Development of a Paper-Based Analytical Method for the Selective Colorimetric Determination of Bismuth in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
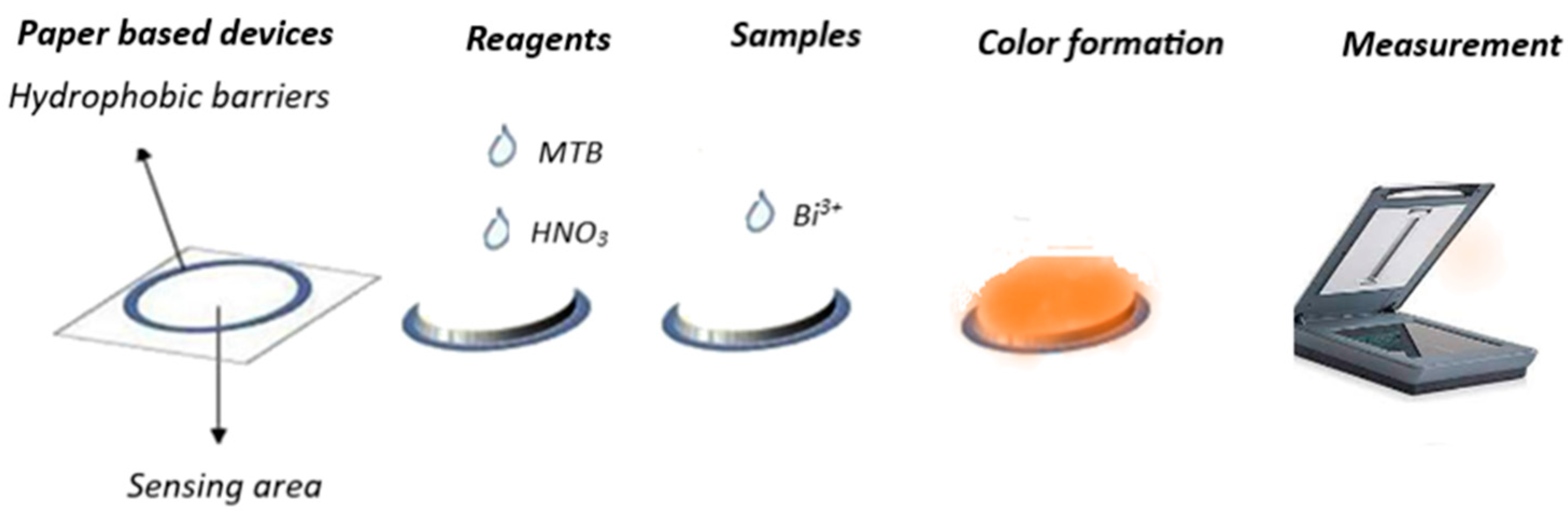
2.3. Fabrication of Paper Devices
2.4. Experimental Procedure
2.5. Real Samples
3. Results and Discussion
3.1. Optimization Procedures
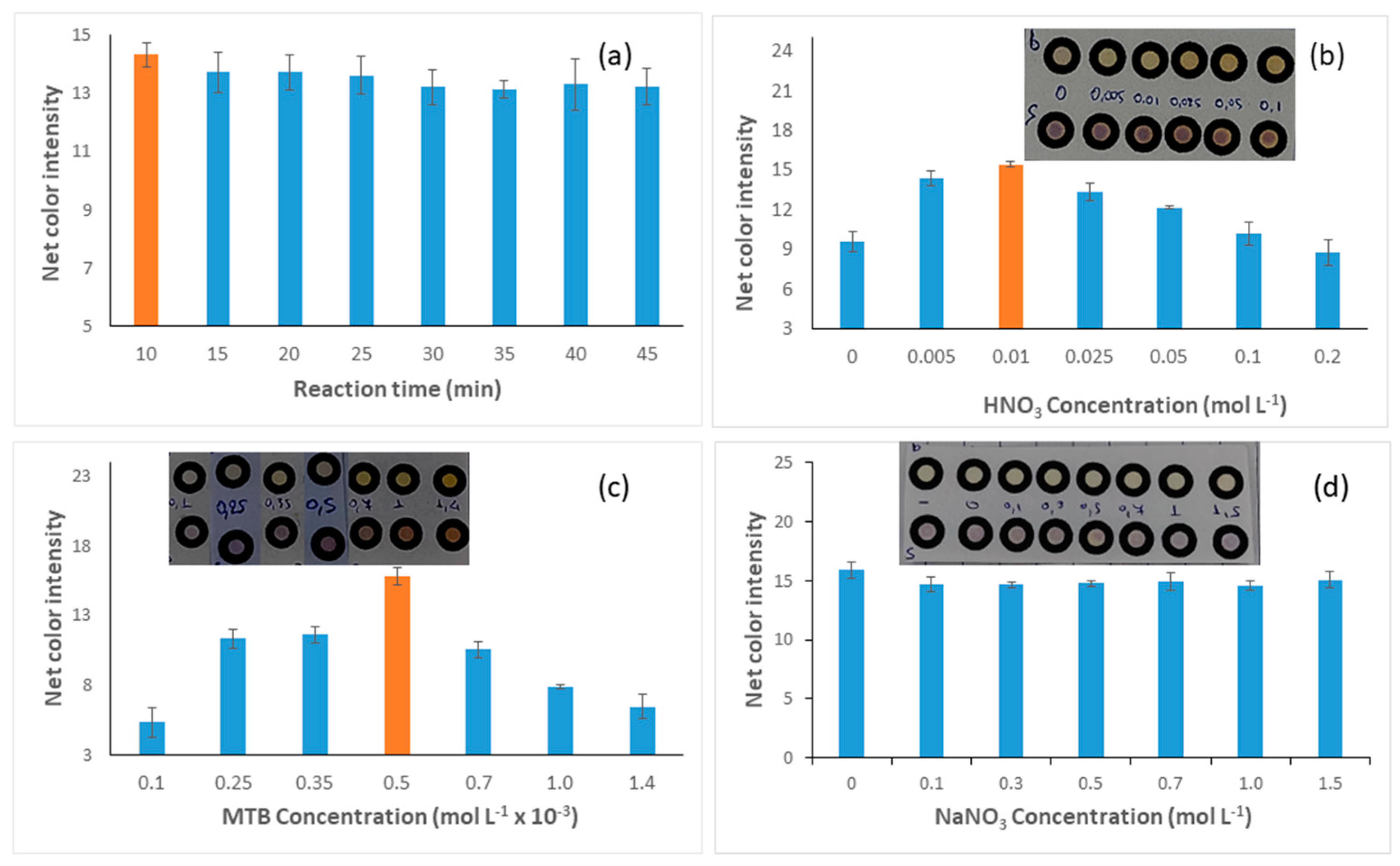
3.1.1. Effect of Reaction Time
3.1.2. Effect of Nitric Acid Concentration
3.1.3. Effect of MTB Concentration
3.1.4. Effect of Ionic Strength
3.1.5. Effect of the Detection Zone Size
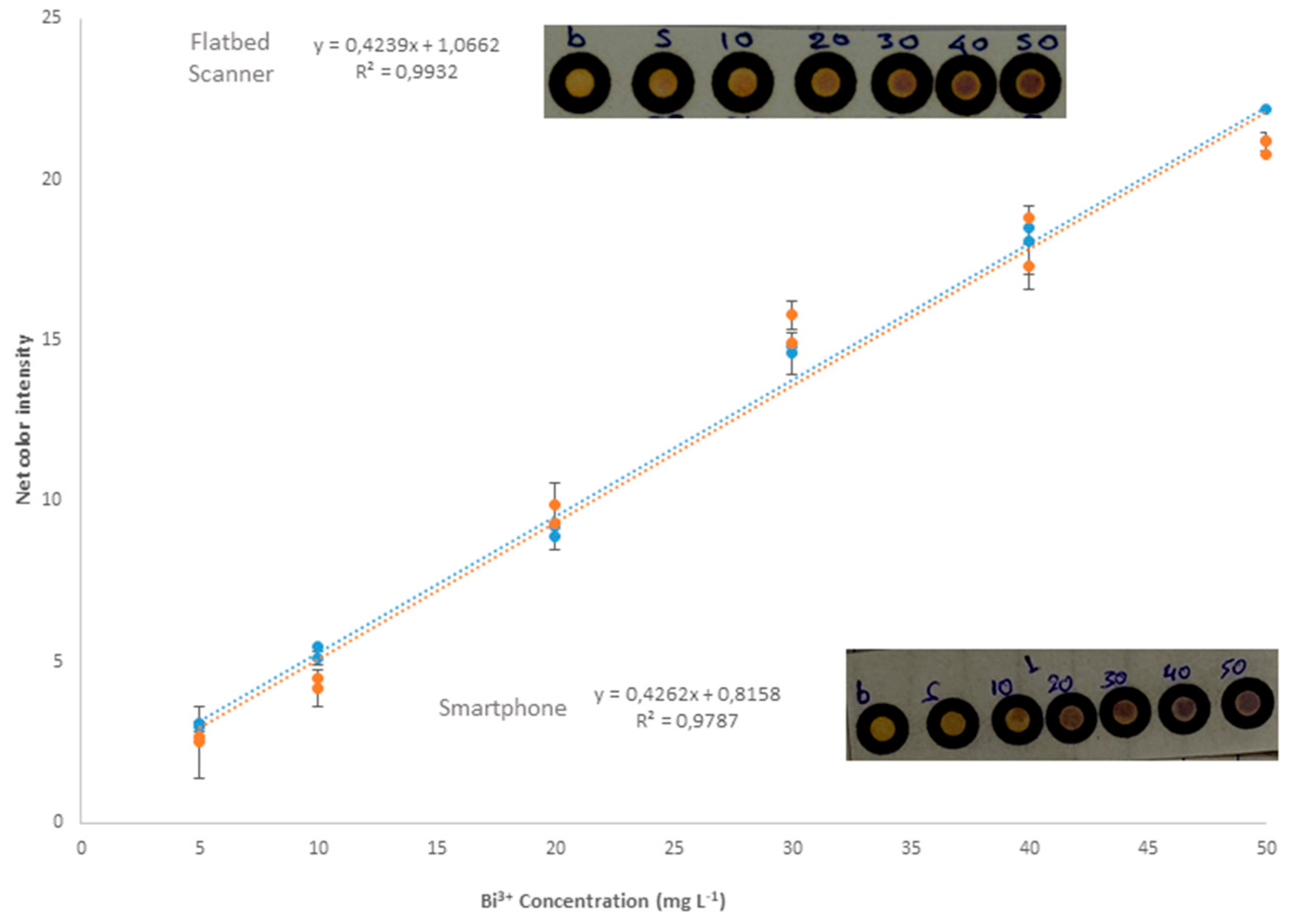
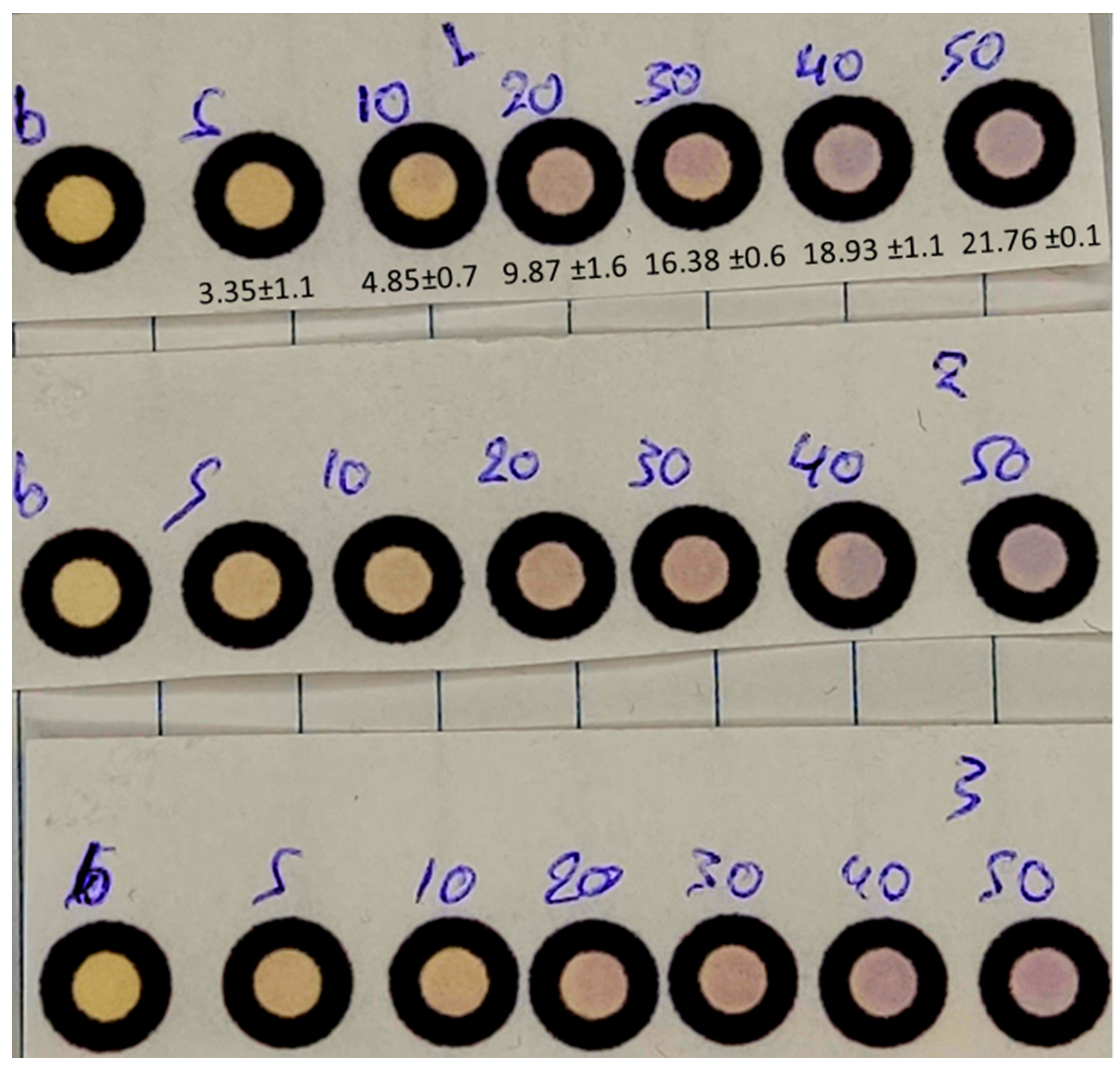
3.1.6. Effect of Photo-Capture Detector
3.2. Analytical Parameters
3.2.1. Linearity, Precision and Limits of Detection (LOD) and Quantification (LOQ)
3.2.2. Selectivity
3.2.3. Stability of the μPADS
3.3. Application in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, M.A.; Rahimi, M.; Fazelirada, H. A sensitive fluorescence quenching method for determination of bismuth with tiron. J. Lumin. 2014, 145, 976–980. [Google Scholar] [CrossRef]
- Calderilla, C.; Avivar, J.; Leal, L.O.; Cerdà, V. Multivariate optimisation of a rapid and simple automated method for bismuth determination in well water samples exploiting long path length spectrophotometry. Int. J. Environ. Anal. Chem. 2016, 96, 653–666. [Google Scholar] [CrossRef]
- Das, A.K.; Chakraborty, R.; Cervera, M.L.; de la Guardia, M. Analytical techniques for the determination of bismuth in solid environmental samples. Trends Anal. Chem. 2006, 25, 599–608. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Dobrzyńska, J.; Gawrońska, B. Determination of bismuth in environmental samples by slurry sampling graphite furnace atomic absorption spectrometry using combined chemical modifiers. Environ. Monit. Assess 2015, 187, 4125–4133. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, C.G.; Nunes, B.R.; Oss Giacomelli, M.B.; da Silva, J.B.B. Direct determination of bismuth in urine samples by electrothermal atomic absorption spectrometry: Study of chemical modifiers. J. Anal. At. Spectrom. 2003, 18, 787–789. [Google Scholar] [CrossRef]
- Matusiewicz, H. Atom trapping and in situ preconcentration techniques for flame atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1997, 52, 1711–1736. [Google Scholar] [CrossRef]
- Bodur, S.; Erarpat, S.; Selali Chormey, D.; Büyükpınar, C.; Bakırdere, S. Determination of bismuth in bottled and mineral water samples at trace levels by T-Shaped slotted quartz tube-atom trap-flame atomic absorption spectrometry. Anal. Lett. 2019, 52, 539–549. [Google Scholar] [CrossRef]
- Sun, M.; Wu, Q. Determination of trace bismuth in human serum by cloud point extraction coupled flow injection inductively coupled plasma optical emission spectrometry. J. Haz. Mat. 2011, 192, 935–939. [Google Scholar] [CrossRef]
- Moyano, S.; Wuilloud, R.G.; Olsina, R.A.; Gásquez, J.A.; Martinez, L.D. On-line preconcentration system for bismuth determination in urine by flow injection hydride generation inductively coupled plasma atomic emission spectrometry. Talanta 2001, 54, 211–219. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Q.; Bao, H.; Mou, Q.; Shi, Z.; Chen, Y.; Gao, Y. Determination of trace bismuth in environmental waters by ICP-MS with cobalt ion-assisted photochemical vapour generation. Geostand. Geoanal. Res. 2020, 44, 617–627. [Google Scholar] [CrossRef]
