Polypyrrole Film Deposited-TiO2 Nanorod Arrays for High Performance Ultraviolet Photodetectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
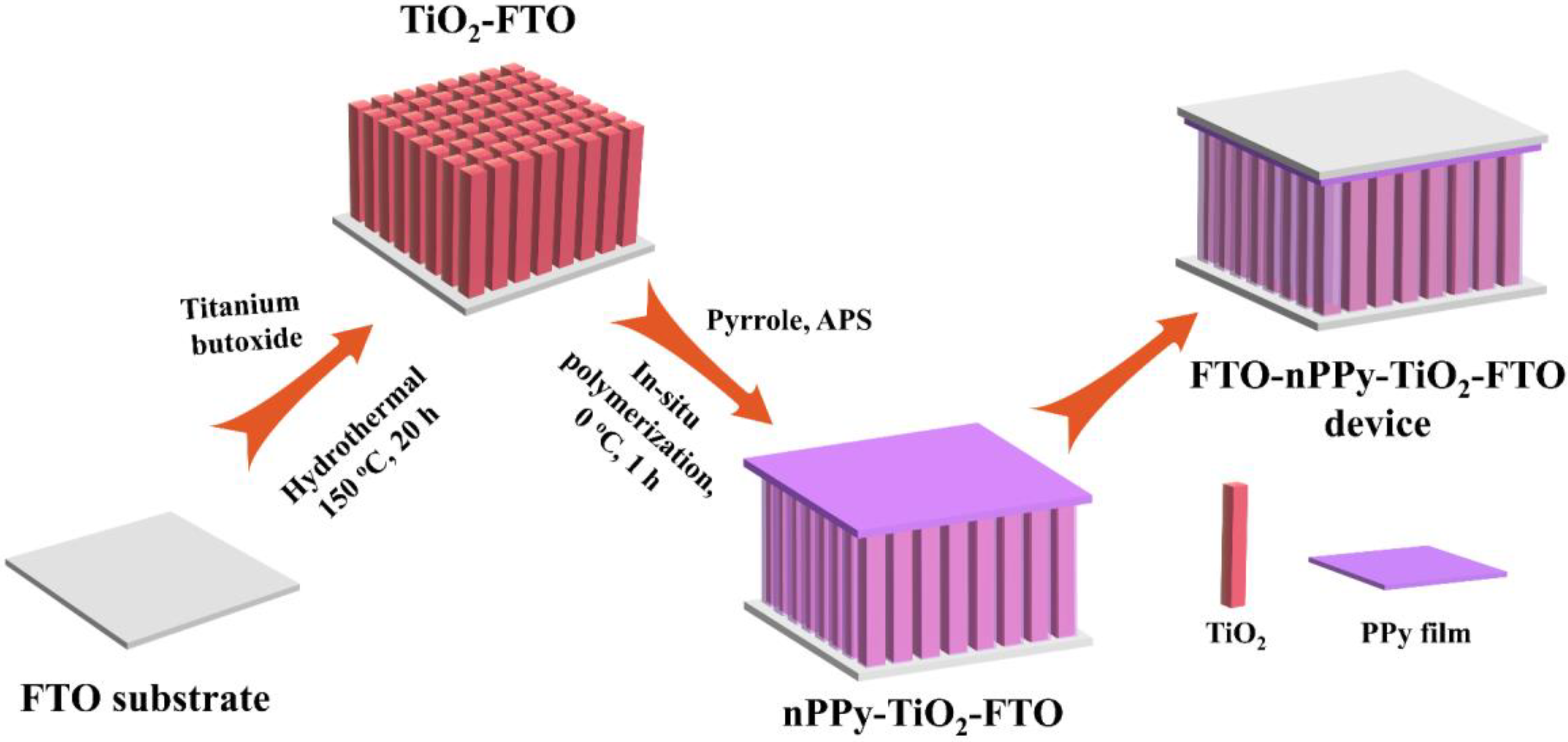
2.2. Preparation of the TiO2 Nanorod Arrays
2.3. Preparation of the PPy-TiO2 Nanocomposites
2.4. Fabrication of the FTO-nPPy-TiO2-FTO Device
2.5. Characterization
3. Results and Discussion
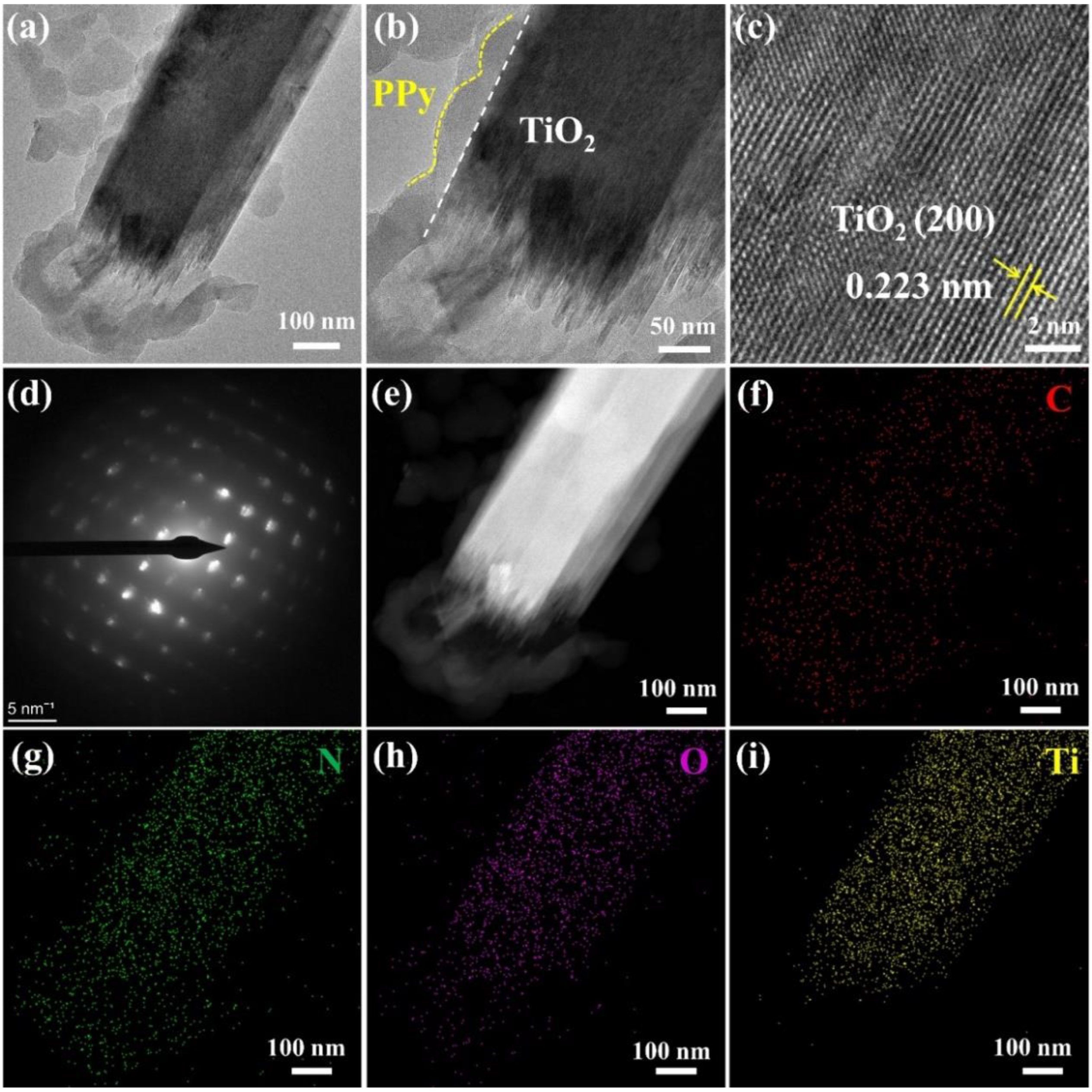
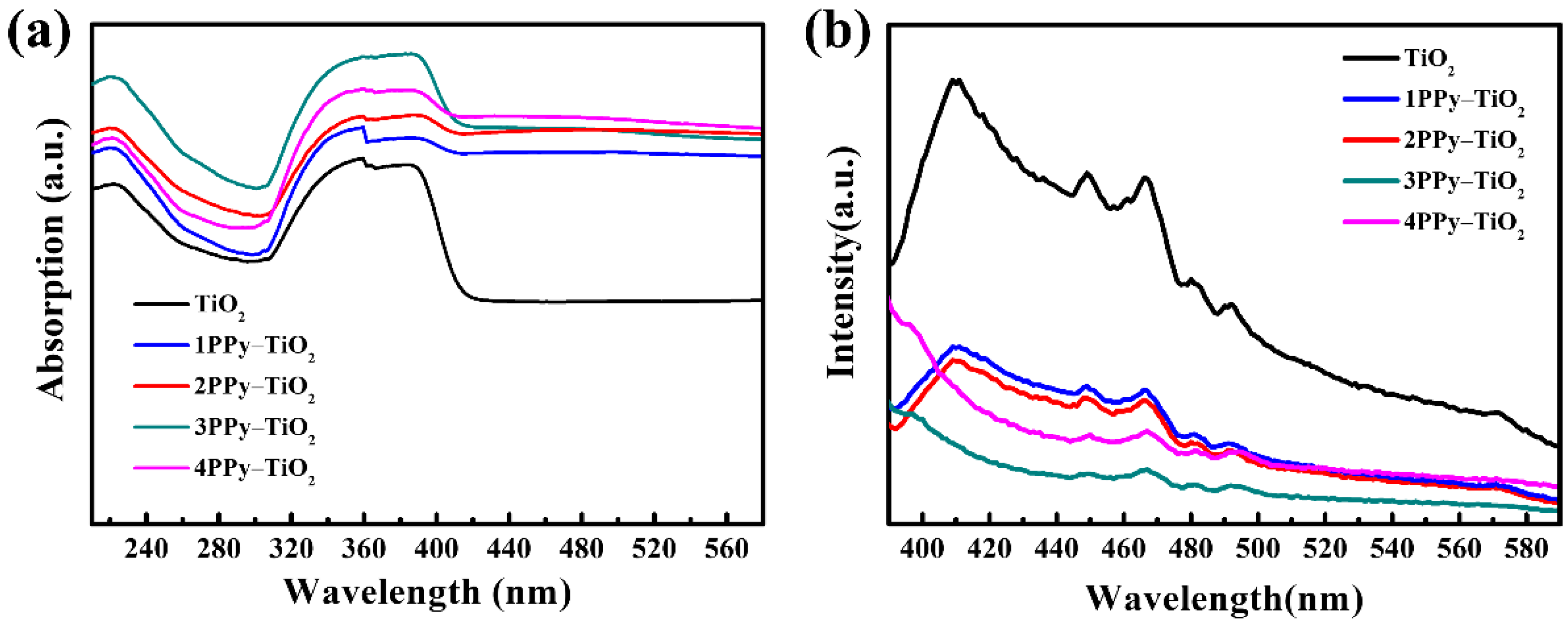
3.1. Structural and Optical Properties of the PPy-TiO2 Nanocomposites
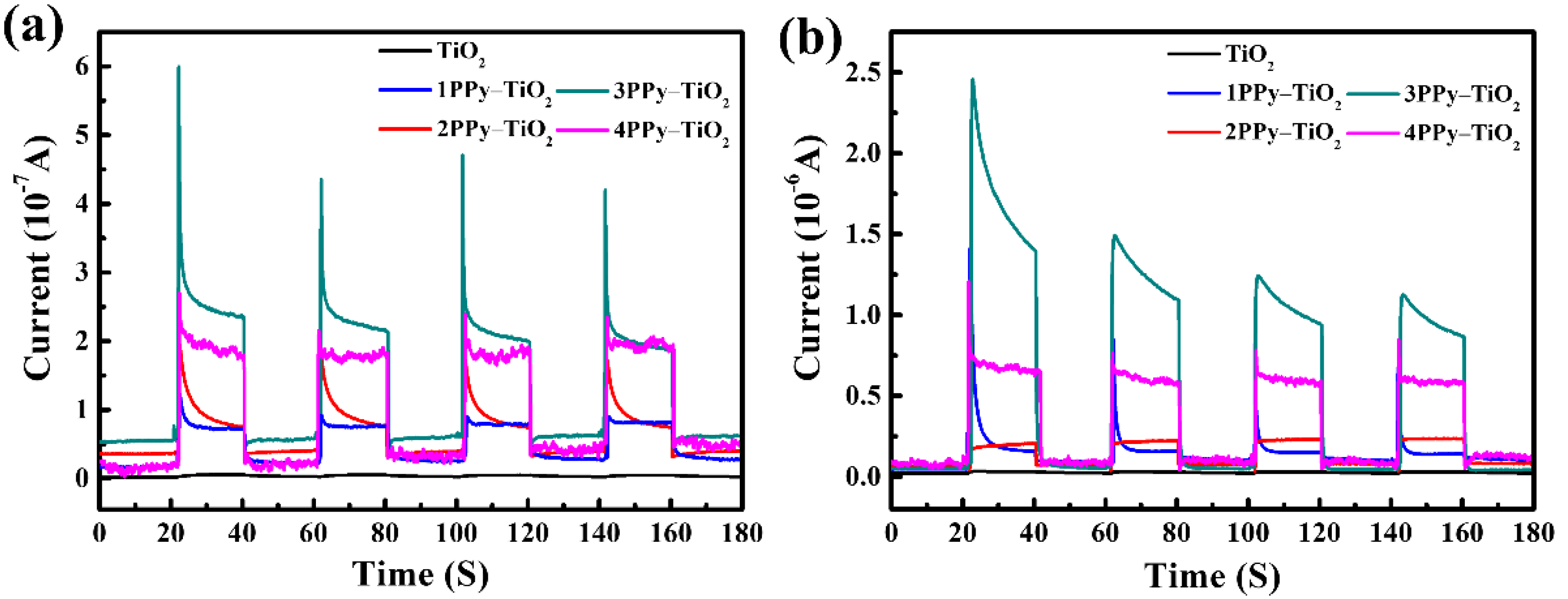
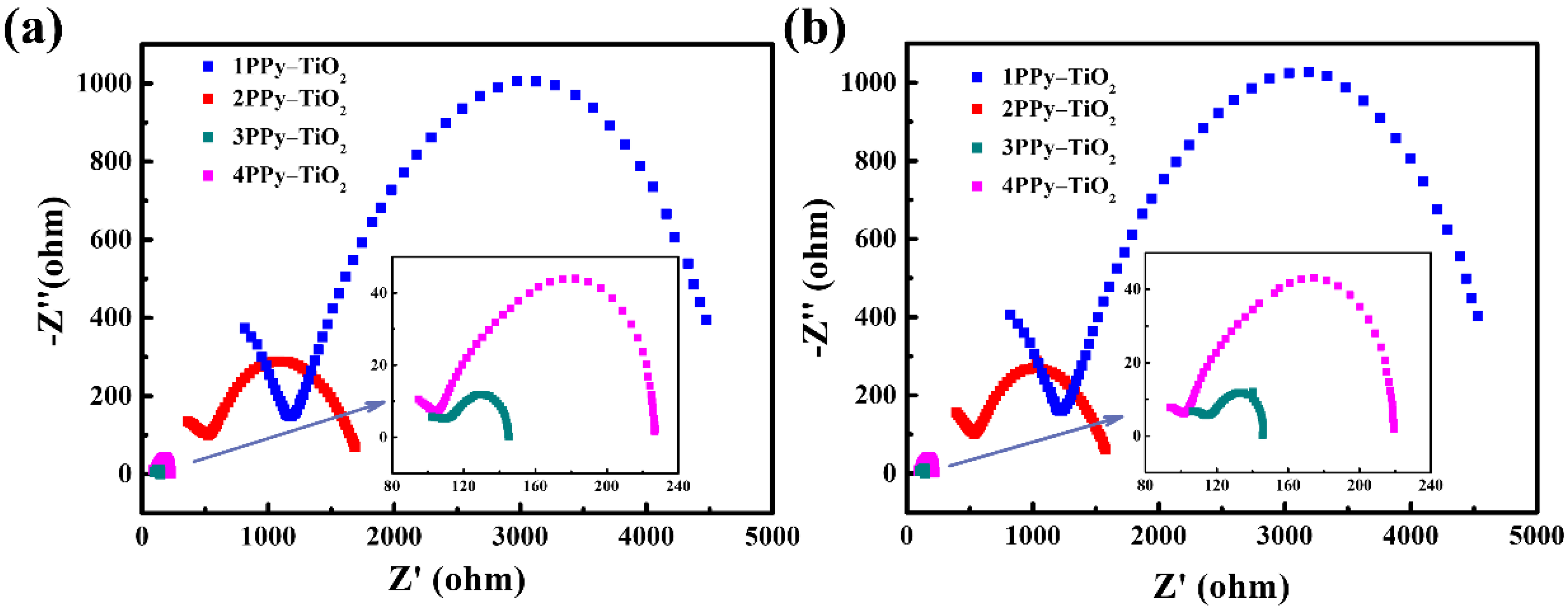
3.2. UV Photo-Detection Properties of the FTO-nPPy-TiO2-FTO Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Ouyang, W.; Yang, W.; He, J.; Fang, X. Recent Progress of Heterojunction Ultraviolet Photodetectors: Materials, Integrations, and Applications. Adv. Funct. Mater. 2020, 30, 1909909. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, H.; Fang, J.; Liu, X.; Wang, S.; Xie, M. Fabrication of Au-Cu Ordered Metallic Nanoparticle Arrays and Their Optical Properties. Chin. J. Rare Met. 2020, 44, 916–926. [Google Scholar]
- Li, S.; Deng, T.; Zhang, Y.; Li, Y.; Yin, W.; Chen, Q.; Liu, Z. Solar-blind ultraviolet detection based on TiO2 nanoparticles decorated graphene field-effect transistors. Nanophotonics 2019, 8, 899–908. [Google Scholar] [CrossRef]
