Gas Sensitive Characteristics of Polyaniline Decorated with Molybdenum Ditelluride Nanosheets
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Synthesis of MoTe2/PANI Composites
2.3. Characterization
2.4. Fabrication of NH3 Gas Sensor
2.5. Gas-Sensing Test
3. Results and Discussion
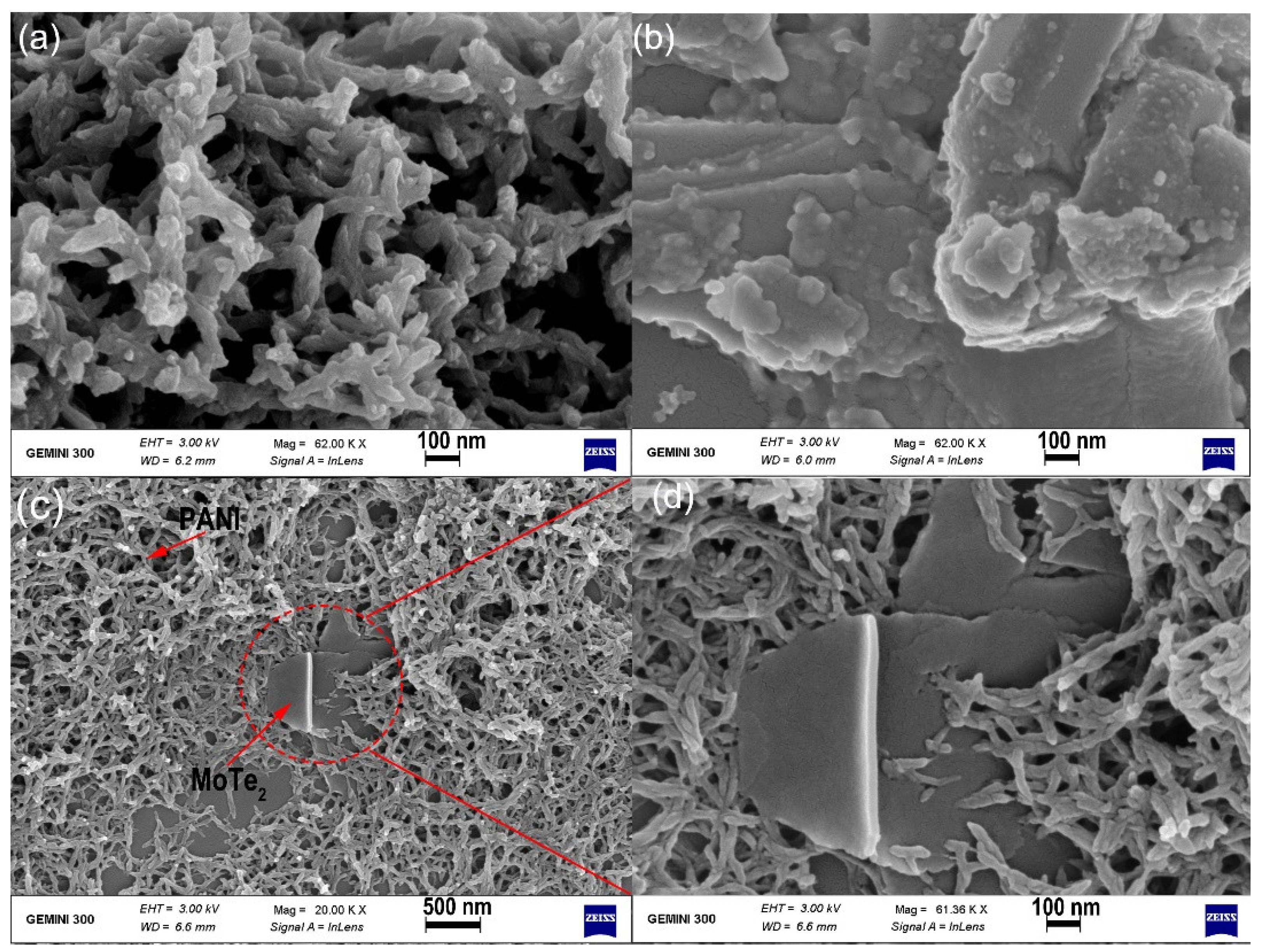
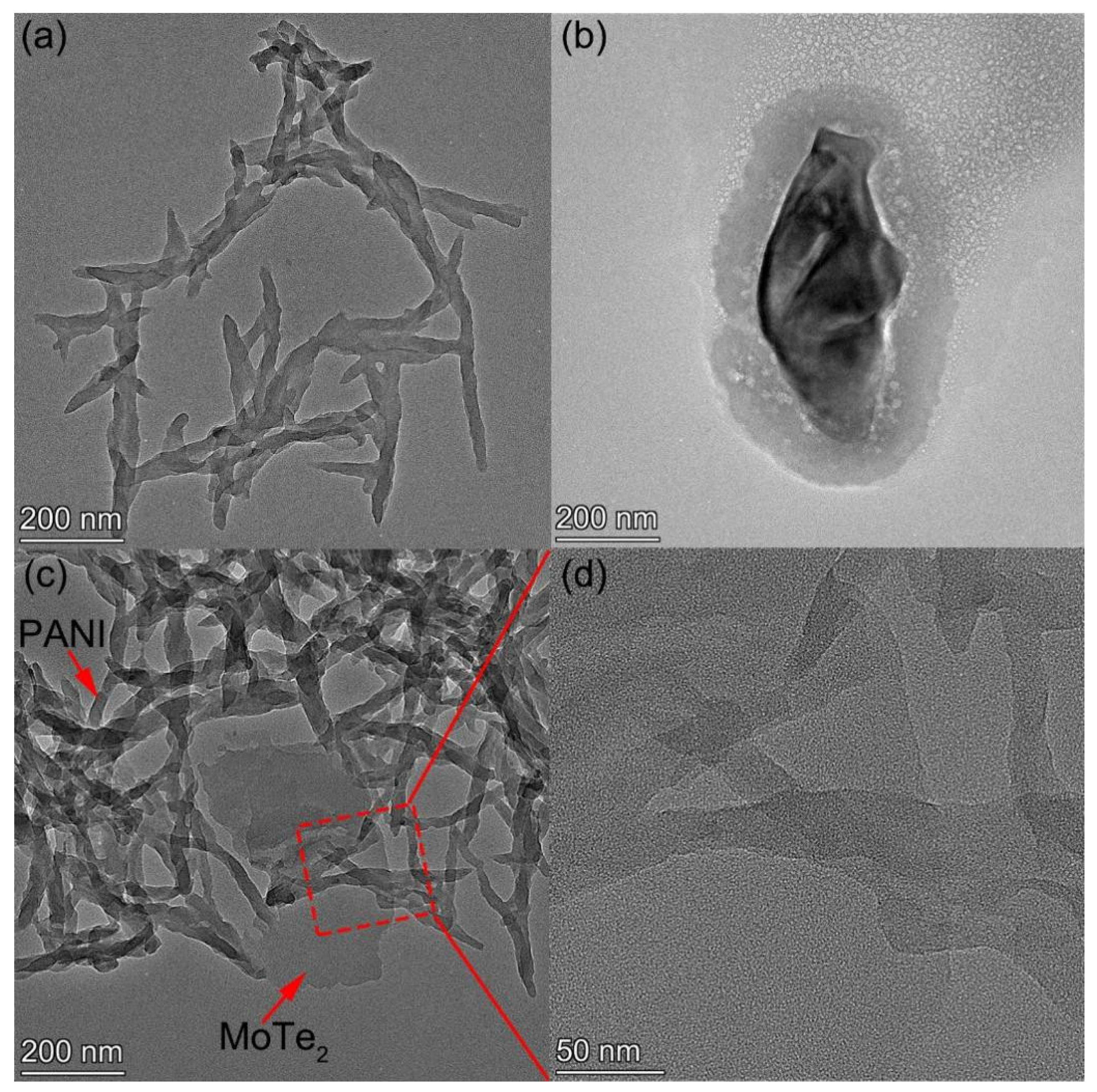
3.1. The Characterization of Morphology and Microstructure
3.2. FTIR Analysis
3.3. NH3 Gas Sensing Properties
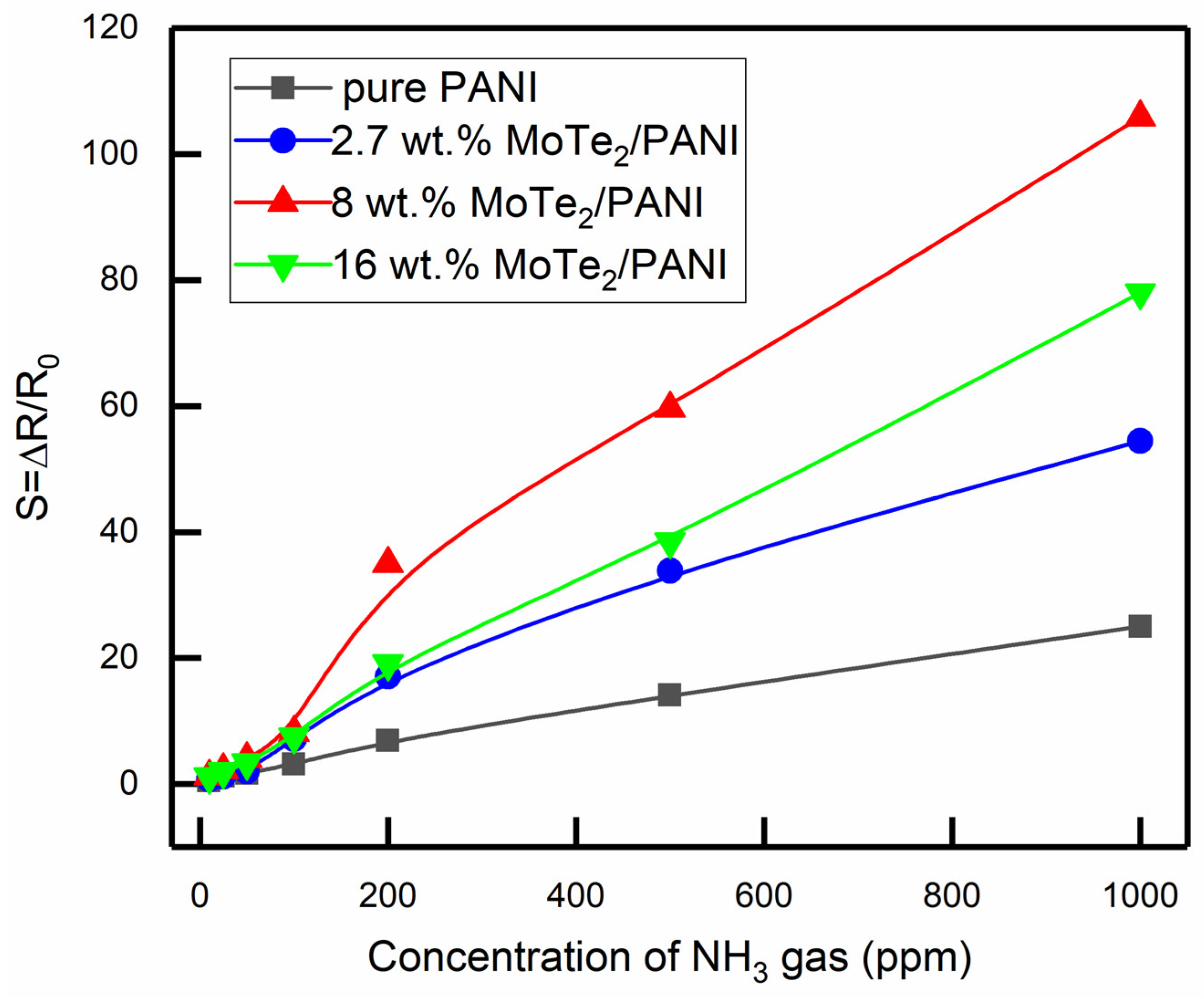
3.3.1. Gas Response
3.3.2. Response and Recovery Times
3.3.3. Repeatability and Selectivity
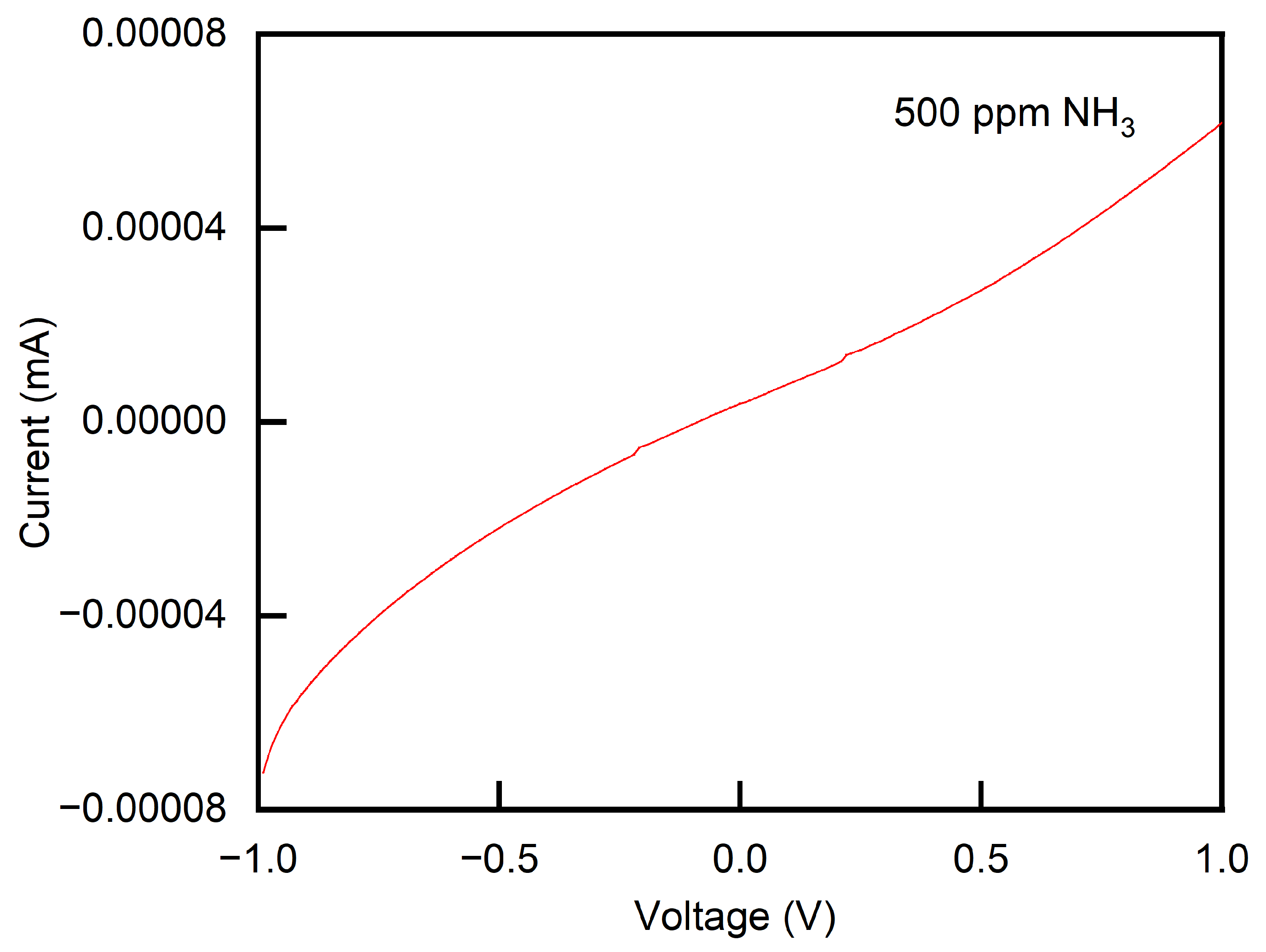
3.3.4. Enhanced Mechanism for the Gas-Sensitive Response of MoTe2/PANI Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Li, T.; Ma, Y.; Wei, Q.; Qiu, W.; Guo, H.; Shi, X.; Zhang, P.; Asiri, A.M.; Chen, L.; et al. Boosted electrocatalytic N2 reduction to NH3 by defect-rich MoS2 nanoflower. Adv. Energy Mater. 2018, 8, 1801357. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Io, J.; Sugiyama, G.; Sakai, Y. Effect of NH3 gas on the electrical conductivity of polyaniline blend films. Synth. Met. 2002, 128, 15–19. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, Y.; Sun, J.; Tian, Y.; Luo, R.; Li, D.; Chen, A. Ultrasensitive room temperature NH3 sensor based on a graphene–polyaniline hybrid loaded on PET thin film. Chem. Commun. 