- Okano, G.; Igarashi, S.; Ohno, O.; Yamamoto, Y.; Saito, S.; Oka, Y. Determination of trace amounts of bismuth in steel by ICP-MS through a cascade-preconcentration and separation method. ISIJ Int. 2015, 55, 332–334. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guo, Y. Simultaneous determination of trace arsenic, antimony, bismuth, and selenium in biological samples by hydride generation-four-channel atomic fluorescence spectrometry. Talanta 2005, 65, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, P.C.A.; Araújo, A.N.; Montenegro, M.C.B.S.M.; Satinský, D.; Solich, P. Colorimetric bismuth determination in pharmaceuticals using a xylenol orange sol–gel sensor coupled to a multicommutated flow system. Anal. Chim. Acta 2004, 504, 235–241. [Google Scholar] [CrossRef]
- Gumus, G.; Filik, H.; Demirata, B. Determination of bismuth and zinc in pharmaceuticals by first derivative UV–Visible spectrophotometry. Anal. Chim. Acta 2005, 547, 138–143. [Google Scholar] [CrossRef]
- Tzanavaras, P.D.; Themelis, D.G.; Economou, A. Sequential injection method for the direct spectrophotometric determination of bismuth in pharmaceutical products. Anal. Chim. Acta 2004, 505, 167–171. [Google Scholar] [CrossRef]
- Themelis, D.G.; Tzanavaras, P.D.; Papadimitriou, J.K. Flow injection manifold for the direct spectrophotometric determination of bismuth in pharmaceutical products using Methylthymol Blue as a chromogenic reagent. Analyst 2001, 126, 247–250. [Google Scholar] [CrossRef]
- Szpunar-Łobińska, J. Extraction flow-injection spectrophotometric determination of bismuth with lead tetramethylenedithiocarbamate. Anal. Chim. Acta 1991, 251, 275–280. [Google Scholar] [CrossRef]