- Yang, T.; Chen, S.; Li, X.; Xu, X.; Gao, F.; Wang, L.; Chen, J.; Yang, W.; Hou, X.; Fang, X. High-Performance SiC Nanobelt Photodetectors with Long-Term Stability against 300 °C up to 180 Days. Adv. Funct. Mater. 2019, 29, 1806250. [Google Scholar] [CrossRef]
- Li, X.; Ma, H.; Wang, X.; Chen, F.; Zeng, X. Structural and Luminescent Properties of Eu, Mg Co-doped GaN. Chin. J. Rare Met. 2020, 44, 1170–1176. [Google Scholar]
- Zheng, K.-G.; Yang, T.-Y.; Guo, Z. Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemiresistive Sensing Performance. Chemosensors 2022, 10, 96. [Google Scholar] [CrossRef]
- Nguyen, T.; Patel, M.; Kim, S.-H.; Mir, R.; Yi, J.-S.; Dao, V.-A.; Kim, J.-D. Transparent photovoltaic cells and self-powered photodetectors by TiO2/NiO heterojunction. J. Power Sources 2021, 481, 228865. [Google Scholar] [CrossRef]
- Wang, H.; Qin, P.; Yi, G.; Zu, X.; Zhang, L.; Hong, W.; Chen, X. A high-sensitive ultraviolet photodetector composed of double-layered TiO2 nanostructure and Au nanoparticles film based on Schottky junction. Mater. Chem. Phys. 2017, 194, 42–48. [Google Scholar] [CrossRef]
- Zheng, W.; Dong, Y.; Li, T.; Chen, J.; Chen, X.; Dai, Y.; He, G. MgO blocking layer induced highly UV responsive TiO2 nanoparticles based self-powered photodetectors. J. Alloys Compd. 2021, 869, 159299. [Google Scholar] [CrossRef]
- Tian, A.; Shi, X.; Tan, H.; Li, B.; Ma, J.; Yang, H. Preparation and Photocatalytic Properties of Ni Doped TiO2 Nanotube. Chin. J. Rare Met. 2021, 45, 41–46. [Google Scholar]
