Synthesis of ZIF-8 Coating on ZnO Nanorods for Enhanced Gas-Sensing Performance
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of ZIF-8-Coated ZnO
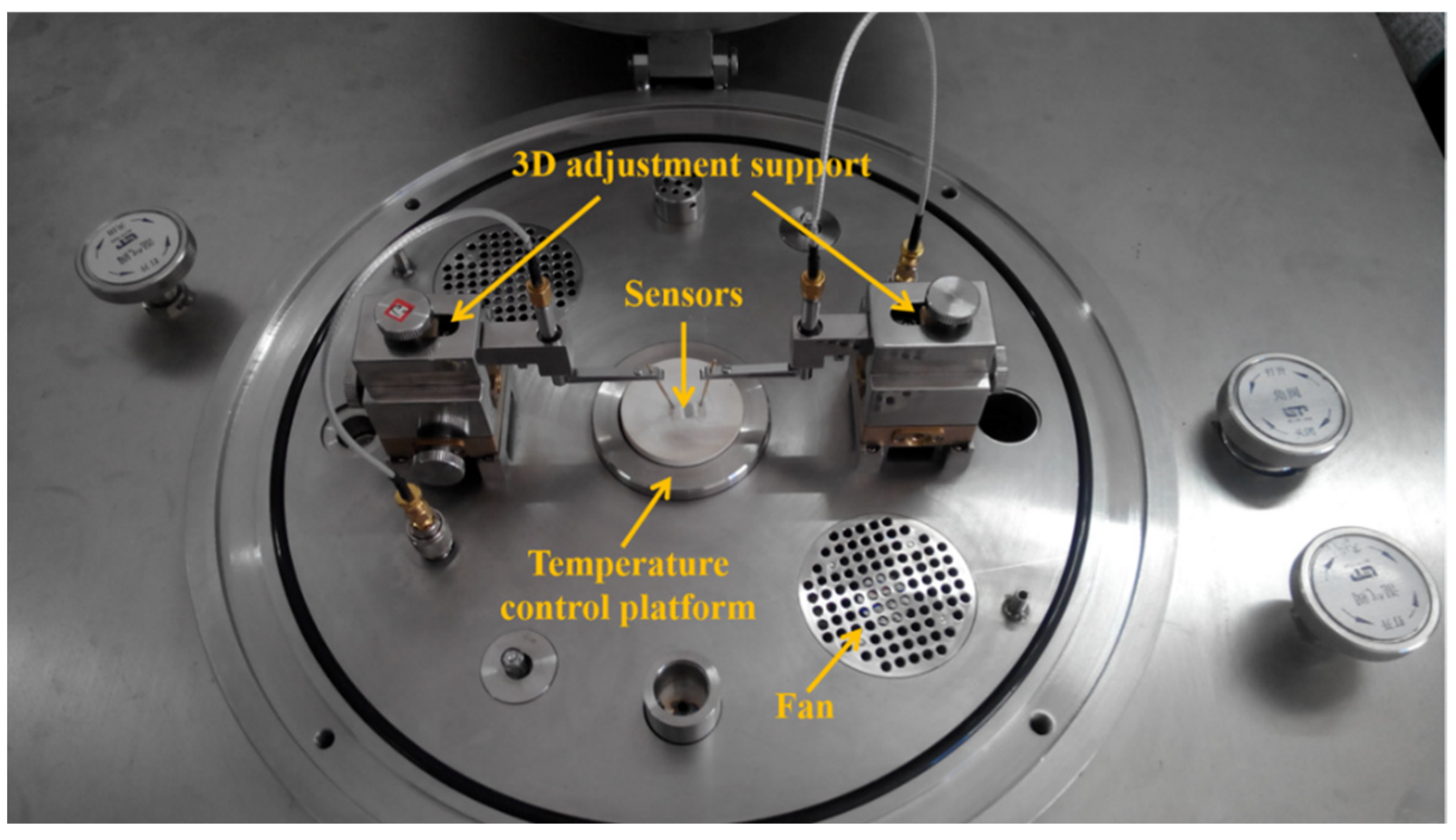
2.2. Preparation of Sensors and Gas-Sensing Measurements
2.3. Material Characterization
3. Results and Discussion
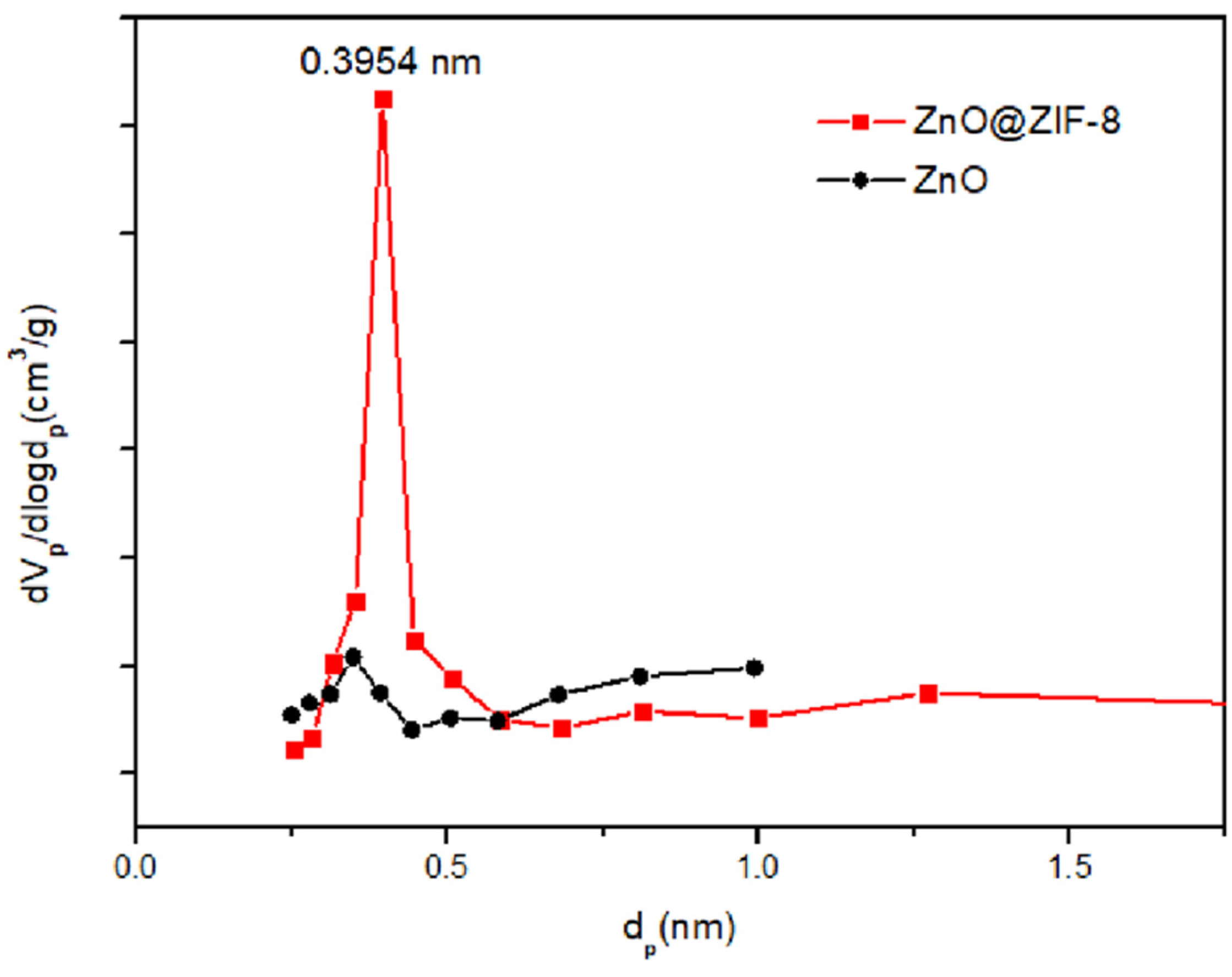
3.1. Microstructure Characterization
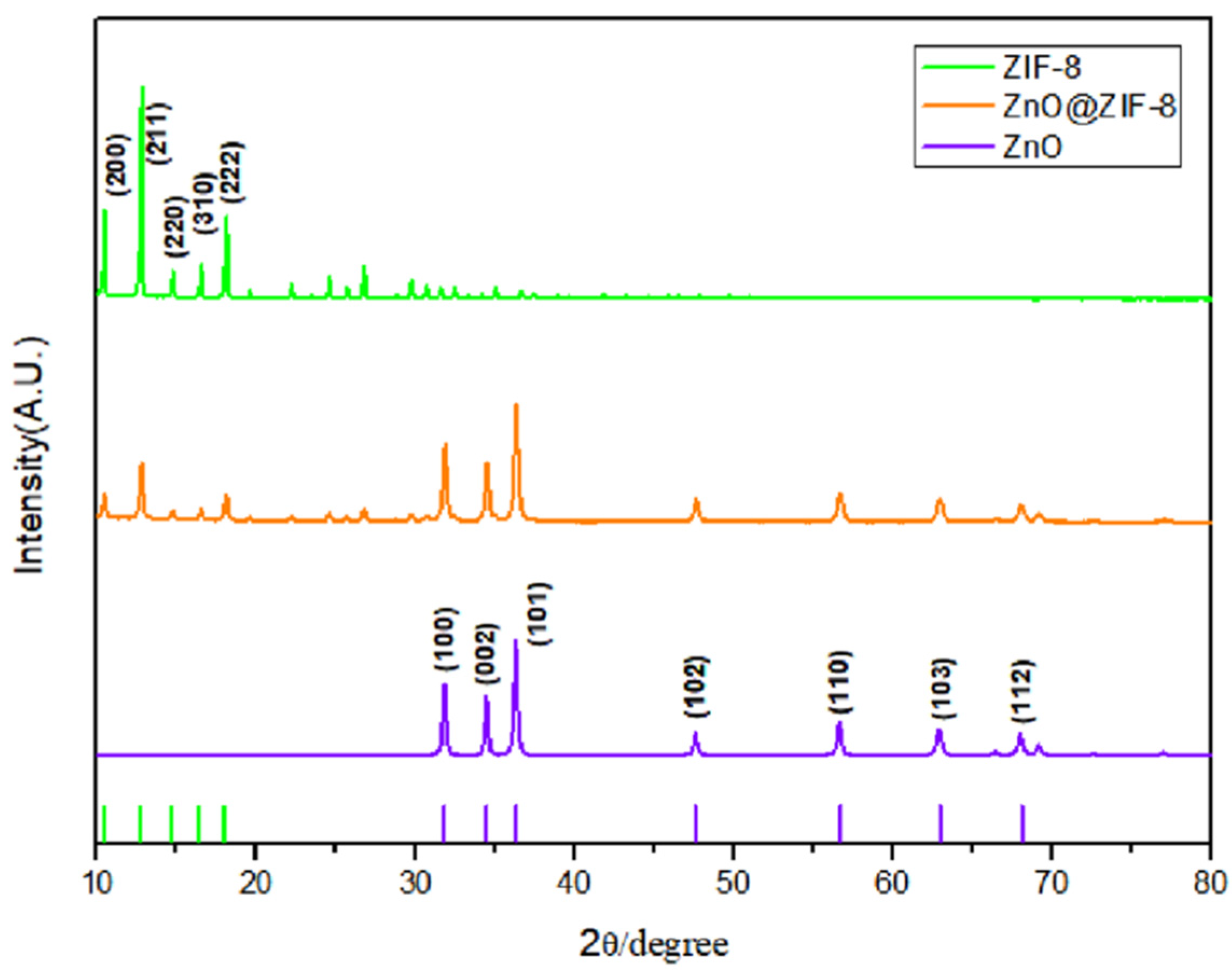
3.1.1. XRD Analysis
3.1.2. SEM and TEM Analysis
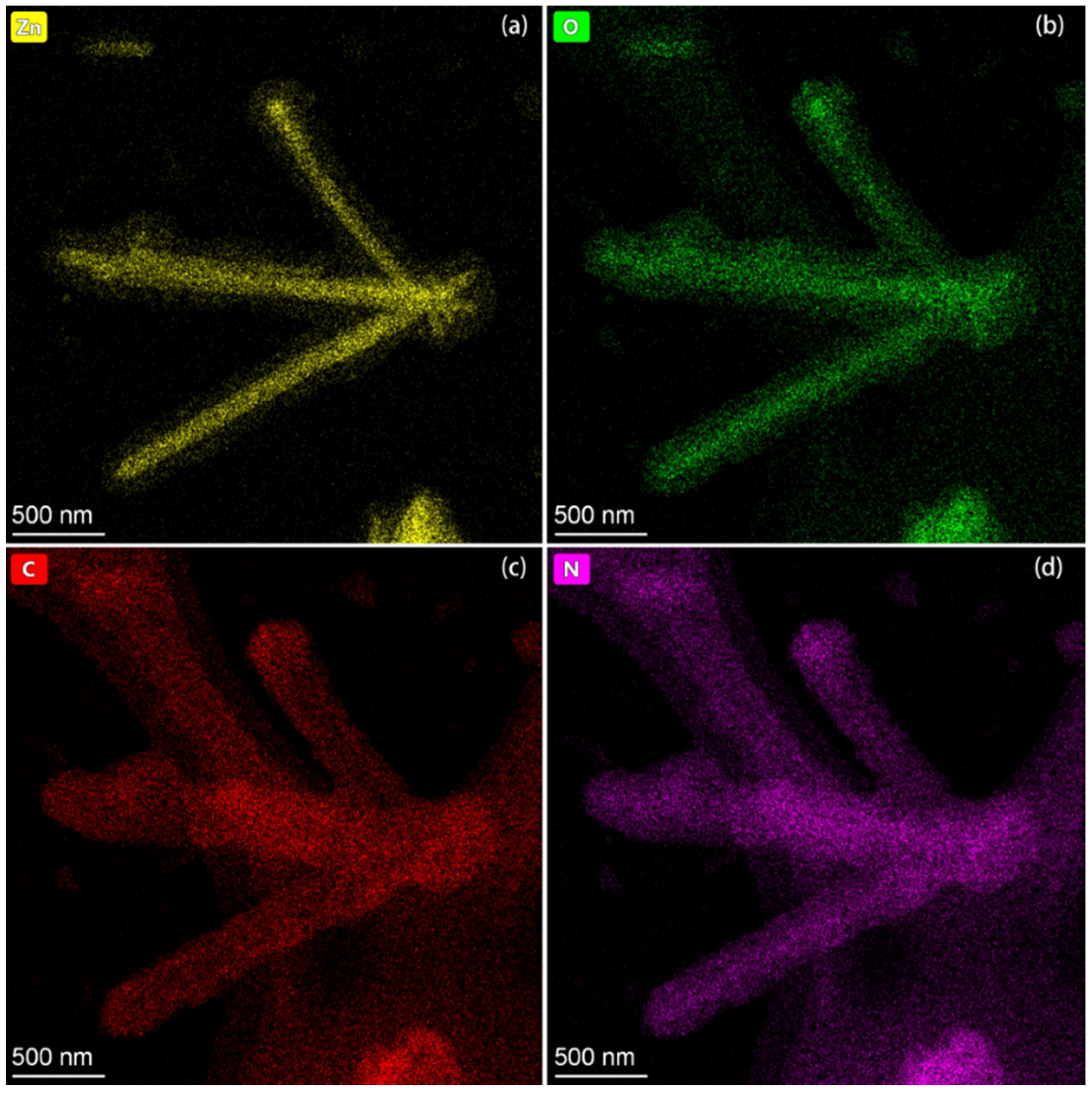
3.1.3. EDS Element Analysis
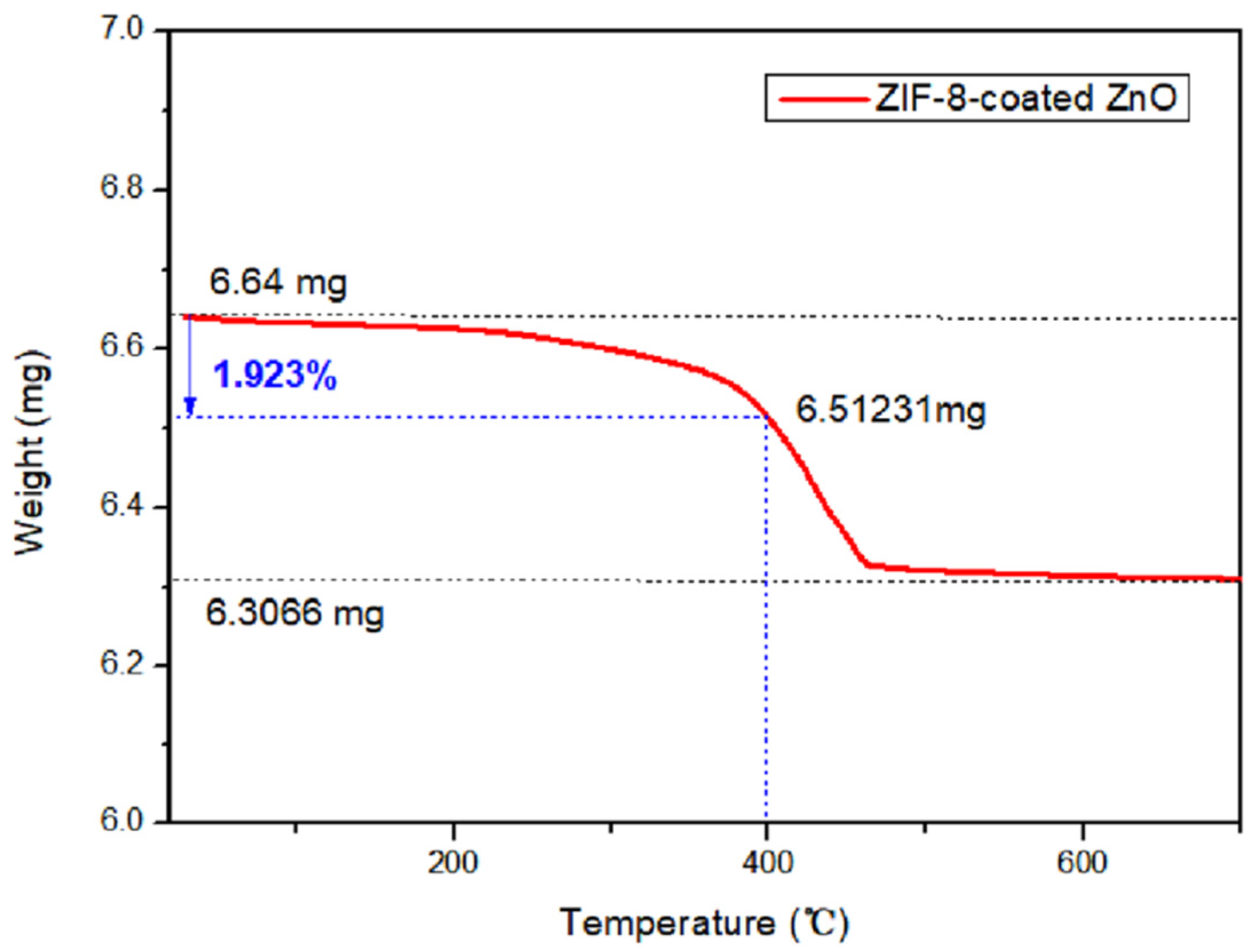
3.1.4. TG Analysis
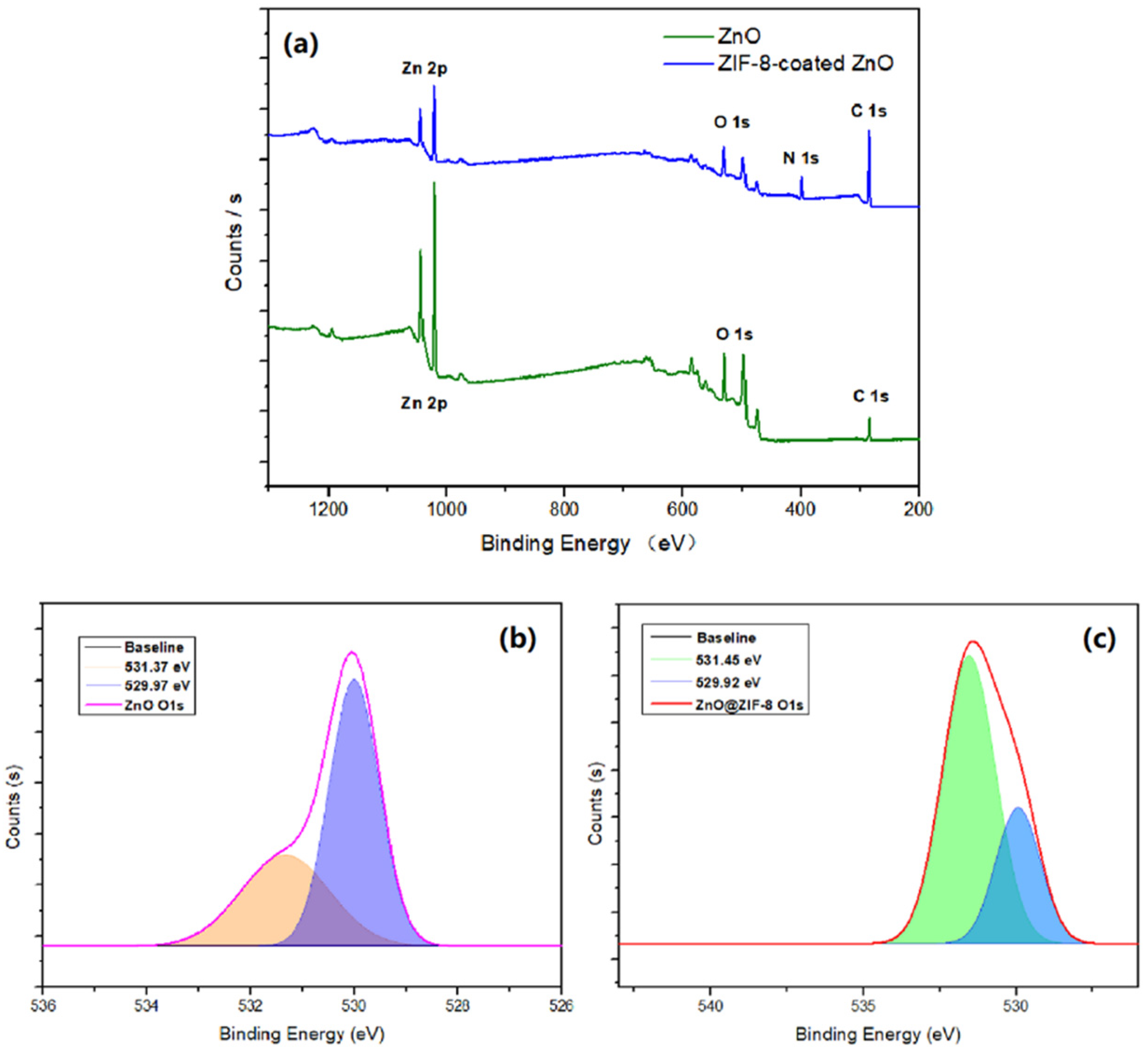
3.1.5. XPS Analysis
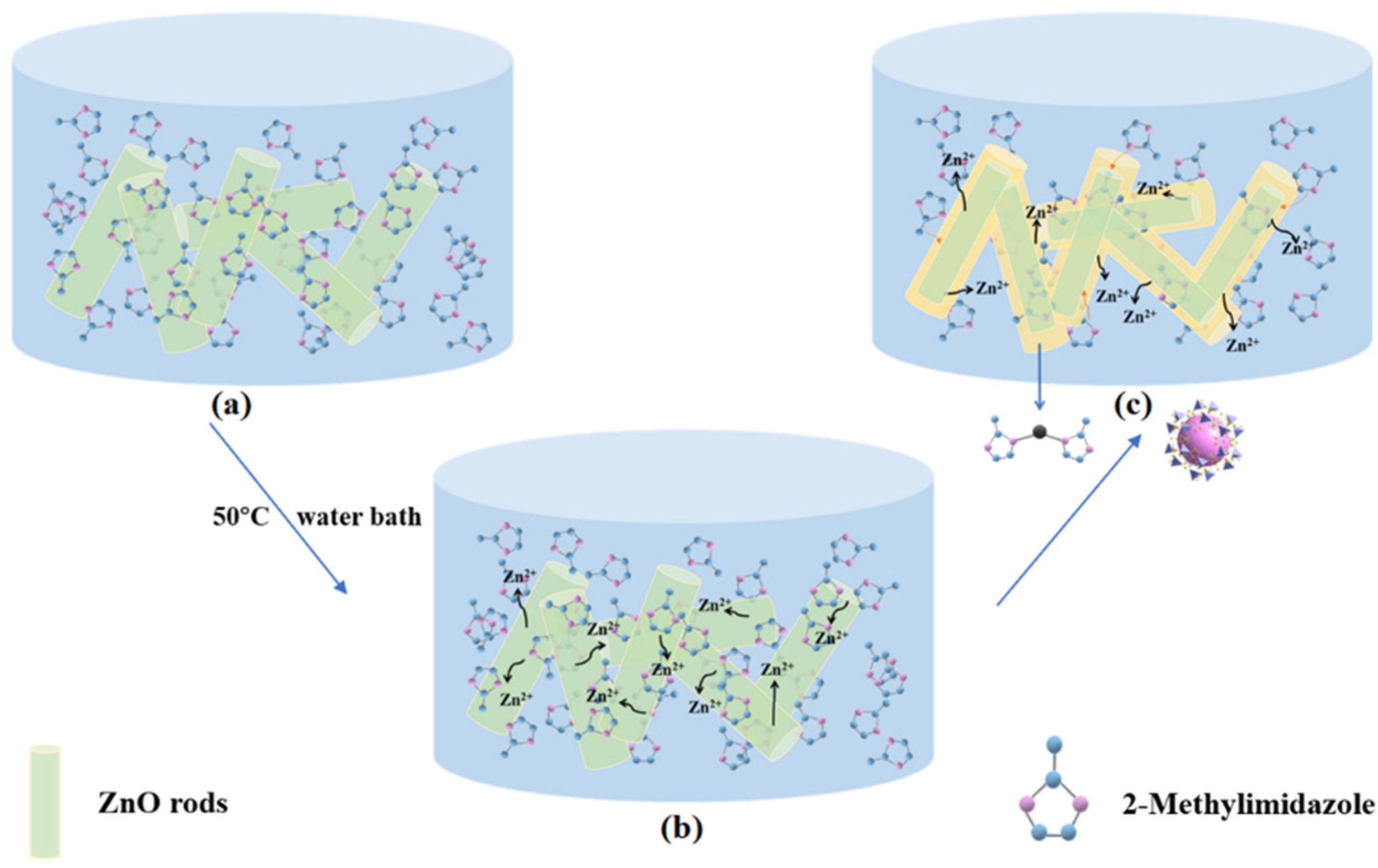
3.2. The Formation of ZIF-8-Coated ZnO Structure
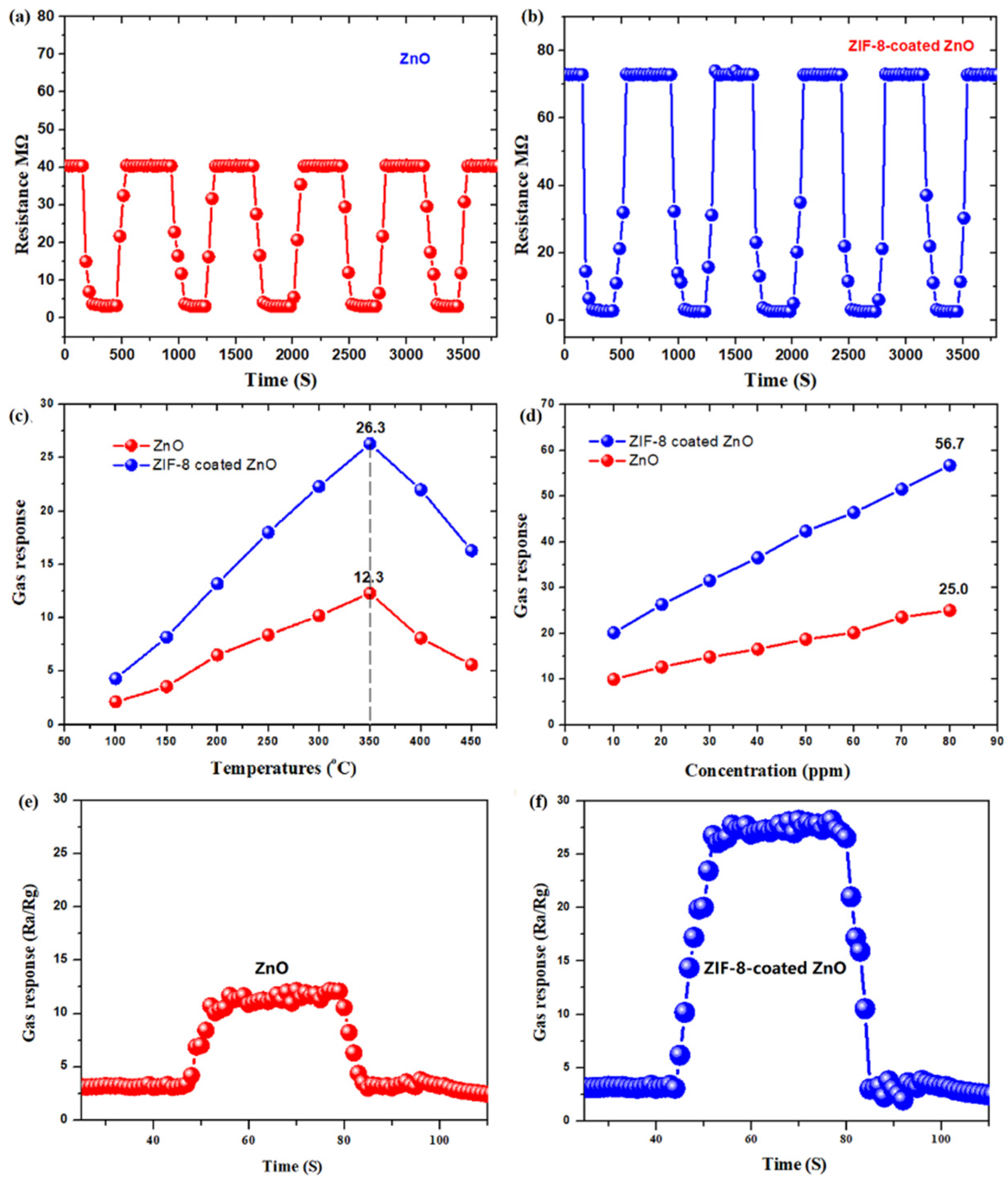
3.3. Gas-Sensing Property
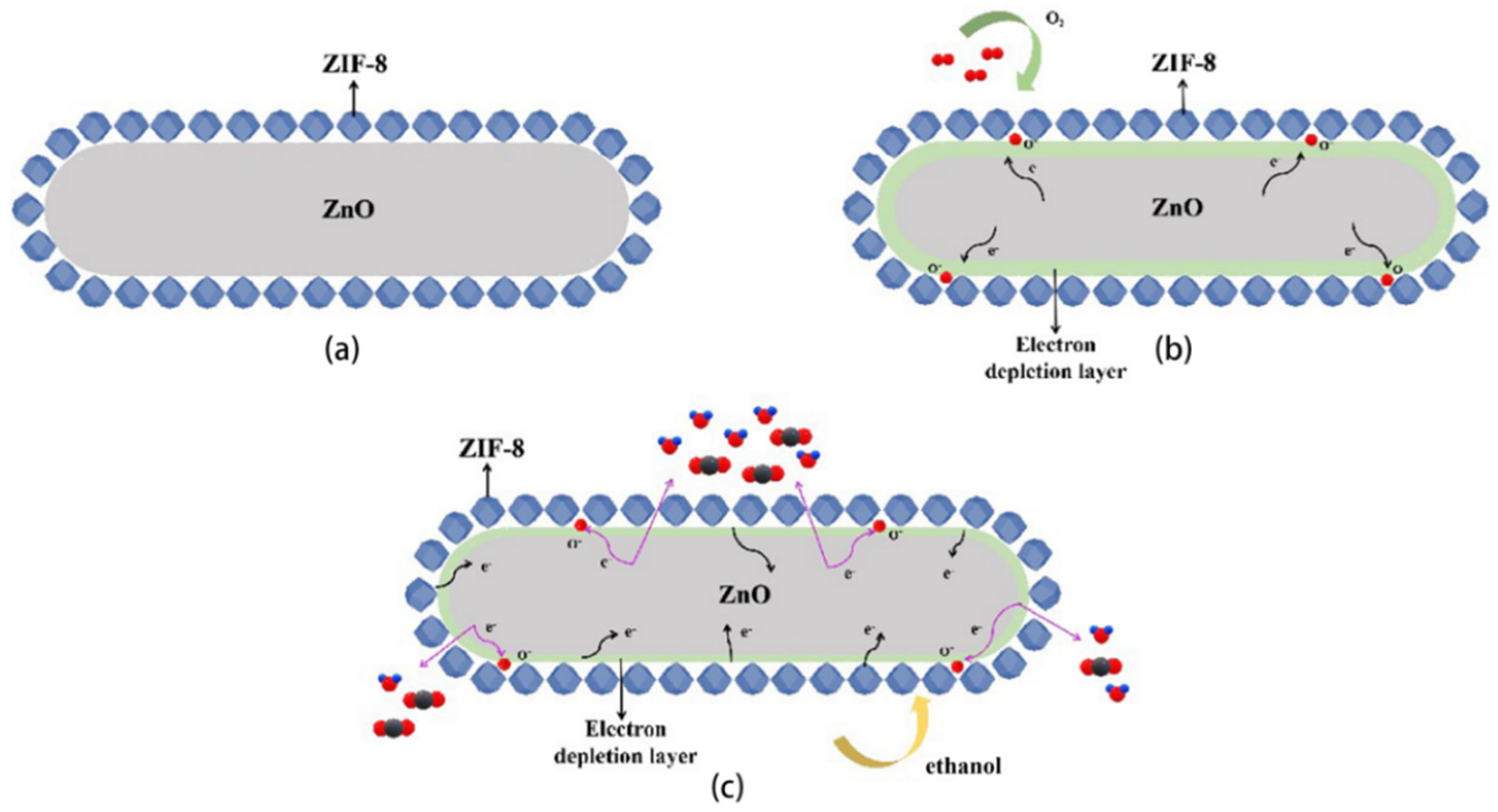
3.4. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Li, B.; Li, H.; Al-Barakani, A. Expansion of city scale, traffic modes, traffic congestion, and air pollution. Cities 2021, 108, 102974. [Google Scholar] [CrossRef]
- Shaddick, G.; Thomas, M.L.; Mudu, P.; Ruggeri, G.; Gumy, S. Half the world’s population are exposed to increasing air pollution. Npj Clim. Atmos. Sci. 2020, 3, 23. [Google Scholar] [CrossRef]
- Molev, M.; Zanina, I.; Chertov, Y.; Shemetov, A. Assessment of urban environment safety for public health. In Proceedings of the International Scientific Conference on Business Technologies for Sustainable Urban Development (SPbWOSCE), Peter Great Saint Petersburg Polytechn University, Institute Ind Management Econ & Tr, St Petersburg, Russia, 20–22 December 2017. [Google Scholar]
- Lajunen, A.; Lipman, T. Lifecycle cost assessment and carbon dioxide emissions of diesel, natural gas, hybrid electric, fuel cell hybrid and electric transit buses. Energy 2016, 106, 329–342. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, C.; Miao, Y.; Xiang, Y.; Chen, L.; Deng, Q. Outdoor particulate air pollution and indoor renovation associated with childhood pneumonia in China. Atmos. Environ. 2018, 174, 76–81. [Google Scholar] [CrossRef]