- González-Portal, A.; Bermejo-Martínez, F.; Baluja-Santos, C.; Diez-Rodríguez, M.C. Spectrophotometric study of bismuth with N-(2-acetamido)iminodiacetic acid (ADA): Determination of bismuth in pharmaceutical formulations. Microchem. J. 1985, 31, 368–374. [Google Scholar] [CrossRef]
- Mohammadi, S.; Khayatian, G. Colorimetric detection of Bi (III) in water and drug samples using pyridine-2,6-dicarboxylic acid modified silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 405–411. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Mohammed, M.M. Spectrophotometric determination of bismuth with alizarin red S and cetylpyridinium chloride—Application to water samples, urine and veterinary preparation. Raf. J. Sci. 2013, 24, 52–63. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Aldhaheri, S.M. Spectrophotometric determination of bismuth (III and V) in water after ion-pair liquid–liquid extraction using tetramethylammonium cation as counter ion. Fresenius J. Anal. Chem. 1996, 354, 200–203. [Google Scholar] [CrossRef]
- Rasouli, Z.; Irani, M.; Jafari, S.; Ghavami, R. Study of interaction of metal ions with methylthymol blue by chemometrics and quantum chemical calculations. Sci. Rep. 2021, 11, 6465–6483. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, Z.; Hassanzadeh, Z.; Ghavami, R. Application of a new version of GA-RBF neural network for simultaneous spectrophotometric determination of Zn(II), Fe(II), Co(II) and Cu(II) in real samples: An exploratory study of their complexation abilities toward MTB. Talanta 2016, 160, 86–98. [Google Scholar] [CrossRef]
- Pourreza, N.; Zavvar Mousavi, H. Solid phase preconcentration of iron as methylthymol blue complex on naphthalene-tetraoctylammonium bromide adsorbent with subsequent flame atomic absorption determination. Talanta 2004, 64, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.; Ruf, H.; Grell, E. Kinetics and mechanism of complex formation between Mg2+ and methylthymol blue. J. Phys. Chem. A 1998, 102, 146–152. [Google Scholar] [CrossRef]