- Haidry, A.; Fatima, Q.; Mehmood, A.; Shahzad, A.; Ji, Y.; Saruhan, B. Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors. Chemosensors 2022, 10, 11. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, C.; Yin, B.; Xu, R.; Zhou, J.; Zhang, X.; Ruan, S. Organics filled one-dimensional TiO2 nanowires array ultraviolet detector with enhanced photo-conductivity and dark-resistivity. Nanoscale 2017, 9, 9095–9103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, Y.; Qin, H.; Ji, Z.; Chi, H. Interface engineering on ZnO/Au based Schottky junction for enhanced photoresponse of UV detector with TiO2 inserting layer. J. Alloys Compd. 2020, 816, 152537. [Google Scholar] [CrossRef]
- Ouyang, W.; Teng, F.; Fang, X. High Performance BiOCl Nanosheets/TiO2 Nanotube Arrays Heterojunction UV Photodetector: The Influences of Self-Induced Inner Electric Fields in the BiOCl Nanosheets. Adv. Funct. Mater. 2018, 28, 1707178. [Google Scholar] [CrossRef]
- Yang, D.; Du, F.; Ren, Y.; Kang, T.; Hu, P.; Teng, F.; Fan, H. A high-performance NiO/TiO2 UV photodetector: The influence of the NiO layer position. J. Mater. Chem. C 2021, 9, 14146–14153. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhou, H.; Wei, K.; Zhu, Z.; Xie, E.; Cao, W.; Han, W. Rutile TiO2 nanowire arrays interconnected with ZnO nanosheets for high performance electrochemical UV sensors. J. Mater. Chem. C 2019, 7, 8011–8018. [Google Scholar] [CrossRef]