- Palacios, J.; Eichholtz, P.; Kok, N.; Aydin, E. The impact of housing conditions on health outcomes. Real Estate Econ. 2021, 49, 1172–1200. [Google Scholar] [CrossRef]
- Ren, P.; Qi, L.; You, K.; Shi, Q. Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved Formaldehyde Gas Sensing. Nanomaterials 2022, 12, 228. [Google Scholar] [CrossRef]
- Dadkhah, M.; Tulliani, J.-M. Nanostructured Metal Oxide Semiconductors towards Greenhouse Gas Detection. Chemosensors 2022, 10, 57. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Tokarev, S.; Fedorova, O.; Bozhev, I.; Rumyantseva, M. In2O3 Based Hybrid Materials: Interplay between Microstructure, Photoelectrical and Light Activated NO2 Sensor Properties. Chemosensors 2022, 10, 135. [Google Scholar] [CrossRef]
- Chizhov, A.; Kutukov, P.; Gulin, A.; Astafiev, A.; Rumyantseva, M. UV-Activated NO2 Gas Sensing by Nanocrystalline ZnO: Mechanistic Insights from Mass Spectrometry Investigations. Chemosensors 2022, 10, 147. [Google Scholar] [CrossRef]
- Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials 2021, 14, 4263. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.-X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO4 nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563–11569. [Google Scholar] [CrossRef]
- Peng, P.; Deng, Y.; Niu, J.; Shi, L.; Mei, Y.; Du, S.; Liu, J.; Xu, D. Fabrication and electrical characteristics of flash-sintered SiO2-doped ZnO-Bi2O3-MnO(2)varistors. J. Adv. Ceram. 2020, 9, 683–692. [Google Scholar] [CrossRef]