- Themelis, D.G.; Tzanavaras, P.D.; Anthemidis, A.N.; Stratis, J.A. Direct, selective flow injection spectrophotometric determination of calcium in wines using methylthymol blue and an on-line cascade dilution system. Anal. Chim. Acta 1999, 402, 259–266. [Google Scholar] [CrossRef]
- Sandell, E.B. Colorimetric Determination of Traces of Metals, 3rd ed.; Interscience Publishers: New York, NY, USA, 1959; Volume III, pp. 157–168. [Google Scholar]
- Sadaaki, M.; Takashi, Y. Equilibria of aluminium (III) complexes with methylthymol blue. Polyhedron 1982, 1, 405–408. [Google Scholar] [CrossRef]
- Cheng, K. Determination of zirconium and hafnium with xylenol orange and methylthymol blue. Anal. Chim. Acta 1963, 28, 41–53. [Google Scholar] [CrossRef]
- Karadakov, B.; Kantcheva, D.; Nenova, P. Spectrophotometric study of the reaction of iron(iii) with methylthymol blue. Talanta 1968, 15, 525–534. [Google Scholar] [CrossRef]
- Kiss, T.A.; Knežević, O.I. Complexometric determination of magnesium in magnesium stearate. Microchem. J. 1968, 13, 459–462. [Google Scholar] [CrossRef]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in paper-based analytical devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattanarat, P.; Dungchai, W.; Cate, D.; Volckens, J.; Chailapakul, O.; Henry, C.S. Multilayer paper-based device for colorimetric and electrochemical quantification of metals. Anal. Chem. 2014, 86, 3555–3562. [Google Scholar] [CrossRef] [PubMed]
- Kappi, F.A.; Tsogas, G.Z.; Routsi, A.M.; Christodouleas, D.C.; Giokas, D.L. Paper-based devices for biothiols sensing using the photochemical reduction of silver halides. Anal. Chim. Acta 2018, 1036, 89–96. [Google Scholar] [CrossRef]