- Mojtabavi, E.A.; Nasirian, S. Flexible self-powered ultraviolet-visible photodetector based on polyaniline-titanium dioxide heterostructures: The study of the rearrangement of layers. Appl. Surf. Sci. 2019, 492, 189–198. [Google Scholar] [CrossRef]
- Mojtabavi, E.A.; Nasirian, S. A self-powered UV photodetector based on polyaniline/titania nanocomposite with long-term stability. Opt. Mater. 2019, 94, 28–34. [Google Scholar] [CrossRef]
- Zhang, H.; Abdiryim, T.; Jamal, R.; Li, J.; Liu, H.; Kadir, A.; Zou, D.; Che, Y.; Serkjan, N. Self-powered TiO2 NRs UV photodetectors: Heterojunction with PTTh and enhanced responsivity by Au nanoparticles. J. Alloys Compd. 2022, 899, 163279. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, H.; Abdiryim, T.; Jamal, R.; Kadir, A.; Helil, Z.; Liu, H. Ultraviolet photodetectors based on TiO2 nanorod arrays/PEDOT-type conducting polymers. Opt. Mater. 2021, 122, 111805. [Google Scholar] [CrossRef]
- Kareem, O.A.; Mohameed, S.J.; Ibrahim, I.M. The importance of doping TiO2 into polypyrrol nanofibers to improve their optical sensitivity. J. Phys. Conf. Ser. 2021, 2114, 012057. [Google Scholar] [CrossRef]
- Qin, P.; Yi, G.; Zu, X.; Wang, H.; Tan, M.; Luo, H. Preparation of graphene-TiO2 nanocomposite films and its photocatalytic performances on degradation of Rhodamine B. Pigm. Resin Technol. 2018, 47, 79–85. [Google Scholar] [CrossRef]