2015, 51, 7524–7527. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Y.; Wan, P.; Zhang, H.; Chen, X.; Sun, X. Flexible transparent electronic gas sensors. Small 2016, 12, 3748–3756. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Jiang, Y.; He, Z.; Liu, B.; Xie, H.; Li, H.; Li, Z.; Wang, Y.; Tai, H. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2Tx hybrid sensitive films based gas sensor. Sens. Actuators B Chem. 2020, 316, 128144. [Google Scholar] [CrossRef]
- Kulkarni, S.; Navale, Y.; Navale, S.; Stadler, F.; Ramgir, N.; Patil, V. Hybrid polyaniline-WO3 flexible sensor: A room temperature competence towards NH3 gas. Sens. Actuators B Chem. 2019, 288, 279–288. [Google Scholar] [CrossRef]
- Hien, H.T.; Giang, H.T.; van Hieu, N.; Trung, T.; van Tuan, C. Elaboration of Pd-nanoparticle decorated polyaniline films for room temperature NH3 gas sensors. Sens. Actuators B Chem. 2017, 249, 348–356. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Liu, B.; Duan, Z.; Pan, H.; Yuan, Z.; Xie, G.; Wang, J.; Fang, Z.; Tai, H. Ultrathin Nb2CTx nanosheets-supported polyaniline nanocomposite: Enabling ultrasensitive NH3 detection. Sens. Actuators B Chem. 2021, 343, 130069. [Google Scholar] [CrossRef]
