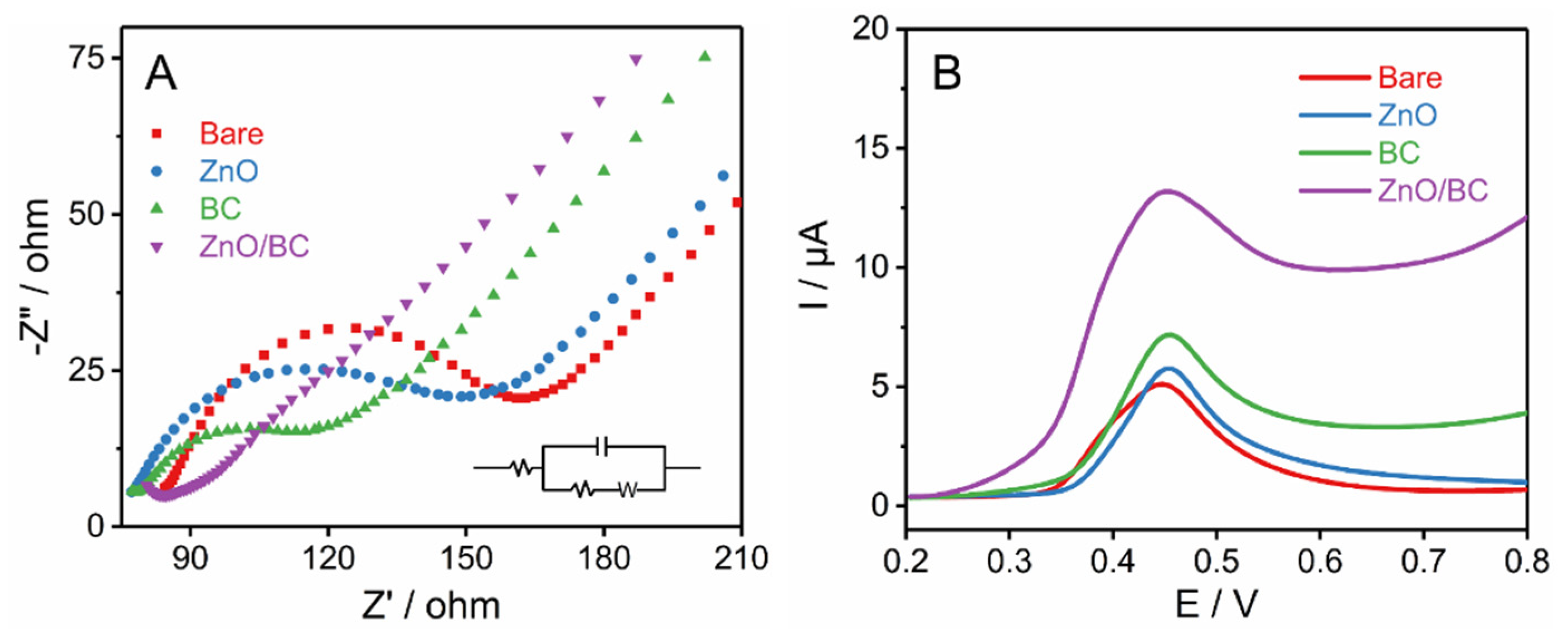
3.3. Mechanism Study of ZnO/BC Sensor for BPA Detection
3.4. Electrochemical Detection of BPA
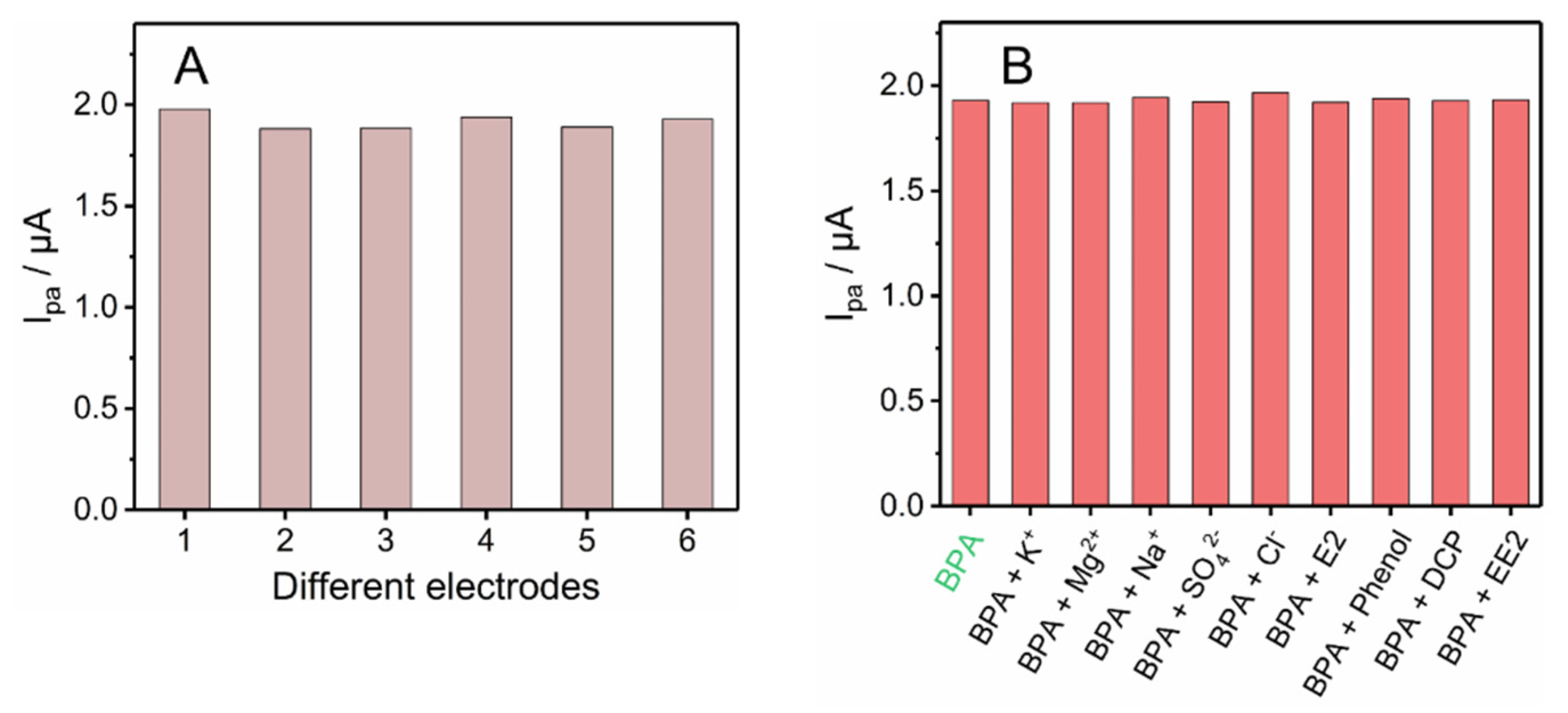
3.5. Reproducibility, Stability, Anti-Interference and Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, Y.J.; Lee, L.S. Aerobic Soil Biodegradation of Bisphenol (BPA) Alternatives Bisphenol S and Bisphenol AF Compared to BPA. Environ. Sci. Technol. 2017, 51, 13698–13704. [Google Scholar] [CrossRef]
- Bahmani, R.; Kim, D.G.; Modareszadeh, M.; Thompson, A.J.; Park, J.H.; Yoo, H.H.; Hwang, S. The mechanism of root growth inhibition by the endocrine disruptor bisphenol A (BPA). Environ. Pollut. 2020, 257, 113516. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, G.; Wang, P.; Zheng, H.; Zheng, Y. Microwave-enhanced Mn-Fenton process for the removal of BPA in water. Chem. Eng. J. 2016, 294, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Mirzajani, H.; Cheng, C.; Wu, J.; Chen, J.; Eda, S.; Najafi Aghdam, E.; Badri Ghavifekr, H. A highly sensitive and specific capacitive aptasensor for rapid and label-free trace analysis of Bisphenol A (BPA) in canned foods. Biosens. Bioelectron. 2017, 89, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Kubica, P.; Kudłak, B.; Rutkowska, A.; Konieczna, A.; Rachoń, D.; Namieśnik, J.; Wasik, A. Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography–tandem mass spectrometry. Sci. Total Environ. 2018, 628–629, 1362–1368. [Google Scholar] [CrossRef]
- Zhang, J.; Cooke, G.M.; Curran, I.H.A.; Goodyer, C.G.; Cao, X.L. GC-MS analysis of bisphenol A in human placental and fetal liver samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Maiolini, E.; Ferri, E.; Pitasi, A.L.; Montoya, A.; Di Giovanni, M.; Errani, E.; Girotti, S. Bisphenol A determination in baby bottles by chemiluminescence enzyme-linked immunosorbent assay, lateral flow immunoassay and liquid chromatography tandem mass spectrometry. Analyst 2014, 139, 318–324. [Google Scholar] [CrossRef]
- Moraes, F.C.; Silva, T.A.; Cesarino, I.; MacHado, S.A.S. Effect of the surface organization with carbon nanotubes on the electrochemical detection of bisphenol A. Sens. Actuators B Chem. 2013, 177, 14–18. [Google Scholar] [CrossRef]