- Li, S.; Yin, W.; Li, Y.; Sun, J.; Zhu, M.; Liu, Z.; Deng, T. High sensitivity ultraviolet detection based on three-dimensional graphene field effect transistors decorated with TiO2 NPs. Nanoscale 2019, 11, 14912–14920. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, J.; Shi, S.; Dong, H.; Cheng, Y.; Wei, C.; Zhang, X.; Yin, S.; Li, L. TiO2 Nanorod Arrays Based Self-Powered UV Photodetector: Heterojunction with NiO Nanoflakes and Enhanced UV Photoresponse. ACS Appl. Mater. Interfaces 2018, 10, 11269–11279. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ma, M.; Tong, Z.; Wang, R.; Bi, Y.; Chen, Y.; Chung, K.-L.; Ma, Y. Fabrication of ZnFe2O4/C@PPy composites with efficient electromagnetic wave absorption properties. J. Colloid Interf. Sci. 2021, 602, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ma, M.; Liu, Y.; Tong, Z.; Wang, R.; Chung, K.-L.; Ma, A.; Wu, G.; Ma, Y.; He, C.; et al. Microwave absorption enhancement of 2-dimensional CoZn/C@ MoS2@ PPy composites derived from metal-organic framework. J. Colloid Interf. Sci. 2021, 600, 209–218. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Shi, L.; Yang, X.; Lu, Y.; Tong, G.; Wu, W. Chemical conversion of Cu2O/PPy core-shell nanowires (CSNWs): A surface/interface adjustment method for high-quality Cu/Fe/C and Cu/Fe3O4/C CSNWs with superior microwave absorption capabilities. Carbon 2020, 166, 205–217. [Google Scholar] [CrossRef]
- Zhao, D.; Dai, M.; Zhao, Y.; Liu, H.; Liu, Y.; Wu, X. Improving electrocatalytic activities of FeCo2O4@FeCo2S4@PPy electrodes by surface/interface regulation. Nano Energy 2020, 72, 104715. [Google Scholar] [CrossRef]