- Katelakha, K.; Nopponpunth, V.; Boonlue, W.; Laiwattanapaisal, W. A simple distance paper-based analytical device for the screening of lead in food matrices. Biosensors 2021, 11, 90. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, D.; Li, W.; Li, X.; Chang, Y.; Zhang, Q.; Liu, M. An origami paper-based analytical device for rapid and sensitive analysis of acrylamide in foods. Micromachines 2022, 13, 13. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, flow control, and applications of microfluidic paper-based analytical devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [Green Version]
- Tarara, M.; Giokas, D.L.; Tsogas, G.Z. Wax-Printed fluidic controls for delaying and accelerating fluid transport on paper-based analytical devices. Chemosensors 2022, 10, 155. [Google Scholar] [CrossRef]
- Kappi, F.A.; Papadopoulos, G.A.; Tsogas, G.Z.; Giokas, D.L. Low-cost colorimetric assay of biothiols based on the photochemical reduction of silver halides and consumer electronic imaging devices. Talanta 2017, 172, 15–22. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Migliorelli, D.; Uttl, L.; Filippini, D.; Pulkrabova, J.; Hajslova, J. A microfluidic paper-based analytical device (μPAD) with smartphone readout for chlorpyrifos-oxon screening in human serum. Talanta 2021, 222, 121535. [Google Scholar] [CrossRef]
- Manori Jayawardane, B.; Wongwilai, W.; Grudpan, K.; Kolev, S.D.; Heaven, M.W.; Nash, D.M.; McKelvie, I.D. Evaluation and application of a paper-based device for the determination of reactive phosphate in soil solution. J. Environ. Qual. 2014, 43, 1081–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Time (Days) | |||