- Kumar, R.R.; Murugesan, T.; Chang, T.-W.; Lin, H.-N. Defect controlled adsorption/desorption kinetics of ZnO nanorods for UV-activated NO2 gas sensing at room temperature. Mater. Lett. 2021, 287, 129257. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Zheng, K.-G.; Yang, T.-Y.; Guo, Z. Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemiresistive Sensing Performance. Chemosensors 2022, 10, 96. [Google Scholar] [CrossRef]
- Leonardi, S.G. Two-Dimensional Zinc Oxide Nanostructures for Gas Sensor Applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Hui, G.; Zhu, M.; Yang, X.; Liu, J.; Pan, G.; Wang, Z. Highly sensitive ethanol gas sensor based on CeO2/ZnO binary heterojunction composite. Mater. Lett. 2020, 278, 128453. [Google Scholar] [CrossRef]
- Xu, X.H.; Ma, S.Y.; Xu, X.L.; Han, T.; Pei, S.T.; Tie, Y.; Cao, P.F.; Liu, W.W.; Wang, B.J.; Zhang, R.; et al. Ultra-sensitive glycol sensing performance with rapid-recovery based on heterostructured ZnO-SnO2 hollow nanotube. Mater. Lett. 2020, 273, 127967. [Google Scholar] [CrossRef]
- Badadhe, S.S.; Mulla, I.S. Effect of aluminium doping on structural and gas sensing properties of zinc oxide thin films deposited by spray pyrolysis. Sens. Actuators B Chem. 2011, 156, 943–948. [Google Scholar] [CrossRef]
- Fan, C.; Sun, F.; Wang, X.; Majidi, M.; Huang, Z.; Kumar, P.; Liu, B. Enhanced H2S gas sensing properties by the optimization of p-CuO/n-ZnO composite nanofibers. J. Mater. Sci. 2020, 55, 7702–7714. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Ibrahim, A.A.; Al-Heniti, S.H.; Kumar, R.; Baskoutas, S.; Raffah, B.M. Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles. Ceram. Int. 2017, 43, 6765–6770. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, Z.; Li, X.; Geng, Y.; Tian, X.; Du, Y.; Qian, Z. Enhanced acetone-sensing properties to ppb detection level using Au/Pd-doped ZnO nanorod. Sens. Actuators B Chem. 2020, 310, 127129. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xie, C.; Wu, J.; Zeng, D.; Liao, Y. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Bulakhe, R.N.; Lokhande, C.D.; Kale, S.N.; Naushad, M.; Mane, R.S. Sprayed zinc oxide films: Ultra-violet light-induced reversible surface wettability and platinum-sensitization-assisted improved liquefied petroleum gas response. J. Colloid Interface Sci. 2016, 480, 109–117. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef]
- Bhat, P.; Kumar, N.S.K.; Nagaraju, P. Synthesis and characterization of ZnO-MWCNT nanocomposites for 1-butanol sensing application at room temperature. Phys. B Condens. Matter 2019, 570, 139–147. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, M.; Yang, M. In-situ grown nanostructured ZnO via a green approach and gas sensing properties of polypyrrole/ZnO nanohybrids. Sens. Actuators B Chem. 2017, 238, 596–604. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Shao, C.; Li, X.; Ma, J.; Zhang, X.; Liu, Y. Composition-controllable p-CuO/n-ZnO hollow nanofibers for high-performance H2S detection. Sens. Actuators B Chem. 2019, 285, 495–503. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Zeng, W. Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance. Chemosensors 2021, 9, 226. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef]
- Campbell, M.G.; Dinca, M. Metal-Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal-Organic Frameworks. Chempluschem 2016, 81, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Kukkar, P.; Kim, K.-H.; Kukkar, D.; Singh, P. Recent advances in the synthesis techniques for zeolitic imidazolate frameworks and their sensing applications. Coord. Chem. Rev. 2021, 446, 214109. [Google Scholar] [CrossRef]
- Duan, C.X.; Yu, Y.; Hu, H. Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Bai, S.; Fu, H.; Guo, J.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Pyrolyzing Co/Zn bimetallic organic framework to form p-n heterojunction of Co3O4/ZnO for detection of formaldehyde. Sens. Actuators B Chem. 2019, 285, 291–301. [Google Scholar] [CrossRef]