- Bi, Y.; Ma, M.; Liao, Z.; Tong, Z.; Chen, Y.; Wang, R.; Ma, Y.; Wu, G. One-dimensional Ni@Co/C@PPy composites for superior electromagnetic wave absorption. J. Colloid Interface Sci. 2022, 605, 483–492. [Google Scholar] [CrossRef]
- Ding, J.; Wang, L.; Zhao, Y.; Xing, L.; Yu, X.; Chen, G.; Zhang, J.; Che, R. Boosted interfacial polarization from multishell TiO2@ Fe3O4@ PPy heterojunction for enhanced microwave absorption. Small 2019, 15, 1902885. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Xie, F.; Wu, J.; Jia, X.; Yang, J.; Song, H.; Zhang, Z. Environmentally safe and porous MS@TiO2@PPy monoliths with superior visible-light photocatalytic properties for rapid oil–water separation and water purification. ACS Sustain. Chem. Eng. 2020, 8, 5347–5359. [Google Scholar] [CrossRef]
- Ha, J.-U.; Lee, J.; Abbas, M.-A.; Lee, M.-D.; Lee, J.; Bang, J.-H. Designing hierarchical assembly of carbon-coated TiO2 nanocrystals and unraveling the role of TiO2/carbon interface in lithium-ion storage in TiO2. ACS Appl. Mater. Interfaces 2019, 11, 11391–11402. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Li, Z.-Q.; Zuo, C.; Fang, X. Application of Nanostructured TiO2 in UV Photodetectors: A Review. Adv. Mater. 2022, 2109083. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Berger, H.; Schmid, P.; Lévy, F.; Burri, G. Photoluminescence in TiO2 anatase single crystals. Solid State Commun. 1993, 87, 847–850. [Google Scholar] [CrossRef]