- Güney, S.; Güney, O. Development of an Electrochemical Sensor Based on Covalent Molecular Imprinting for Selective Determination of Bisphenol-A. Electroanalysis 2017, 29, 2579–2590. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Song, J.; Liang, X.; Zhang, Z.; Men, D.; Wang, D.; Zhang, X.E. Chemical nature of electrochemical activation of carbon electrodes. Biosens. Bioelectron. 2019, 144, 111534. [Google Scholar] [CrossRef] [PubMed]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Soozanipour, A.; Low, Z.X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for wastewater monitoring: A review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, T.; Wang, Y.; Miao, P. Electrochemical aptasensors for detection of small molecules, macromolecules, and cells. Rev. Anal. Chem. 2016, 35, 201–211. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjugate Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Yan, B.; Zhang, H. An electrochemical sensor for the determination of bisphenol A using glassy carbon electrode modified with reduced graphene oxide-silver/poly-L-lysine nanocomposites. J. Electroanal. Chem. 2017, 805, 39–46. [Google Scholar] [CrossRef]
- Verma, D.; Yadav, A.K.; Das Mukherjee, M.; Solanki, P.R. Fabrication of a sensitive electrochemical sensor platform using reduced graphene oxide-molybdenum trioxide nanocomposite for BPA detection: An endocrine disruptor. J. Environ. Chem. Eng. 2021, 9, 105504. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Zeng, T.; Chen, X.; Wang, W.; Wan, Q.; Yang, N. Graphene nanoplatelet supported CeO2 nanocomposites towards electrocatalytic oxidation of multiple phenolic pollutants. Anal. Chim. Acta 2019, 1088, 45–53. [Google Scholar] [CrossRef] [PubMed]
- He, M.Q.; Wang, K.; Wang, J.; Yu, Y.L.; He, R.H. A sensitive aptasensor based on molybdenum carbide nanotubes and label-free aptamer for detection of bisphenol A. Anal. Bioanal. Chem. 2017, 409, 1797–1803. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Rajagopal, V.; Suryanarayanan, V. Highly sensitive and selective amperometric determination of BPA on carbon black/f-MWCNT composite modified GCE. J. Alloys Compd. 2019, 786, 698–706. [Google Scholar] [CrossRef]
- Campos, A.M.; Raymundo-Pereira, P.A.; Cincotto, F.H.; Canevari, T.C.; Machado, S.A.S. Sensitive determination of the endocrine disruptor bisphenol A at ultrathin film based on nanostructured hybrid material SiO2/GO/AgNP. J. Solid State Electrochem. 2016, 20, 2503–2507. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Peluso, M.A.; Thomas, H.; Damonte, L.C.; Sambeth, J.E. Structural and optical properties of ZnO and manganese-doped ZnO. J. Alloys Compd. 2016, 689, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Yin, F.; Zhang, C.; Guo, W.; Han, L.; Yuan, Y. Three-Dimensional Ordered Mesoporous Carbon Spheres Modified with Ultrafine Zinc Oxide Nanoparticles for Enhanced Microwave Absorption Properties. Nano-Micro Lett. 2021, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, Z.H.; Chen, Z.H.; Shafiq, I.; Zapien, J.A.; Bello, I. Different ZnO Nanostructures in Array Configurations & DESIGN 2009. Cryst. Growth Des. 2009, 9, 3222–3227. [Google Scholar]