- Yao, M.-S.; Li, W.-H.; Xu, G. Metal-organic frameworks and their derivatives for electrically-transduced gas sensors. Coord. Chem. Rev. 2021, 426, 213479. [Google Scholar] [CrossRef]
- Huang, B.; Zeng, W.; Li, Y. Synthesis of ZnO@ZIF-8 Nanorods with Enhanced Response to VOCs. J. Electrochem. Soc. 2022, 169, 047508. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Li, M.; Ma, L. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. Acs Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Zhan, K.; Xing, Y.; Zhu, Y.; Yan, J.; Chen, Y. Carbon tetrachloride vapor sensing based on ZIF-8@ZnO/TiO2 one-dimensional top-defect photonic crystals. Sens. Actuators A Phys. 2020, 314, 112249. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Matatagui, D.; Sainz-Vidal, A.; Gracia, I.; Figueras, E.; Cane, C.; Saniger, J.M. Chemoresistive gas sensor based on ZIF-8/ZIF-67 nanocrystals. Sens. Actuators B Chem. 2018, 274, 601–608. [Google Scholar] [CrossRef]
- Du, B.; Cai, M.; Wang, X.; Qian, J.; He, C.; Shui, A. Enhanced electromagnetic wave absorption property of binary ZnO/NiCo2O4 composites. J. Adv. Ceram. 2021, 10, 832–842. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. Acs Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Sun, Y.; Wu, C.; Wang, X.; Tang, X.; Zhang, T.; Wang, H.; Xie, C.; Zeng, D. Gas Adsorption at Metal Sites for Enhancing Gas Sensing Performance of ZnO@ZIF-71 Nanorod Arrays. Langmuir 2019, 35, 3248–3255. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, S.; Mao, Z.; Hu, S.; Long, X. A Designed ZnO@ZIF-8 Core-Shell Nanorod Film as a Gas Sensor with Excellent Selectivity for H-2 over CO. Chem. A Eur. J. 2017, 23, 7969–7975. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.; Zhang, Q.; Wang, W.; Lin, Y.; Zhang, S. ZnO@ZIF-8 Core-Shell Structure Gas Sensors with Excellent Selectivity to H-2. Sensors 2021, 21, 4069. [Google Scholar] [CrossRef]