- Jha, R.K.; Wan, M.; Jacob, C.; Guha, P.K. Ammonia vapour sensing properties of in situ polymerized conducting PANI-nanofiber/WS2 nanosheet composites. New J. Chem. 2018, 42, 735–745. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.; Jernigan, G.; Jonker, B.T. Chemical vapor sensing with monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Doneux, T.; Bougouma, M. Single-layer MoSe2 based NH3 gas sensor. Appl. Phys. Lett. 2014, 105, 233103. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, Y.; Chen, J.; Yu, Y.; Zheng, S.; Zhang, R.; Li, Q.; Chen, X.; Sun, C.; Zhang, H.; et al. Highly sensitive MoTe2 chemical sensor with fast recovery rate through gate biasing. 2D Mater. 2017, 4, 025018. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Zhu, X.; Wang, Y.; Ren, H.; Wang, Y.; Gao, C.; Guo, Y. Humidity enhanced ammonia sensing of porous polyaniline/tungsten disulfide nanocomposite film. Sens. Actuators B Chem. 2020, 323, 128699. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sens. Actuators B Chem. 2018, 258, 895–905. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, Y.; Pol, V.G.; Gedanken, A. Synthesis of α-MoTe2 nanorods via annealing te-seeded amorphous MoTe2 particles. Inorg. Chem. 2004, 43, 6061–6066. [Google Scholar] [CrossRef]