- Shaikh, H.M.; Siva Kumar, N.; Mahmood, A. Synthesis of carbon nanoparticles/zinc oxide/zinc cobalt oxide composite and its electrochemical properties. Ceram. Int. 2020, 46, 18096–18100. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Du, W.; Shi, D.; Ning, W.; Lu, X.; Hou, Z. Metal-organic framework derived Cu/ZnO catalysts for continuous hydrogenolysis of glycerol. Appl. Catal. B Environ. 2017, 203, 146–153. [Google Scholar] [CrossRef]
- Guo, H.L.; Zhu, Q.; Wu, X.L.; Jiang, Y.F.; Xie, X.; Xu, A.W. Oxygen deficient ZnO1-x nanosheets with high visible light photocatalytic activity. Nanoscale 2015, 7, 7216–7223. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, Y.; Kim, J.; Yao, L.; Dong, X.; Li, T.; Piao, Y. Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol A sensing in water. Chem. Eng. J. 2020, 384, 123276. [Google Scholar] [CrossRef]
- Pang, Y.H.; Huang, Y.Y.; Wang, L.; Shen, X.F.; Wang, Y.Y. Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor. Environ. Pollut. 2020, 263, 114616. [Google Scholar] [CrossRef]
- Chakraborty, U.; Bhanjana, G.; Kaur, G.; Kaushik, A.; Chaudhary, G.R. Electro-active silver oxide nanocubes for label free direct sensing of bisphenol A to assure water quality. Mater. Today Chem. 2020, 16, 100267. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption characteristics and mechanism of bisphenol a by magnetic biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, X.; Liu, X.; Wang, L.; Liu, H.; Wang, H. Electrochemical determination of bisphenol A at ordered mesoporous carbon modified nano-carbon ionic liquid paste electrode. Talanta 2016, 148, 362–369. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Yang, D.; Luo, X.; Tang, Y.; Li, H. Sensitivity and selectivity determination of bisphenol A using SWCNT-CD conjugate modified glassy carbon electrode. J. Hazard. Mater. 2012, 199–200, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Malitesta, C.; Margapoti, E. Direct electrochemical detection of bisphenol A at PEDOT-modified glassy carbon electrodes. Anal. Bioanal. Chem. 2013, 405, 3587–3592. [Google Scholar] [CrossRef]