- Yao, M.-S.; Tang, W.-X.; Wang, G.-E.; Nath, B.; Xu, G. MOF Thin Film-Coated Metal Oxide Nanowire Array: Significantly Improved Chemiresistor Sensor Performance. Adv. Mater. 2016, 28, 5229–5244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Zhang, T.; Liu, S.; Fei, T. Zeolitic imidazolate framework-8 (ZIF-8)-coated In2O3 nanofibers as an efficient sensing material for ppb-level NO2 detection. J. Colloid Interface Sci. 2019, 541, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, T.; Zhang, Y.; Tang, L.; Guo, Q.; Wang, M.; Xie, C.; Zeng, D. Synthesis of core-shell flower-like WO3@ZIF-71 with enhanced response and selectivity to H2S gas. Solid State Ion. 2020, 350, 115278. [Google Scholar] [CrossRef]
- Dmello, M.E.; Sundaram, N.G.; Kalidindi, S.B. Assembly of ZIF-67 Metal-Organic Framework over Tin Oxide Nanoparticles for Synergistic Chemiresistive CO2 Gas Sensing. Chem. A Eur. J. 2018, 24, 9220–9223. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Wang, X.; Wu, C.; Zeng, D.; Xie, C. Pore size dependent gas-sensing selectivity based on ZnO@ZIF nanorod arrays. Sens. Actuators B Chem. 2018, 258, 1099–1106. [Google Scholar] [CrossRef]
- Cui, F.; Chen, W.; Jin, L.; Zhang, H.; Jiang, Z.; Song, Z. Fabrication of ZIF-8 encapsulated ZnO microrods with enhanced sensing properties for H-2 detection. J. Mater. Sci. Mater. Electron. 2018, 29, 19697–19709. [Google Scholar] [CrossRef]
- Ji, P.; Hu, X.; Tian, R.; Zheng, H.; Sun, J.; Zhang, W.; Peng, J. Atom-economical synthesis of ZnO@ZIF-8 core-shell heterostructure by dry gel conversion (DGC) method for enhanced H-2 sensing selectivity. J. Mater. Chem. C 2020, 8, 2927–2936. [Google Scholar] [CrossRef]
- Nair, S.S.; Illyaskutty, N.; Tam, B.; Yazaydin, A.O.; Emmerich, K.; Steudel, A.; Hashem, T.; Schoettner, L.; Woell, C.; Kohler, H.; et al. ZnO@ZIF-8: Gas sensitive core-shell hetero-structures show reduced cross-sensitivity to humidity. Sens. Actuators B Chem. 2020, 304, 127184. [Google Scholar] [CrossRef]