- Maurya, A.; Chauhan, P.; Mishra, S.K.; Srivastava, R.K. Structural, optical and charge transport study of rutile TiO2 nanocrystals at two calcination temperatures. J. Alloys Compd. 2011, 509, 8433–8440. [Google Scholar] [CrossRef]
- Serpone, N.; Lawless, D.; Khairutdinov, R. Size effects on the photophysical properties of colloidal anatase TiO2 particles: Size quantization versus direct transitions in this indirect semiconductor? J. Phys. Chem. 1995, 99, 16646–16654. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Lee, K.-M.; Huang, J.T.-W.; Lin, C.-Y.; Lee, C.-H.; Wang, L.-P.; Tsai, S.-Y.; Ho, K.-C. EIS analysis on low temperature fabrication of TiO2 porous films for dye-sensitized solar cells. Electrochim. Acta 2008, 53, 7514–7522. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, P.; Hu, K.; Teng, F.; Chen, H.; Fang, X. Scalable-production, self-powered TiO2 nanowell–organic hybrid UV photodetectors with tunable performances. ACS Appl. Mater. Interfaces 2016, 8, 33924–33932. [Google Scholar] [CrossRef]
- Zheng, L.; Deng, X.; Wang, Y.; Chen, J.; Fang, X.; Wang, L.; Shi, X.; Zheng, H. Self-powered flexible TiO2 fibrous photodetectors: Heterojunction with P3HT and boosted responsivity and selectivity by Au nanoparticles. Adv. Funct. Mater. 2020, 30, 2001604. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Meng, L.; Wang, D.; Chen, Y.; Tian, Y.; Yan, S.; Mei, L.; Jiao, J. High-performance self-powered UV detector based on SnO2-TiO2 nanomace arrays. Nanoscale Res. Lett. 2018, 13, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Wu, B.; Zhang, X.; Cao, S.; Ma, P.; Wang, K.; Fan, Z.; Su, M. Preparation and UV photoelectric properties of aligned ZnO–TiO2 and TiO2–ZnO core–shell structured heterojunction nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 38490–38498. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Qin, P.; Feng, Y.-H.; Sun, H.-L.; Wu, H.-X.; Liao, B.-K.; Xi, Y.-B.; Wang, W. Polypyrrole Film Deposited-TiO2 Nanorod Arrays for High Performance Ultraviolet Photodetectors. Chemosensors 2022, 10, 277. https://doi.org/10.3390/chemosensors10070277
Wang H, Qin P, Feng Y-H, Sun H-L, Wu H-X, Liao B-K, Xi Y-B, Wang W. Polypyrrole Film Deposited-TiO2 Nanorod Arrays for High Performance Ultraviolet Photodetectors. Chemosensors. 2022; 10(7):277. https://doi.org/10.3390/chemosensors10070277
Chicago/Turabian StyleWang, Huan, Pei Qin, Yun-Hui Feng, Hui-Liang Sun, Hui-Xiang Wu, Bo-Kai Liao, Yue-Bin Xi, and Wei Wang. 2022. "Polypyrrole Film Deposited-TiO2 Nanorod Arrays for High Performance Ultraviolet Photodetectors" Chemosensors 10, no. 7: 277. https://doi.org/10.3390/chemosensors10070277
APA StyleWang, H., Qin, P., Feng, Y.-H., Sun, H.-L., Wu, H.-X., Liao, B.-K., Xi, Y.-B., & Wang, W. (2022). Polypyrrole Film Deposited-TiO2 Nanorod Arrays for High Performance Ultraviolet Photodetectors. Chemosensors, 10(7), 277. https://doi.org/10.3390/chemosensors10070277