- Panigrahi, P.; Hussain, T.; Karton, A.; Ahuja, R. Elemental substitution of two-dimensional transition metal dichalcogenides (MoSe2 and MoTe2): Implications for enhanced gas sensing. ACS Sens. 2019, 4, 2646–2653. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Kim, J.H.; Zhao, J.; Seok, J.; Keum, D.H.; Baik, J.; Choe, D.H.; Chang, K.J. Phase patterning for ohmic homojunction contact in MoTe2. Science 2015, 349, 625–628. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; He, Z.; Shen, Q. One-pot synthesis of non-precious metal RGO/1T′-MoTe2: Cu heterohybrids for excellent catalytic hydrogen evolution. Mater. Sci. Eng. B 2020, 260, 114659. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Ding, X.; Yu, X.; Yu, X. Gas-sensitive enhancement of rGO/HMWCNTs/PANI ternary composites. IEEE Sens. J. 2021, 22, 1905–1915. [Google Scholar] [CrossRef]
- Trchová, M.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Yan, C.; Zou, L.; Short, R. Single-walled carbon nanotubes and polyaniline composites for capacitive deionization. Desalination 2012, 290, 125–129. [Google Scholar] [CrossRef]
- Ginic-Markovic, M.; Matisons, J.G.; Cervini, R.; Simon, G.P.; Fredericks, P.M. Synthesis of new polyaniline/nanotube composites using ultrasonically initiated emulsion polymerization. Chem. Mater. 2006, 18, 6258–6265. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A.J.S. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, N.; Wang, H.; Xu, J.; Pan, F. 3D conductive network-based free-standing PANI–RGO–MWNTs hybrid film for high-performance flexible supercapacitor. J. Mater. Chem. A 2014, 2, 12340–12347. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Singh, B.P.; Mathur, R.B.; Dhawan, S.K. Polyaniline–MWCNT nanocomposites for microwave absorption and EMI shielding. Mater. Chem. Phys. 2009, 113, 919–926. [Google Scholar] [CrossRef]
- Andre, R.S.; Shimizu, F.M.; Miyazaki, C.M.; Riul, A., Jr.; Manzani, D.; Ribeiro, S.J.; Oliveira, O.N., Jr.; Mattoso, L.H.; Correa, D.S. Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sens. Actuators B Chem. 2017, 238, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Bandgar, D.K.; Navale, S.T.; Navale, Y.H.; Ingole, S.M.; Stadler, F.J.; Ramgir, N.; Aswal, D.K.; Gupta, S.K.; Mane, R.S.; Patil, V.B. Flexible camphor sulfonic acid-doped PAni/α-Fe2O3 nanocomposite films and their room temperature ammonia sensing activity. Mater. Chem. Phys. 2017, 189, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Ramgir, N.S.; Debnath, A.K.; Gadkari, S.C.; Gupta, S.K.; Aswal, D.K.; Patil, V.B. Enhanced ammonia sensing characteristics of tungsten oxide decorated polyaniline hybrid nanocomposites. Org. Electron. 2017, 45, 65–73. [Google Scholar] [CrossRef]