|---|---|---|---|
| 2 | 4 | 6 | |
| Temperature (°C) | % Recovery | ||
| 25 | 98.5 | 92.8 | 86.2 |
| 4 | 101.9 | 98.5 | 94.4 |
| −18 | 101.3 | 97.6 | 95.5 |
| Samples | Spiked (mg L−1) | % Recovery (± RSD, n = 3) |
|---|---|---|
| River water 1 | 10 | 105.9 ± 3.2 |
| 30 | 89.5 ± 3.7 | |
| River water 2 | 10 | 83.7 ± 5.1 |
| 30 | 90.5 ± 3.6 | |
| Bottled water 1 | 10 | 89.9 ± 5.6 |
| 30 | 87.2 ± 5.9 | |
| Bottled water 2 | 10 | 111.4 ± 9.8 |
| 30 | 82.8 ± 2.7 | |
| Tap water | 10 | 115.4 ± 6.9 |
| 30 | 83.7 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizirtsakis, P.A.; Tarara, M.; Tsiasioti, A.; Tzanavaras, P.D.; Tsogas, G.Z. Development of a Paper-Based Analytical Method for the Selective Colorimetric Determination of Bismuth in Water Samples. Chemosensors 2022, 10, 265. https://doi.org/10.3390/chemosensors10070265
Bizirtsakis PA, Tarara M, Tsiasioti A, Tzanavaras PD, Tsogas GZ. Development of a Paper-Based Analytical Method for the Selective Colorimetric Determination of Bismuth in Water Samples. Chemosensors. 2022; 10(7):265. https://doi.org/10.3390/chemosensors10070265
Chicago/Turabian StyleBizirtsakis, Panagiotis A., Maria Tarara, Apostolia Tsiasioti, Paraskevas D. Tzanavaras, and George Z. Tsogas. 2022. "Development of a Paper-Based Analytical Method for the Selective Colorimetric Determination of Bismuth in Water Samples" Chemosensors 10, no. 7: 265. https://doi.org/10.3390/chemosensors10070265
APA StyleBizirtsakis, P. A., Tarara, M., Tsiasioti, A., Tzanavaras, P. D., & Tsogas, G. Z. (2022). Development of a Paper-Based Analytical Method for the Selective Colorimetric Determination of Bismuth in Water Samples. Chemosensors, 10(7), 265. https://doi.org/10.3390/chemosensors10070265