- Zhan, W.-W.; Kuang, Q.; Zhou, J.-Z.; Kong, X.-J.; Xie, Z.-X.; Zheng, L.-S. Semiconductor@Metal-Organic Framework Core-Shell Heterostructures: A Case of ZnO@ZIF-8 Nanorods with Selective Photoelectrochemical Response. J. Am. Chem. Soc. 2013, 135, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Khudiar, A.I.; Elttayef, A.K.; Khalaf, M.K.; Oufi, A.M. Fabrication of ZnO@ZIF-8 gas sensors for selective gas detection. Mater. Res. Express 2019, 6, 126450. [Google Scholar] [CrossRef]
- Yao, M.-S.; Cao, L.-A.; Tang, Y.-X.; Wang, G.-E.; Liu, R.-H.; Kumar, P.N.; Wu, G.-D.; Deng, W.-H.; Hong, W.-J.; Xu, G. Gas transport regulation in a MO/MOF interface for enhanced selective gas detection. J. Mater. Chem. A 2019, 7, 18397–18403. [Google Scholar] [CrossRef]
- Park, S. High-response and selective hydrogen sensing properties of porous ZnO nanotubes. Curr. Appl. Phys. 2016, 16, 1263–1269. [Google Scholar] [CrossRef]
- Lian, X.-x.; Li, Y.; Lv, T.; Zou, Y.-l.; An, D.; Zhang, N. Preparation of ZnO Nanoparticles by Combustion Method and Their Gas Sensing Properties. Electron. Mater. Lett. 2016, 12, 24–31. [Google Scholar] [CrossRef]
- Song, L.; Yue, H.; Li, H.; Liu, L.; Li, Y.; Du, L.; Duan, H.; Klyui, N.I. Hierarchical porous ZnO microflowers with ultra-high ethanol gas-sensing at low concentration. Chem. Phys. Lett. 2018, 699, 1–7. [Google Scholar] [CrossRef]
- Feng, S.; Jia, X.; Yang, J.; Li, Y.; Wang, S.; Song, H. One-pot synthesis of core-shell ZIF-8@ZnO porous nanospheres with improved ethanol gas sensing. J. Mater. Sci. Mater. Electron. 2020, 31, 22534–22545. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Islam, M.N.; Ghosh, T.B.; Chopra, K.L.; Acharya, H.N. XPS and X-ray diffraction studies of aluminum-doped zinc oxide transparent conducting films. Thin Solid Film. 1996, 280, 20–25. [Google Scholar] [CrossRef]