- Tohidi, S.; Parhizkar, M.; Bidadi, H.; Mohamad-Rezaei, R.J.N. High-performance chemiresistor-type NH3 gas sensor based on three-dimensional reduced graphene oxide/polyaniline hybrid. Nanotechnology 2020, 31, 415501. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Duan, Z.; Zhao, Q.; Zhang, Y.; Xie, G.; Jiang, Y.; Li, S.; Tai, H. PANI nanofibers-supported Nb2CTx nanosheets-enabled selective NH3 detection driven by TENG at room temperature. Sens. Actuators B Chem. 2021, 327, 128923. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Hu, Z.; Zhou, Z.; Deng, Y. Ultrasensitive NH3 gas sensor from polyaniline nanograin enchased TiO2 fibers. J. Phys. Chem. C 2010, 114, 9970–9974. [Google Scholar] [CrossRef]
- Rigoni, F.; Drera, G.; Pagliara, S.; Perghem, E.; Pintossi, C.; Goldoni, A.; Sangaletti, L. Gas sensing at the nanoscale: Engineering SWCNT-ITO nano-heterojunctions for the selective detection of NH3 and NO2 target molecules. Nanotechnology 2016, 28, 035502. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Wang, X. A high performance sensor based on PANI/ZnTi-LDHs nanocomposite for trace NH3 detection. Org. Electron. 2019, 66, 102–109. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, E.; Zhang, J.; Hu, X.; Zhang, D.; Liu, J. Gate-tunable photodetection/voltaic device based on BP/MoTe2 heterostructure. ACS Appl. Mater. Interfaces 2019, 11, 14215–14221. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Metal-organic frameworks-derived zinc oxide nanopolyhedra/S, N: Graphene quantum dots/polyaniline ternary nanohybrid for high-performance acetone sensing. Sens. Actuators B Chem. 2019, 288, 232–242. [Google Scholar] [CrossRef]


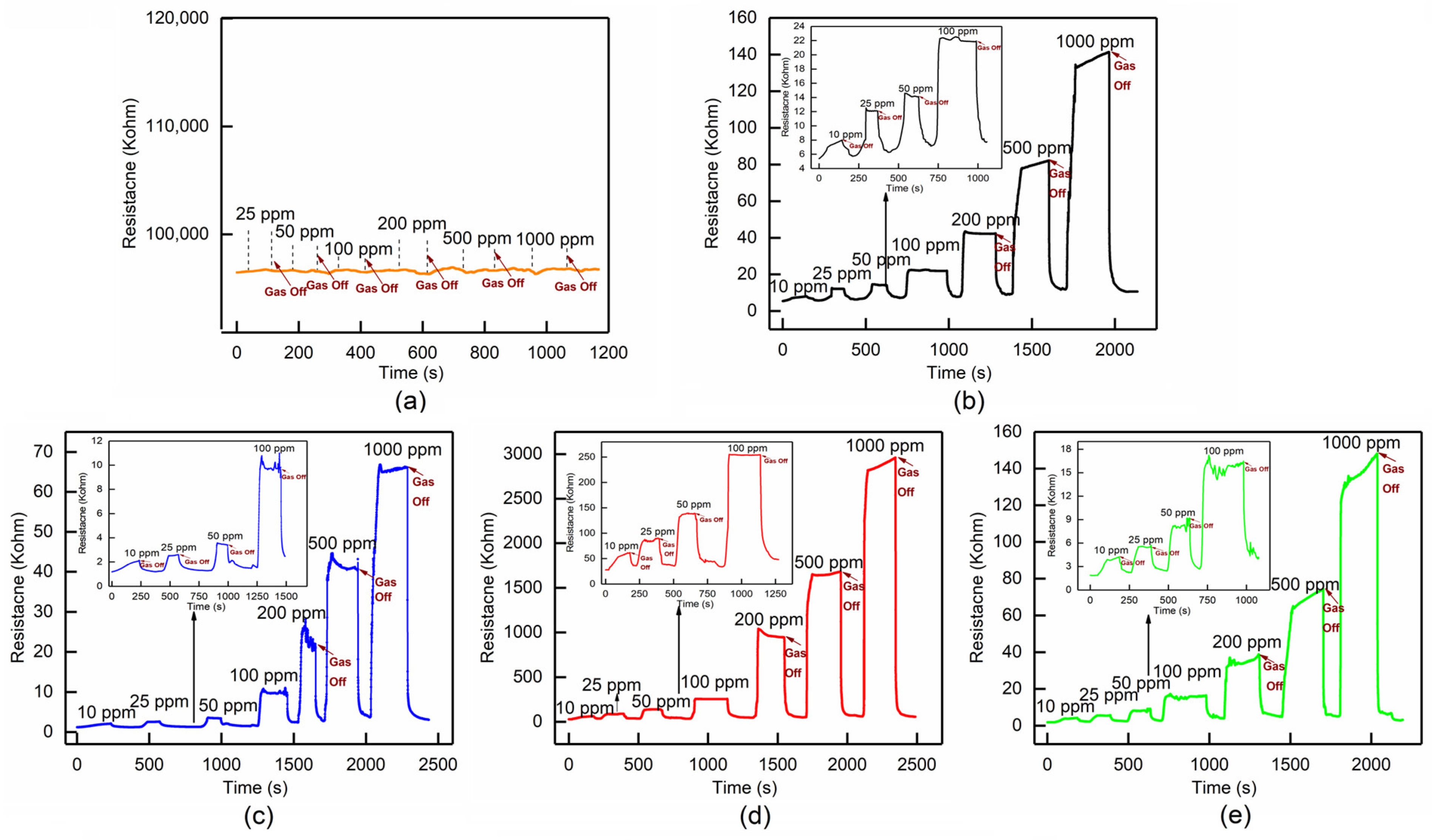

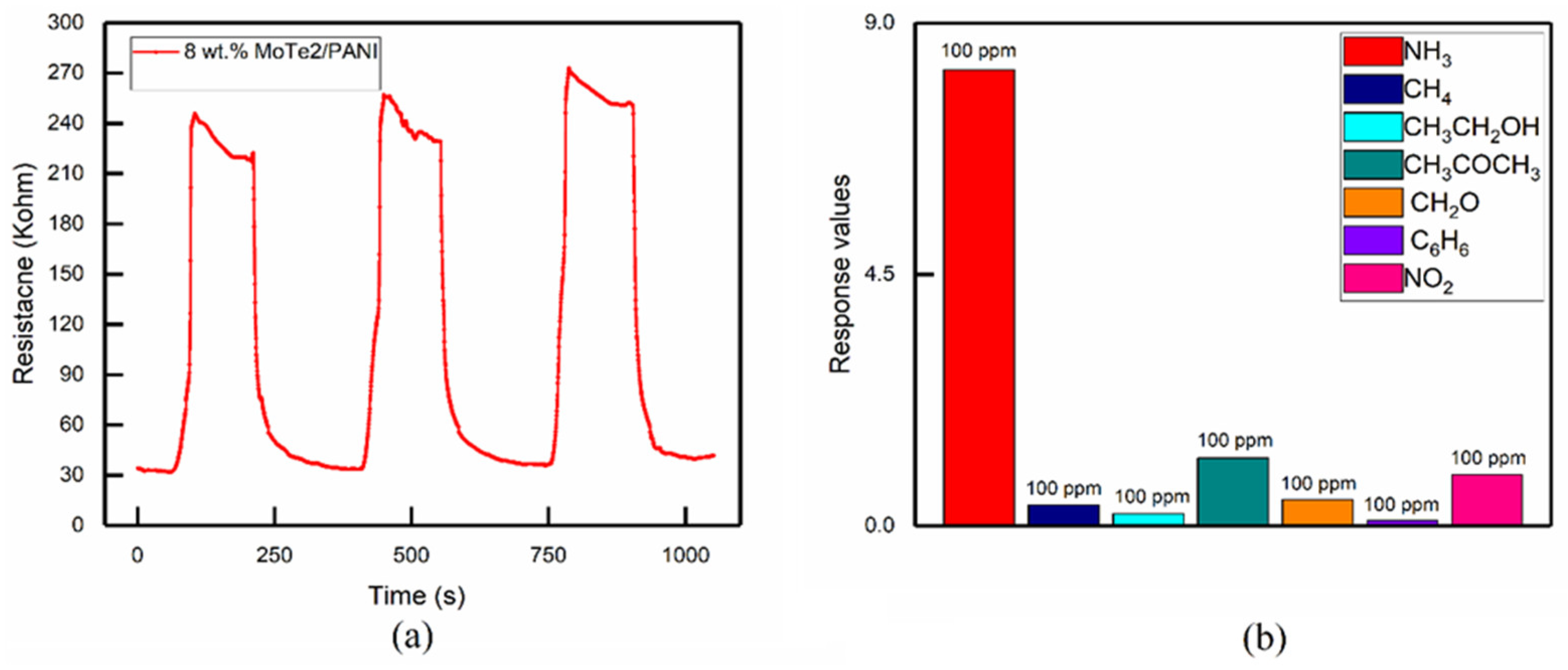

| NH3 Gas Concentration Response | 10 ppm | 25 ppm | 50 ppm | 100 ppm | 200 ppm | 500 ppm | 1000 ppm |
|---|---|---|---|---|---|---|---|
| pure PANI | 0.48 | 1.24 | 1.69 | 3.15 | 6.91 | 14.12 | 25.01 |
| 2.7 wt.% MoTe2/PANI composites | 0.76 | 1.19 | 1.99 | 7.16 | 17.12 | 33.9 | 54.44 |
| 8 wt.% MoTe2/PANI composites | 1.23 | 2.24 | 4.12 | 8.16 | 35 | 59.62 | 105.9 |
| 16 wt.%MoTe2/PANI composites | 1.14 | 1.97 | 3.37 | 7.5 | 19.1 | 38.48 | 77.99 |
| Sensitive Film | Pure PANI | 2.7 wt.% MoTe2/PANI | 8 wt.% MoTe2/PANI | 16 wt.% MoTe2/PANI |
|---|---|---|---|---|
| Response times (T1) | 36 s | 25 s | 26 s | 25 s |
| Recovery times (T2) | 27 s | 12 s | 24 s | 24 s |
| Material | Response (×100%) | T1 (s) | T2 (s) | Type of Transducer | Reference |
|---|---|---|---|---|---|
| PANI/GO/PANI/ZnO | 38.31%@100 ppm | 30 | — | Impedance | [30] |