- Pogacean, F.; Biris, A.R.; Socaci, C.; Coros, M.; Magerusan, L.; Rosu, M.C.; Lazar, M.D.; Borodi, G.; Pruneanu, S. Graphene-bimetallic nanoparticle composites with enhanced electro-catalytic detection of bisphenol A. Nanotechnology 2016, 27, 484001. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Senthil Kumar, S. Stable and Sensitive Amperometric Determination of Endocrine Disruptor Bisphenol A at Residual Metal Impurities Within SWCNT. Electroanalysis 2018, 30, 445–452. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Xu, J.; Ai, S.; Cui, L.; Zhu, L. Amperometric biosensor based on tyrosinase immobilized onto multiwalled carbon nanotubes-cobalt phthalocyanine-silk fibroin film and its application to determine bisphenol A. Anal. Chim. Acta 2010, 659, 144–150. [Google Scholar] [CrossRef]

| Sensor 1 | Linear Range (μM) | Detection Limit (μM) | References |
|---|---|---|---|
| PEDOT/GCE | 90–410 and 40–410 | 50 and 22 | [34] |
| Au/ssDNA/SWCNT | 0.5–3.8 | 0.011 | [8] |
| Au/Gr-AgCu | 0.1–100 | 1.31 | [35] |
| SiO2/GO/AgNP/GCE | 0.1–2.6 | 0.045 | [18] |
| SWCNT/GCE | 10–100 | 7.3 | [36] |
| RGO-Ag/PLL/GCE | 1–80 | 0.54 | [15] |
| CTpPa-2/GCE | 0.1–50 | 0.02 | [28] |
| ZnO/BC/GCE | 0.5–100 | 0.1 | This work |
| Samples | Detected by the ZnO/BC Sensor | Detected by HPLC | ||||
|---|---|---|---|---|---|---|
| Added (μM) | Average found 1 (μM) | Recovery (%) | RSD (%) | Average found 1 (μM) | Recovery (%) | |
| Groundwater | 1.0 | 0.96 | 96.00 | 3.92 | 0.95 | 95.00 |
| 10.0 | 10.04 | 103.70 | 4.67 | 10.28 | 102.80 | |
| 100.0 | 100.06 | 100.06 | 5.41 | 99.01 | 99.01 | |
| Tap water | 1.0 | 1.02 | 102.00 | 2.94 | 0.99 | 99.15 |
| 10.0 | 9.92 | 99.17 | 3.28 | 9.70 | 96.99 | |
| 100.0 | 99.55 | 99.55 | 1.44 | 95.91 | 95.91 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Mao, D.; Duan, P.; Li, K.; Lin, Y.; Wang, X.; Piao, Y. Green Synthesis of ZnO/BC Nanohybrid for Fast and Sensitive Detection of Bisphenol A in Water. Chemosensors 2022, 10, 163. https://doi.org/10.3390/chemosensors10050163
Hu J, Mao D, Duan P, Li K, Lin Y, Wang X, Piao Y. Green Synthesis of ZnO/BC Nanohybrid for Fast and Sensitive Detection of Bisphenol A in Water. Chemosensors. 2022; 10(5):163. https://doi.org/10.3390/chemosensors10050163
Chicago/Turabian StyleHu, Jiafeng, Dongpeng Mao, Penghu Duan, Kelan Li, Yuqing Lin, Xinyao Wang, and Yunxian Piao. 2022. "Green Synthesis of ZnO/BC Nanohybrid for Fast and Sensitive Detection of Bisphenol A in Water" Chemosensors 10, no. 5: 163. https://doi.org/10.3390/chemosensors10050163
APA StyleHu, J., Mao, D., Duan, P., Li, K., Lin, Y., Wang, X., & Piao, Y. (2022). Green Synthesis of ZnO/BC Nanohybrid for Fast and Sensitive Detection of Bisphenol A in Water. Chemosensors, 10(5), 163. https://doi.org/10.3390/chemosensors10050163