- Wei, X.Q.; Man, B.Y.; Liu, M.; Xue, C.S.; Zhuang, H.Z.; Yang, C. Blue luminescent centers and microstructural evaluation by XPS and Raman in ZnO thin films annealed in vacuum, N-2 and O-2. Phys. B Condens. Matter 2007, 388, 145–152. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. Acs Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, Z.; Yang, W.; Faheem, M.; Xing, J.; Zou, X.; Pan, Q.; Zhu, G.; Du, Y. ZnO@ZIF-8 core-shell microspheres for improved ethanol gas sensing. Sens. Actuators B Chem. 2019, 284, 421–427. [Google Scholar] [CrossRef]
- Jafari, N.; Zeinali, S.; Shadmehr, J. Room temperature resistive gas sensor based on ZIF-8/MWCNT/AgNPs nanocomposite for VOCs detection. J. Mater. Sci. Mater. Electron. 2019, 30, 12339–12350. [Google Scholar] [CrossRef]
- Japip, S.; Liao, K.-S.; Xiao, Y.; Chung, T.-S. Enhancement of molecular-sieving properties by constructing surface nano-metric layer via vapor cross-linking. J. Membr. Sci. 2016, 497, 248–258. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Zeng, W.; Li, Y. Synthesis of ZIF-8 Coating on ZnO Nanorods for Enhanced Gas-Sensing Performance. Chemosensors 2022, 10, 297. https://doi.org/10.3390/chemosensors10080297
Huang B, Zeng W, Li Y. Synthesis of ZIF-8 Coating on ZnO Nanorods for Enhanced Gas-Sensing Performance. Chemosensors. 2022; 10(8):297. https://doi.org/10.3390/chemosensors10080297
Chicago/Turabian StyleHuang, Bo, Wen Zeng, and Yanqiong Li. 2022. "Synthesis of ZIF-8 Coating on ZnO Nanorods for Enhanced Gas-Sensing Performance" Chemosensors 10, no. 8: 297. https://doi.org/10.3390/chemosensors10080297
APA StyleHuang, B., Zeng, W., & Li, Y. (2022). Synthesis of ZIF-8 Coating on ZnO Nanorods for Enhanced Gas-Sensing Performance. Chemosensors, 10(8), 297. https://doi.org/10.3390/chemosensors10080297