| PANI-α-Fe2O3 | 72%@100 ppm | 50 | 1575 | Resistive | [31] |
| PANI/WS2 | 81%@200 ppm | 260 | 790 | Resistive | [12] |
| PAni-WO3 | 158%@100 ppm | 39 | 377 | Resistive | [32] |
| 3D RGO/PANI hybrid | 10.8%@100 ppm | 370 | 675 | Resistive | [33] |
| PANI/Nb2CTx nanosheets | 301%@100 ppm | 105 | 143 | Voltage | [34] |
| MoTe2/PANI | 816%@100 ppm | 25 | 24 | Resistive | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, X.; Ding, X.; Yu, X. Gas Sensitive Characteristics of Polyaniline Decorated with Molybdenum Ditelluride Nanosheets. Chemosensors 2022, 10, 264. https://doi.org/10.3390/chemosensors10070264
Chen X, Chen X, Ding X, Yu X. Gas Sensitive Characteristics of Polyaniline Decorated with Molybdenum Ditelluride Nanosheets. Chemosensors. 2022; 10(7):264. https://doi.org/10.3390/chemosensors10070264
Chicago/Turabian StyleChen, Xinpeng, Xiangdong Chen, Xing Ding, and Xiang Yu. 2022. "Gas Sensitive Characteristics of Polyaniline Decorated with Molybdenum Ditelluride Nanosheets" Chemosensors 10, no. 7: 264. https://doi.org/10.3390/chemosensors10070264
APA StyleChen, X., Chen, X., Ding, X., & Yu, X. (2022). Gas Sensitive Characteristics of Polyaniline Decorated with Molybdenum Ditelluride Nanosheets. Chemosensors, 10(7), 264. https://doi.org/10.3390/chemosensors10070264





